In this issue of Blood, Zinzani et al present long-term follow-up data demonstrating that pembrolizumab, an inhibitor of programmed cell death protein 1 (PD-1), is safe and effective in patients with relapsed/refractory primary mediastinal B-cell lymphoma (PMBCL).1 In patients who achieve a complete response, the response is durable at 4 years of follow-up with no additional therapy, suggesting that some patients with relapsed PMBCL may be cured with checkpoint blockade alone.
PMBCL is a rare subtype of non-Hodgkin lymphoma with clinical and biological overlap with classic Hodgkin lymphoma. The key molecular features of PMBCL include frequent copy number gains/amplifications in 9p24.1, resulting in upregulation of programmed cell death ligand 1 (PD-L1) and PD-L2 and recurrent genetic abnormalities in B2M, CIITA, CD58, CD274, and PDC1LG2, which contribute to an immunosuppressive tumor microenvironment.2 This constellation of molecular characteristics makes PMBCL an ideal tumor to target with immune checkpoint blockade.
Previous trials have investigated the role of PD-1 inhibitors in the relapsed setting with promising early signals of safety and efficacy. In a phase 1b trial, pembrolizumab was studied in patients with relapsed/refractory PMBCL to evaluate safety and tolerability.3 Of patients, 61% experienced a drug-related adverse event (AE), most of which were grade 1 to 2, and no patient discontinued treatment due to toxicity. The overall response rate (ORR) was 41% (7/17), including 2 patients who achieved a complete response (CR) and 5 patients who achieved a partial response (PR). The subsequent phase 2 KEYNOTE-170 trial evaluated pembrolizumab in 53 patients with relapsed/refractory PMBCL.4 With a median follow-up of 12.5 months, the ORR was 45% (13% CR). The median duration of response was not reached. On the basis of these trials, pembrolizumab was approved by the US Food and Drug Administration for patients with PMBCL who have received at least 2 prior lines of therapy.5 The PD-1 inhibitor nivolumab has also been evaluated in relapsed/refractory PMBCL in combination with the anti-CD30 antibody drug conjugate brentuximab vedotin.6 In a phase 2 trial, the ORR to the combination was 73% (37% CR) with a median follow-up of 11.1 months. Collectively, these studies provide strong support for checkpoint inhibition in relapsed/refractory PMBCL, albeit with short-term follow-up. Long-term follow-up is important to understand the durability of responses as well as the emergence of late adverse effects, which are particularly relevant with checkpoint blockade, where immune-mediated adverse events can emerge at later time points.
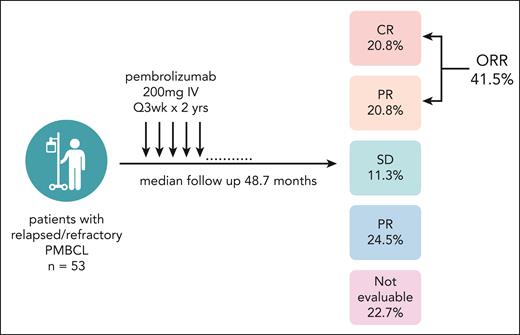
Here, Zinzani and colleagues present the final report from KEYNOTE-170 with a median follow-up of 48.7 months (figure 1). This trial provides the longest follow-up of patients with PMBCL treated with checkpoint inhibition and an opportunity to understand response durability and late toxicities. This trial enrolled patients aged ≥18 years with relapsed/refractory PMBCL who had either progressed after hematopoietic stem cell transplant or were ineligible for transplant and had received at least 2 prior therapies. Pembrolizumab was given at 200 mg IV every 3 weeks for up to 2 years. Of patients, 24% completed all 2 years of treatment. The ORR was 41.5% (20.8% CR), and the 4-year progression-free survival and overall survival rates were 33% and 45%, respectively. Grade 3/4 treatment-related AEs occurred in 22.6% of patients, and 7.5% of patients interrupted treatment due to a treatment-related AE. Nine immune-mediated AEs occurred in a total of 6 patients, including 1 case of grade 3/4 pneumonitis. This safety profile is reassuring; however, larger cohorts will be needed to understand the full spectrum of AEs in this population.
Study schema and best overall response. Among the patients who were not evaluable, 9 (17%) died before the first assessment, 2 (3.7%) received subsequent anti-cancer therapy before the first assessmenet and 1 (1.9%) was not assessed. Image created with biorender.com.
Study schema and best overall response. Among the patients who were not evaluable, 9 (17%) died before the first assessment, 2 (3.7%) received subsequent anti-cancer therapy before the first assessmenet and 1 (1.9%) was not assessed. Image created with biorender.com.
There are several key findings from this trial that will inform treatment decisions in PMBCL. First, there were a significant subset of patients who converted from a PR to a CR (7 of 18 patients with an initial PR). The median time to CR among all patients was 2.7 months (range, 2.0-5.5 months). This suggests that patients with an initial PR should continue therapy with close observation. Second, among the 11 patients who achieved a CR, all remained in a CR at the time of data lockout without consolidative stem cell transplant or other additional therapy. This is a remarkable response in a heavily pretreated population (median of 3 prior therapies; range, 2-8). Given the aggressive nature of PMBCL, with most relapses occurring early, this finding suggests that single-agent pembrolizumab may be curative in a subset of patients who achieve a CR. Last, in a post hoc analysis, patients with primary refractory disease (n = 16) had inferior outcomes, with an ORR of 25% and no patients achieving a CR. In this higher-risk group, additional therapy beyond single-agent pembrolizumab is likely needed.
As this trial firmly establishes the role for checkpoint inhibition in third-line treatment for PMBCL, the next big question in PMBCL is the role for checkpoint inhibition in the second-line and even front-line settings. Standard approaches to second-line therapy include chemotherapy followed by autologous stem cell transplant and/or radiation for cases with disease confined to the mediastinum.7 CD19 chimeric antigen receptor (CAR)-T cell therapy has also shown activity in relapsed/refractory PMCBL; however, the landmark trials of CAR-T in B-cell lymphoma have only included a small number of patients with PMCBL,8 leaving the role for CAR-T in relapsed/refractory PMBCL undefined. Where checkpoint inhibitors fit into second-line treatment options for PMBCL remains unseen and will require future studies. The integration of checkpoint inhibition into frontline chemoimmunotherapy is also of great interest as it may improve outcomes and reduce the number of patients requiring radiation therapy. Our team is leading a randomized phase 3 trial that is investigating the addition of nivolumab to standard therapy for patients with previously untreated PMCBL (NCT04759586). This trial, which is currently open to enrollment, should define the role for checkpoint inhibition in the frontline management of PMBCL.
In summary, the data presented by Zinzani and colleagues in this issue of Blood provide the strongest evidence available to date supporting the use of checkpoint inhibitors in PMBCL. This work establishes the role for checkpoint inhibition in the third-line setting and paves the way for studies to investigate checkpoint inhibitors in earlier settings.
Conflict-of-interest disclosure: L.G.-R. is a consultant to Merck and Roche.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal