Key Points
Pembrolizumab monotherapy provides sustained antitumor activity in heavily pretreated R/R PMBCL.
Complete responses were maintained after ∼4 years of follow-up.
Abstract
Previous analyses of the phase 2 KEYNOTE-170 (NCT02576990) study demonstrated effective antitumor activity and acceptable safety of pembrolizumab 200 mg given every 3 weeks for up to 35 cycles (∼2 years) in patients with relapsed/refractory (R/R) primary mediastinal B-cell lymphoma (PMBCL) whose disease progressed after or who were ineligible for autologous stem cell transplantation. The end points included objective response rate (ORR), progression-free survival (PFS), and duration of response (DOR) according to the investigator per 2007 Response Criteria; overall survival (OS); and safety. In this final analysis, median duration of follow-up was 48.7 months (range, 41.2-56.2). The ORR was 41.5% (complete response, 20.8%; partial response, 20.8%). The median DOR was not reached; no patients who achieved a complete response progressed at the data cutoff. The median PFS was 4.3 months; the 4-year PFS rate was 33.0%. The median OS was 22.3 months; the 4-year OS rate was 45.3%. At the data cutoff, 30 patients (56.6%) had any-grade treatment-related adverse events (AEs); the most common were neutropenia, asthenia, and hypothyroidism. Grade 3/4 treatment-related AEs occurred in 22.6% of the patients; no grade 5 AEs occurred. After 4 years of follow-up, pembrolizumab continued to provide durable responses, with promising trends for long-term survival and acceptable safety in R/R PMBCL.
Introduction
Primary mediastinal B-cell lymphoma (PMBCL) is a rare and aggressive lymphoma predominantly affecting young women.1 Although morphologically similar to diffuse large B-cell lymphoma, genomic studies have shown that PMBCL shares more similarities with classical Hodgkin lymphoma and in particular demonstrates frequent amplification or translocation events at 9p24.1, resulting in overexpression of programmed death ligand 1 and programmed death ligand 2.2-6 These genetic features of PMBCL provided the rationale for testing programmed death 1 (PD-1) blockade in this disease, as did the very limited treatment options for patients with relapsed/refractory disease, for whom prognosis is poor.7 In the phase 1b KEYNOTE-013 study (NCT01953692), patients with relapsed/refractory (R/R) PMBCL treated with the PD-1 inhibitor pembrolizumab achieved an objective response rate (ORR) of 41% and durable responses (median duration of response [DOR], not reached after 11.3 months of follow-up [range, 3.4-27.4]), supporting pembrolizumab as a possible treatment option for these patients.8 The open-label, phase 2 KEYNOTE-170 study (NCT02576990) of pembrolizumab monotherapy in patients with PMBCL was designed to confirm these findings.9 Earlier results from this study led to approval by the US Food and Drug Administration of pembrolizumab for patients with R/R PMBCL after ≥2 previous lines of therapy.10
Because PMBCL is an aggressive malignancy with few salvage options, the long-term outcomes of patients treated with pembrolizumab remain a salient clinical question. In particular, the DOR and predictors of durable responses inform both prognosis and decisions regarding consolidation therapy, particularly for stem cell transplantation (SCT). In this final analysis of the KEYNOTE-170 study, we present data on the efficacy and safety of pembrolizumab in patients with R/R PMBCL with ∼4 years of follow-up.
Study design
Patients
The detailed inclusion and exclusion requirements and study methodology for KEYNOTE-170 have been previously described.9 Key eligibility requirements included patients aged at least 18 years who had experienced disease progression after or ineligibility for (after at least 2 previous lines of therapy) autologous SCT. Patients received pembrolizumab 200 mg by intravenous infusion every 3 weeks for up to 35 cycles (2 years) or documented disease progression by investigator assessment, unacceptable toxicity, or patient withdrawal.9
Assessments
Diagnostic imaging via computed tomography and positron emission tomography was performed at weeks 12 and 24 for confirmation of complete response (CR) and every 12 weeks thereafter by computed tomography only. Survival outcomes were assessed every 12 weeks during follow-up.9 Adverse events (AEs) were collected and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, throughout the study and up to 30 days after the last study treatment dose or 90 days after the last study dose for serious AEs. Immune-mediated AEs were based on a list of terms specified by the sponsor, regardless of the attribution to the study treatment or immune relatedness by the investigator.
End points
The primary end points for the original analysis were ORR by blinded independent central review using the International Working Group 2007 criteria11 and safety.9 Secondary end points included ORR, DOR, and progression-free survival (PFS) according to the investigator assessment per International Working Group 2007 criteria and overall survival (OS).
Statistical analysis
The primary analysis population for both efficacy and safety assessments included all patients who received at least 1 dose of study pembrolizumab. ORR by the investigator assessment was assessed by point estimate with an associated 2-sided 90% confidence interval (CI). The final ORR analysis concluded once the last patient had discontinued the study treatment. PFS, DOR, and OS were estimated using the Kaplan-Meier method, with missing data censored at the last assessment. The final analysis of KEYNOTE-170 was based only on investigator assessment. The data cutoff date was 23 October 2020.
Results and discussion
Fifty-three patients were enrolled in this study. The median duration of follow-up, defined as the time from the first study dose to the data cutoff date, was 48.7 months (range, 41.2-56.2). Patients had received a median of 3 (range, 2-8) previous lines of therapy, and 16 patients (30.2%) had primary refractory disease (patients who were refractory to all regimens received). All patients had received previous rituximab, 17 (32.1%) had received previous radiation therapy, and 14 (26.4%) had previous autologous SCT.
Among all treated patients, 13 (24.5%) completed 2 years of study treatment; 40 (75.5%) discontinued treatment because of progressive disease (PD; 34.0%), clinical progression (22.6%), AEs (11.3%), and physician decision (5.7%). Although this was not prespecified in the protocol, 1 patient (1.9%) discontinued treatment before completing the 2 years of study treatment after achieving CR.
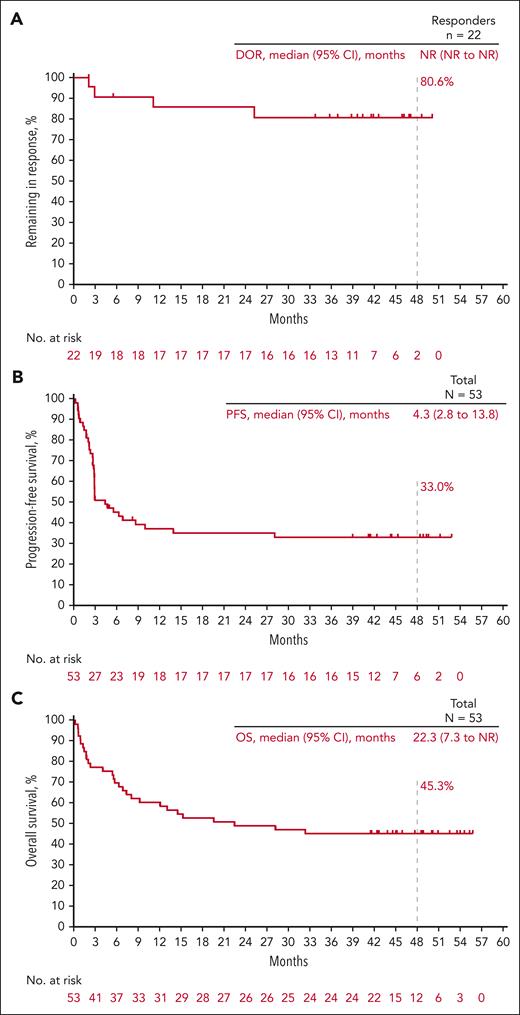
Among the 53 patients included in the efficacy analysis set, the ORR based on investigator assessment was 41.5% (90% CI, 30.0-53.7). The best overall response (BOR) to CR was observed in 11 patients (20.8%) and the BOR to PR was observed in 11 patients (20.8%). Six patients (11.3%) had a BOR for stable disease, 13 (24.5%) had PD, 11 (20.8%) did not reach the first assessment time point because of death (n = 9) or starting subsequent anticancer therapy (n = 2) before the first assessment, and 1 (1.9%) was not assessed. Eighteen patients had a first response to PR. Of these patients, 7 improved to CR, 8 remained in PR, and 3 had PD. The median (95% CI) DOR was not reached (not reached-not reached); the estimated percentage of responders continuing in response was 80.6% at 48 months (Figure 1A). For patients with CR, the median (range) time to CR was 2.7 months (2.0-5.5) and the median (range) duration of CR was not reached (33.7+ to 50.0+ months [“+” indicates there is no PD by the time of last disease assessment]). Notably, none of the patients with CR progressed at the data cutoff, and none received consolidation SCT or subsequent therapy. Eighteen patients had a first response to PR. Of those patients, 7 improved to CR, 8 remained in PR, and 3 had PD. In a post hoc analysis of patients with primary refractory disease (n = 16), the ORR was 25.0% (4 PRs) and the median (95% CI) DOR was not reached (11.1 months-not reached). For patients without primary refractory disease (n = 37), the ORR was 46.9% (11 CRs, 7 PRs) and the median (95% CI) DOR was not reached (not reached-not reached). Seven patients underwent SCT (autologous, 5; allogeneic, 2) after pembrolizumab treatment. The BOR to pembrolizumab for the 5 patients who received subsequent autologous SCT was PR (n = 1; experienced PD before transplantation), stable disease (n = 1), and PD (n = 3). Among the patients who underwent allogeneic SCT, the BOR for pembrolizumab was PR (n = 2). Among all treated patients, the median PFS was 4.3 months, and the PFS rate at 4 years was 33.0% (Figure 1B). The median OS was 22.3 months, and the OS rate at 4 years was 45.3% (Figure 1C).
Kaplan-Meier estimates of response duration, progression-free survival and overall survival. (A) DOR; (B) PFS; (C) OS. NR, not reached.
Kaplan-Meier estimates of response duration, progression-free survival and overall survival. (A) DOR; (B) PFS; (C) OS. NR, not reached.
At the data cutoff, treatment-related AEs occurred in 30 of the 53 patients (56.6%). The most-reported treatment-related AEs (incidence >5%) were neutropenia (18.9%), asthenia (9.4%), hypothyroidism (7.5%), and fatigue and pyrexia (5.7% each; Table 1). Serious treatment-related AEs occurred in 4 patients (7.5%); 1 patient discontinued treatment because of a serious treatment-related AE. Four patients (7.5%) discontinued treatment because of treatment-related AE. Grade 3/4 treatment-related AEs occurred in 12 patients (22.6%), most commonly neutropenia (n = 7; 13.2%). Three patients died because of AEs unrelated to treatment (myocardial infarction, cardiac tamponade, and Aspergillus infection) and no deaths were due to treatment-related AEs. Nine immune-mediated AEs were observed in 6 patients (hyperthyroidism, n = 2; hypothyroidism, n = 4; pneumonitis, n = 1; thyroiditis, n = 1). One patient had a grade 3/4 immune-mediated AE (grade 4 pneumonitis)
With ∼4 years of follow-up, this study provides long-term data on patients with PMBCL treated with PD-1 blockade. Data from this study confirmed that pembrolizumab monotherapy was associated with frequent responses in heavily pretreated patients with R/R PMBCL. Responses were durable, particularly among patients with CR, none of whom had relapsed or received SCT consolidation at the time of data cutoff. Given the long duration of follow-up after treatment, the durable responses were strongly suggestive of a curative potential for pembrolizumab in this subgroup and supported observation rather than SCT for complete responders. In addition, the depth of radiographic response appears to be the strongest predictor of outcome to date, which is similar to the response data observed with PD-1 inhibitors in Hodgkin lymphoma.12,13
The phase 2 CheckMate 436 study of the PD-1 inhibitor nivolumab plus brentuximab vedotin (BV) in patients with R/R PMBCL after autologous SCT also demonstrated effective antitumor activity (ORR = 73% by investigator review) and durable responses (median DOR not reached) as well as an acceptable safety profile,14 despite the limited single-agent activity of BV in this disease.15 Further studies may shed additional light on the potential therapeutic benefit of adding BV to PD-1 blockade in PMBCL.
The results of KEYNOTE-170 confirmed that PD-1 blockade is an effective and safe treatment option for R/R PMBL, and an ongoing study (NCT04759586) is currently testing its role as first-line therapy.
Acknowledgments
The authors thank the patients and their families and caregivers and all the primary investigators and site personnel. Medical writing and editorial assistance were provided by Dominic Singson and Matthew Grzywacz of ApotheCom (Yardley, PA).
This study and assistance with manuscript preparation were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
Authorship
Contribution: P.L.Z., M.A.S., S.T., P.M., and P.A. contributed to the conception, design, or planning of the study; C.T., V.M., K.B., J.W., A.M., L.F., A.M.G.-S., B.C., M.Ö., G.F.P., and P.A. contributed to acquisition of the data; B.C., Z.G., S.C., and S.T. contributed to the analysis of the data; P.L.Z., C.T., J.W., A.M.G.-S., B.C., Z.G., H.G., M.A.S., S.C., S.T., and P.A. contributed to interpretation of the results; Z.G. and S.C. contributed to drafting the manuscript; and P.L.Z., C.T., V.M., K.B., J.W., A.M., L.F., A.M.G.-S., B.C., M.Ö., G.F.P., H.G., M.A.S., S.C., S.T., P.M., and P.A. contributed to critically reviewing or revising the manuscript for important intellectual content.
Conflict-of-interest disclosure: P.L.Z. reports advisory/consultancy roles with Secura Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, EUSA Pharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte, and BeiGene; and speaker bureau roles with Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therapeutics, Takeda, Roche, EUSA Pharma, Kyowa Kirin, Novartis, Incyte, and BeiGene. C.T. reports advisory/consultancy roles and travel accommodations/expenses with Roche, Incyte, Novartis, Kite/Gilead, Amgen, Takeda, and BMS. K.B. reports honoraria from Takeda, Kite-Gilead, Sandoz, and AbbVie; advisory/consultancy roles with Takeda and Kite-Gilead; and research funding from AbbVie and Takeda. J.W. reports advisory/consultancy roles with Novartis; and research grant/funding from AstraZeneca, BMS Celgene, Epizyme, Gilead, GSK, Incyte, Janssen-Cilag, Karyopharm, Morphosys, MSD, Nanovector, Polish Lymphoma Research Group, Polish Myeloma Consortium, Regeneron, Roche, Seagen, Takeda, and TG Therapeutics. L.F. reports honoraria from AstraZeneca. A.M.G.-S. reports honoraria from Roche, BMS/Celgene, Janssen, Servier, Gilead/Kite, Takeda, EUSA Pharma, and Novartis; advisory/consultancy roles with Roche, BMS/Celgene, Kyowa Kirin, Clinigen, EUSA Pharma, Novartis, Gilead/Kite, Servier, Incyte, Lilly, Takeda, ADC Therapeutics America, and Miltenyi; research grant/funding from Janssen and Teva; and travel/accommodation/expenses from Gilead/Kite, Janssen, Roche, BMS/Celgene, Servier, and Kern Pharma. B.C. reports advisory/consultancy roles with Genentech, MorphoSys, ADC Therapeutics, and GenMab; and research grant/funding from Genentech/Roche, Celgene/BMS, Acerta, Triphase, MorphoSys, SeaGen, and Millennium. Z.G. reports an advisory/consultancy role paid to the institution with BMS and a speaker bureau role paid to the institution with Amgen. M.Ö. reports research grant/funding from AbbVie, Bayer, Janssen, Acerta, Reddy’s, MSD, Roche, and Takeda and travel/accommodation/expenses from AbbVie, Jazz, and Roche. G.F.P. reports advisory/consultancy roles with Janssen, AbbVie, Takeda, and AstraZeneca; speaker bureau roles with Janssen, AbbVie, Takeda, AstraZeneca, and MSD; and travel/accommodation/expenses from Janssen, Takeda, AstraZeneca, and MSD. H.G. reports honoraria from Gilead, Roche, BMS, and AbbVie and advisory/consultancy roles with Gilead and Roche. M.A.S. reports research grant/funding from AstraZeneca, Bayer, BMS, and AbbVie. S.T. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co, Inc, Rahway, NJ. S.C. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co, Inc, Rahway, NJ. P.M. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co, Inc, Rahway, NJ. P.A. reports advisory/consultancy roles with Merck, BMS, Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech, and Xencor and research grant/funding from Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM, and Kite. The remaining authors declare no competing financial interests.
Correspondence: Pier Luigi Zinzani, IRCCS Azienda Ospedaliero-Universitaria di Bologna Istituto di Ematologia ‘Seràgnoli,’ Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università degli Studi, Bologna, via Massarenti 9, 40138 Bologna, Italy; e-mail: pierluigi.zinzani@unibo.it.
References
Author notes
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials to conduct legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with the MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.