In this issue of Blood, Jodele et al demonstrate that using C5 complement inhibitor, eculizumab, is associated with high survival rates in children and young adults with hematopoietic stem cell transplant-associated thrombotic microangiopathy (TA-TMA) and multiorgan dysfunction.1
This trial is a culmination of over a decade of deliberate work led by Jodele and colleagues. During the past decade, the investigators systematically established diagnostic and prognostic criteria for TA-TMA, highlighted the efficacy of eculizumab in a retrospective study, and established a pharmacokinetic and pharmacodynamic model-based eculizumab dosing strategy.2-4 These prior studies laid the groundwork for the current successful trial. The trial results are remarkable for several reasons. First, this is the first prospective multicenter clinical trial to examine the role of anticomplement therapy with eculizumab in TA-TMA. Second, the investigators were able to apply rigorous biomarker-based diagnostic and prognostic criteria to diagnose and treat seriously ill children and young adults. Finally, the study results in 21 patients highlight an impressive survival rate of 71% at 6 months after diagnosis of TA-TMA and multiorgan dysfunction, compared with a dismal survival of 18% at 6 months in a prior prospective observational study from the same group.2 The trial also demonstrated improvement in organ function in 73% of patients.
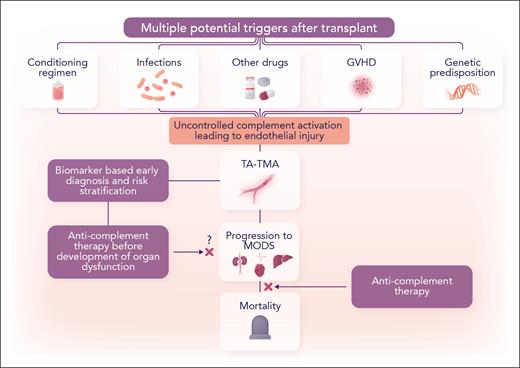
TA-TMA is a difficult condition to diagnose and has poor prognosis. Many predisposing factors can result in complement-mediated endothelial injury, TA-TMA, and multiorgan dysfunction (see figure). In clinical practice, differentiating TA-TMA from other causes, such as calcineurin inhibitor toxicity, is not always obvious. A recent international expert panel guideline highlighted this diagnostic challenge and identified validation of more specific diagnostic and prognostic biomarkers of TA-TMA as a top priority.5 In children and young adults, Jodele et al have previously studied biomarker-based diagnostic and prognostic criteria and applied these in their routine clinical practice to monitor TA-TMA in the early posttransplant period. The biomarkers include elevated soluble terminal complement complex (sC5b-9) and proteinuria measured by random urine protein/creatinine ratio. In their prior study, elevated levels of sC5b-9 and proteinuria (≥1 mg/mg) were associated with a higher risk of nonrelapse mortality and identified patients with high-risk TA-TMA. The current trial used the criteria of Jodele et al for diagnosing TA-TMA and enrolled patients with biomarker-based high-risk TA-TMA, all of whom had developed multiorgan dysfunction.
Complement blockade as a therapeutic strategy in TA-TMA. GVHD, graft-versus-host disease; MODS, multiorgan dysfunction. Professional illustration by Somersault18:24.
Complement blockade as a therapeutic strategy in TA-TMA. GVHD, graft-versus-host disease; MODS, multiorgan dysfunction. Professional illustration by Somersault18:24.
The trial used the results of a prior prospective observational study as a historical control. A randomized placebo-controlled trial in this rare disease was not considered ethical because of the high mortality in the historical controls. The trial enrolled patients undergoing allogeneic or autologous hematopoietic stem cell transplants. The median time to diagnosis of TA-TMA was 22 days after transplant. Eculizumab therapy included intensive weight-based loading doses, followed by induction and maintenance doses. The adequacy of complement blockade was confirmed by measuring eculizumab serum concentrations (goal, >100 μg/ml) as well as levels of sC5b-9 (goal, normal level) and total hemolytic complement (CH50) (goal, <10% of the lower normal value). Infections are feared complications associated with the use of eculizumab. With antimicrobial prophylaxis, no patient developed Neisseria meningitidis; however, 33% of patients had bacteremia, and 2 patients developed candidemia. Such rates of infection are within the expected range in this patient population. Although viremia was common (57%), most cases were present before the diagnosis of TA-TMA. The use of eculizumab resulted in an impressive 4-fold improvement in 6-month survival compared with untreated historical controls, demonstrating a high survival benefit associated with the use of eculizumab in this multicenter trial.
The trial raises a few questions and issues that would need further investigation. Of all patients, 29% did not survive beyond 6 months, and 27% of survivors had residual organ dysfunction at 6 months. What factors led to the failure of this approach in about a quarter of patients despite meticulous complement inhibition and dose adjustment based on eculizumab serum concentration? Acute GVHD occurred in 7 patients (38%); 3 of these patients died because of uncontrolled GVHD or multiorgan dysfunction, perhaps highlighting that success in treating the underlying causes, such as GVHD, also plays an important role. Patients with gastrointestinal tract bleeding represent a group of patients at a particularly high risk of poor outcomes, who may benefit from more frequent doses of eculizumab, a longer course of anticomplement therapy, and strategies to reduce the risk of infection.1,3 The trial measured sC5-9b level to both assist in the diagnosis of TA-TMA and identify high-risk features. Could difficulty in obtaining the results of sC5-9b level or eculizumab drug concentration make it challenging to translate the trial results to routine clinical practices? In cases where sC5-9b is not measured and TA-TMA is diagnosed based on other clinical factors, or if sC5-9b levels are not elevated, how effective would complement blockade be? How do the study results in children and young adults translate to adults with TA-TMA? Given the success of complement blockade in patients who have developed multiorgan dysfunction, the trial results also raise an important question of whether patients with biomarker-based high-risk TA-TMA could do better if eculizumab is started earlier in the disease course (eg, before the development of organ dysfunction)? Although several unanswered questions exist, the trial has made a giant forward stride and opened up avenues to further build on the success of this study.
Conflict-of-interest disclosure: K.G. serves on an advisory board for Autolus and BMS. V.R.B. participates in the Safety Monitoring Committee for Protagonist; serves as an associate editor for the journal Current Problems in Cancer and as a contributor for BMJ Best Practice; receives consulting fees from Imugene, research funding (institutional) from MEI Pharma, Actinium Pharmaceutical, Sanofi US Services, AbbVie, Pfizer, Incyte, Jazz, and National Marrow Donor Program; and receives drug support (institutional) from Chimerix for a trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal