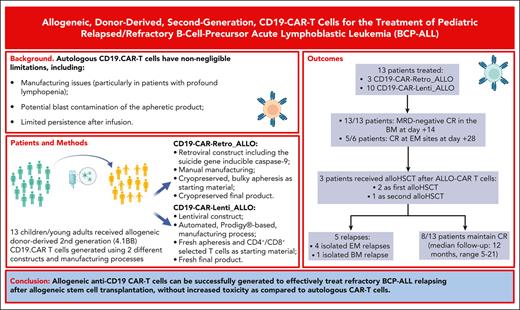
Key Points
Allogeneic, donor-derived CD19 CAR-T cells can be readily generated for the treatment of pediatric patients with relapsed/refractory BCP-ALL.
Allogeneic CD19 CAR-T cells are effective, and compared with autologous CAR, they neither increase toxicity, nor induce high GVHD incidence.
Abstract
Autologous CD19-directed chimeric antigen receptor (CAR)-T cells have shown unprecedented efficacy in children with relapsed/refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL). However, patients either relapsing after allogeneic hematopoietic stem cell transplantation (allo-HSCT) or displaying profound lymphopenia and/or rapidly progressing disease often cannot access autologous products. These hurdles may be overcome by allogeneic, donor-derived CAR-T cells. We tested donor-derived T cells transduced with a second-generation (4.1BB) CD19-directed CAR for treatment of patients with BCP-ALL in a hospital-exemption setting. Two constructs were tested: a retroviral construct incorporating the suicide gene inducible caspase-9 (CD19-CAR–Retro_ALLO) first and then a lentiviral construct and an automated, Prodigy-based manufacturing process (CD19-CAR–Lenti_ALLO). Thirteen children/young adults received ALLO–CAR-T cells between March 2021 and October 2022. Doses ranged between 1.0 × 106 and 3.0 × 106 CAR-T cells per kg. The toxicity profile was comparable with that of autologous CAR-T cells, characterized mainly by cytopenia, cytokine release syndrome (maximum grade 1), and grade 2 immune-effector cell–associated neurotoxicity syndrome. One case of acute graft-versus-host disease (GVHD) occurred and was rapidly controlled with steroids and ruxolitinib. None of the other patients, including 3 given ALLO–CAR-T cells from an HLA-haploidentical donor, experienced GVHD. Two patients received ALLO–CAR-T cells before HSCT and showed a significant expansion of CAR-T cells without any sign of GVHD. All patients obtained complete remission (CR) with absence of minimal residual disease in the bone marrow. With a median follow-up of 12 months (range, 5-21), 8 of 13 patients maintained CR. Allogeneic anti-CD19 CAR-T cells can effectively treat highly refractory BCP-ALL relapsing after allo-HSCT without showing increased toxicity as compared with autologous CAR-T cells.
Introduction
The engineering of T cells to express a second-generation chimeric antigen receptor (CAR) directed toward the target antigen CD19 represents an unprecedented revolution in the treatment of children and young adults with relapsed/refractory (r/r) B-cell precursor acute lymphoblastic leukemia (BCP-ALL).1,2 However, in a substantial proportion of patients, some limitations of the approach emerged, including the impossibility of manufacturing a drug product for patients with profound lymphopenia or rapidly progressing disease requiring urgent treatment and the limited persistence of CAR-T cells after infusion, resulting in the occurrence of CD19+ relapse. The use of allogeneic, donor-derived, CAR-T cells for patients relapsing after allogeneic hematopoietic stem cell transplantation (HSCT) may potentially overcome these hurdles by providing a high number of healthy T cells readily available and never exposed to chemotherapy and steroids and, therefore, likely characterized by better functionality. Moreover, an allogeneic donor abrogates the risk of contamination of the drug product with leukemia blasts that could be inadvertently transduced with the CAR construct, inducing an intrinsic mechanism of resistance to CAR T-cell therapy.3-5 However, one of the main limitations to the use of allogeneic CAR-T cells is related to the risk of promoting graft-versus-host disease (GVHD), a potentially fatal complication due to the alloreactive components of donor T cells.
We report our experience with donor-derived T cells transduced with second-generation (4.1BB) CD19-CAR (ALLO–CAR-T cells) for the treatment of pediatric/young adult patients with r/r BCP-ALL occurring after HSCT. Two patients with a history of extremely refractory BCP-ALL and a HLA-matched family donor (MFD) were treated with ALLO–CAR-T cells before receiving allogeneic HSCT.
Methods
Patient selection
At Ospedale Pediatrico Bambino Gesù in Rome, we treated patients with donor-derived CD19-directed CAR-T cells (CD19 CAR-T cells) in a hospital exemption setting. Patients were evaluated for the allogeneic CAR T-cell program in case of relapse after HSCT or profound lymphopenia and/or rapidly progressing disease with high levels of circulating blasts, both of which affecting the possibility of producing autologous CD19 CAR-T cells. Two selected patients were treated before receiving allogeneic HSCT because of extreme refractoriness to all the therapies, profound lymphopenia (ie, less than 200 CD3+ cells per μL), and the availability of a MFD. The authorization for the treatment was obtained for each patient from both the institutional review board and the National Competent Authority (Agenzia Italiana del Farmaco). Each patient or legal representative provided written informed consent.
CD19 CAR-T cells manufacturing and infusion
Two different constructs and manufacturing processes were explored: (1) a retroviral construct incorporating the suicide gene inducible caspase-9, iC9 (CD19-CAR–Retro_ALLO) and a manual manufacturing process; (2) a lentiviral construct and an automated, Prodigy-based manufacturing process (CD19-CAR–Lenti_ALLO). The 2 constructs have been previously described and are both second-generation (containing 4.1BB as costimulatory molecule) CD19 CAR-T cells.6,7 In addition, CD19-CAR–Lenti_ALLO cells were produced and released as fresh products, and the manufacturing included an initial selection of CD4+/CD8+ cells.
All patients received a lymphodepleting regimen consisting of fludarabine (30 mg/m2 on days −5, −4, and −3) and cyclophosphamide (500 mg/m2 on days −4 and −3), and ALLO–CAR-T cells were subsequently administered as a single infusion.
Immune monitoring of patients infused with ALLO–CAR-T cells
Details on the immune monitoring of the patients are reported in the supplemental Appendix, available on the Blood website.
Toxicity grading, safety approach, and response evaluation
Toxicity was graded using the Common Terminology Criteria for Adverse Events (version 5.0), the Lee criteria for cytokine release syndrome (CRS), and the American Society for Transplantation and Cellular Therapy Consensus Grading for immune-effector cell–associated neurotoxicity syndrome (ICANS).8,9 Acute GVHD (aGVHD) was staged according to the criteria of the MAGIC Consortium.10
To reduce the risk of severe toxicities potentially associated with the use of allogeneic products, we treated the first patients with the CD19-CAR–Retro_ALLO because the suicide gene iC9 present in the CAR construct offered us the possibility of a prompt elimination of CAR-T cells after the infusion in case of the occurrence of severe toxicity and severe GVHD. We then switched to the freshly infused CD19-CAR–Lenti_ALLO product, for which we increased the doses.
To evaluate the response, bone marrow (BM) and cerebrospinal fluid examinations were performed on days +14 and +28 after ALLO–CAR T-cell infusion. Criteria for minimal residual disease–negative (MRD–) complete remission (CR) were <1 × 10−5 BM MRD and no evidence of extramedullary leukemia. The data cutoff date for data analysis was 15 March 2023.
Clinical and laboratory evaluation of the patients
Details on the clinical and laboratory evaluation of the patients can be found in the supplemental Appendix.
Results
Patients characteristics
Thirteen children and young adults, median age 15 years (range, 4-33 years), received ALLO–CAR-T cells between March 2021 and October 2022; in detail, 3 patients were given CD19-CAR–Retro_ALLO and 10 were given CD19-CAR–Lenti_ALLO. Characteristics of the patients and the infused drug product are reported in Table 1. All patients were heavily pretreated for either extremely refractory disease or multiple relapses, and 3 patients had previously failed treatment with either academic or commercial autologous CD19 CAR-T cells (2 were given stand-alone treatment, and 1 was consolidated with allo-HSCT). The criteria for proceeding with ALLO–CAR-T cells were profound cytopenia in 7 of 13 patients (54%) refractoriness to all the treatments and cytopenia in 2 of 13 patients (15%), high levels of circulating blasts in 2 of 13 patients (15%; associated with cytopenia in 1 patient and treatment failure of previous autologous CD19 CAR-T cells in the other), and failure of previous autologous CD19 CAR-T cells in 2 of 13 patients (15%). Nine out of 13 patients (69%) had high disease burden before infusion, defined, as previously described, by the presence of either extramedullary disease (6 patients) and/or >5% blasts in the BM (4 patients).11
Characteristics of patients and infused drug products
| Pt ID . | Sex . | Age (y) . | Cytogenetic anomalies . | Disease phase at infusion . | Donor and HLA matching . | CAR-T product . | CAR+ T cells (×106 cells per kg) . | CAR− T cells (×106 cells per kg) . | Disease status at LD (%) . | Response at d +28 . | Site of relapse . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allo-CD19 CAR001 | F | 17 | iAmp21 | Second relapse, very early after HSCT | MUD 10/10 | CD19-CAR–Retro_ALLO | 3.0 | 4.24 | BM (0.3) | CR (MRD–) | CNS (CD19–) |
| Allo-CD19 CAR002 | M | 21 | MEF2D/BCL9 | First refractory relapse | MFD | CD19-CAR–Retro_ALLO | 3.0 (before HSCT) | 3.65 | BM (12) + bone (>10 spots) + liver | BM: CR (MRD–); liver: CR; bone: 3 spots | n.a. |
| Allo-CD19 CAR003 | M | 29 | KMT2A | Fifth relapse | MFD | CD19-CAR–Retro_ALLO | 3.0 | 1.01 | Pelvic lymph nodes + CNS | CR (BM: MRD–; EM: neg) | Pelvic lymph nodes + CNS (CD19+) |
| CD19-LENTI-ALLO001 | M | 11 | TEL/AML1 | Fourth relapse | MFD | CD19-CAR–Lenti_ALLO | 1.0 | 1.92 | BM (0.2) + bone and kidney | CR (BM: MRD–; EM: neg) | Bone |
| CD19-LENTI-ALLO002 | M | 6 | None | Fifth relapse (after 2 HSCTs) | Haplo | CD19-CAR–Lenti_ALLO | 2.0 | 1.49 | BM (83.7) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO003 | M | 16 | None | Second relapse, very early after HSCT | MFD | CD19-CAR–Lenti_ALLO | 2.0 | 4.17 | BM (0.2) | CR (MRD–) | Pancreas |
| CD19-LENTI-ALLO004 | M | 8 | IKAROS+ | Third relapse, after HSCT | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 7.56 | BM (1.6) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO005 | M | 7 | t(9;22) | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 (before HSCT) | 4.3 | BM (0.03) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO006 | M | 17 | None | Second refractory relapse | Haplo | CD19-CAR–Lenti_ALLO | 3.0 | 4.21 | BM (0.01) + bone | CR (BM: MRD–; EM: neg) | n.a. |
| CD19-LENTI-ALLO007 | M | 4 | 47, XY (+21) | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 3.15 | BM (0.02) + ocular disease | CR (BM: MRD–; EM: neg) | BM (CD19+) |
| CD19-LENTI-ALLO008 | F | 26 | None | Fourth relapse (after HSCT and autologous CD19-CAR) | Haplo | CD19-CAR–Lenti_ALLO | 3.0 | 1.86 | BM (0.03) + mammary gland + bone | CR (BM: MRD–; EM: neg) | n.a. |
| CD19-LENTI-ALLO009 | M | 9 | None | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 5.33 | BM (55) | CR (MRD–) | n.a |
| CD19-LENTI-ALLO010 | F | 33 | KMT2-A | First relapse after HSCT, very early (2 mo) | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 5.33 | BM (35) | CR (MRD–) | n.a. |
| Pt ID . | Sex . | Age (y) . | Cytogenetic anomalies . | Disease phase at infusion . | Donor and HLA matching . | CAR-T product . | CAR+ T cells (×106 cells per kg) . | CAR− T cells (×106 cells per kg) . | Disease status at LD (%) . | Response at d +28 . | Site of relapse . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allo-CD19 CAR001 | F | 17 | iAmp21 | Second relapse, very early after HSCT | MUD 10/10 | CD19-CAR–Retro_ALLO | 3.0 | 4.24 | BM (0.3) | CR (MRD–) | CNS (CD19–) |
| Allo-CD19 CAR002 | M | 21 | MEF2D/BCL9 | First refractory relapse | MFD | CD19-CAR–Retro_ALLO | 3.0 (before HSCT) | 3.65 | BM (12) + bone (>10 spots) + liver | BM: CR (MRD–); liver: CR; bone: 3 spots | n.a. |
| Allo-CD19 CAR003 | M | 29 | KMT2A | Fifth relapse | MFD | CD19-CAR–Retro_ALLO | 3.0 | 1.01 | Pelvic lymph nodes + CNS | CR (BM: MRD–; EM: neg) | Pelvic lymph nodes + CNS (CD19+) |
| CD19-LENTI-ALLO001 | M | 11 | TEL/AML1 | Fourth relapse | MFD | CD19-CAR–Lenti_ALLO | 1.0 | 1.92 | BM (0.2) + bone and kidney | CR (BM: MRD–; EM: neg) | Bone |
| CD19-LENTI-ALLO002 | M | 6 | None | Fifth relapse (after 2 HSCTs) | Haplo | CD19-CAR–Lenti_ALLO | 2.0 | 1.49 | BM (83.7) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO003 | M | 16 | None | Second relapse, very early after HSCT | MFD | CD19-CAR–Lenti_ALLO | 2.0 | 4.17 | BM (0.2) | CR (MRD–) | Pancreas |
| CD19-LENTI-ALLO004 | M | 8 | IKAROS+ | Third relapse, after HSCT | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 7.56 | BM (1.6) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO005 | M | 7 | t(9;22) | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 (before HSCT) | 4.3 | BM (0.03) | CR (MRD–) | n.a. |
| CD19-LENTI-ALLO006 | M | 17 | None | Second refractory relapse | Haplo | CD19-CAR–Lenti_ALLO | 3.0 | 4.21 | BM (0.01) + bone | CR (BM: MRD–; EM: neg) | n.a. |
| CD19-LENTI-ALLO007 | M | 4 | 47, XY (+21) | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 3.15 | BM (0.02) + ocular disease | CR (BM: MRD–; EM: neg) | BM (CD19+) |
| CD19-LENTI-ALLO008 | F | 26 | None | Fourth relapse (after HSCT and autologous CD19-CAR) | Haplo | CD19-CAR–Lenti_ALLO | 3.0 | 1.86 | BM (0.03) + mammary gland + bone | CR (BM: MRD–; EM: neg) | n.a. |
| CD19-LENTI-ALLO009 | M | 9 | None | First refractory relapse | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 5.33 | BM (55) | CR (MRD–) | n.a |
| CD19-LENTI-ALLO010 | F | 33 | KMT2-A | First relapse after HSCT, very early (2 mo) | MFD | CD19-CAR–Lenti_ALLO | 3.0 | 5.33 | BM (35) | CR (MRD–) | n.a. |
F, female; FUP, follow-up; Haplo, haploidentical donor; M, male; MUD, matched unrelated donor; n.a. not applicable; pt, patient.
The first 3 patients were treated with the CD19-CAR–Retro_ALLO and received 3 × 106 CAR+ T cells per kg, a dose already tested in the autologous setting in the academic phase 1/2 clinical trial opened at Ospedale Pediatrico Bambino Gesù (NCT03373071), for which we had demonstrated the safety and efficacy. After documenting the absence of relevant toxicities with CD19-CAR–Retro_ALLO, all the subsequent patients received the freshly infused CD19-CAR–Lenti_ALLO at a dose of 1 × 106 CAR+ T cells per kg (n = 1), 2 × 106 CAR+ T cells per kg (n = 2), and 3 × 106 CAR+ T cells per kg (n = 7).
Drug product characteristics
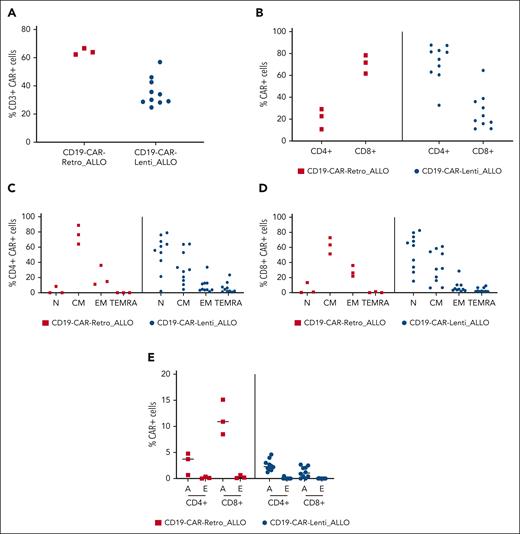
The designed dose was successfully produced for all patients, obtaining a median of 3.87 × 109 (range, 3.31 × 109 to 4.32 × 109) total cells and a median transduction efficiency of 64.3% ± 2.22% (range, 62.3%-66.7%) for the CD19-CAR–Retro_ALLO and a median of 4.71 × 109 (range, 2.25 × 109 to 6.92 × 109) total cells with a median transduction efficiency of 41.35% (range, 28.4%-61.7%) for the CD19-CAR–Lenti_ALLO (Figure 1A). As shown in Figure 1, CD19-CAR–Retro_ALLO was characterized by a prevalence of CD8+ CAR+ cells, and both CD4+ and CD8+ cells had mainly a central memory (CM) and effector memory (EM) phenotype. By contrast, CD19-CAR–Lenti_ALLO were characterized by a higher proportion of CD4+ CAR+ cells and a prevalence of naive and CM cells (Figure 1B-D). In addition, the characterization of the activation/exhaustion profile of drug products (in terms of CD69+ T cells and coexpression of TIM-3, LAG-3 and PD1 on T cells, respectively) showed that for both CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO, the proportion of exhausted CAR+ T cells was negligible (Figure 1E).
Characterization of the drug products. CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO were characterized via flow cytometry in terms of levels of transduction (A), CD4+ and CD8+ subpopulations (B); memory phenotype, with identification of naive (N), CM, EM, and terminally differentiated effector memory cells reexpressing CD45ra (TEMRA) (C-D); and activation (A)/exhaustion (E) profile.
Characterization of the drug products. CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO were characterized via flow cytometry in terms of levels of transduction (A), CD4+ and CD8+ subpopulations (B); memory phenotype, with identification of naive (N), CM, EM, and terminally differentiated effector memory cells reexpressing CD45ra (TEMRA) (C-D); and activation (A)/exhaustion (E) profile.
Characterization of ALLO–CAR-T cells after infusion
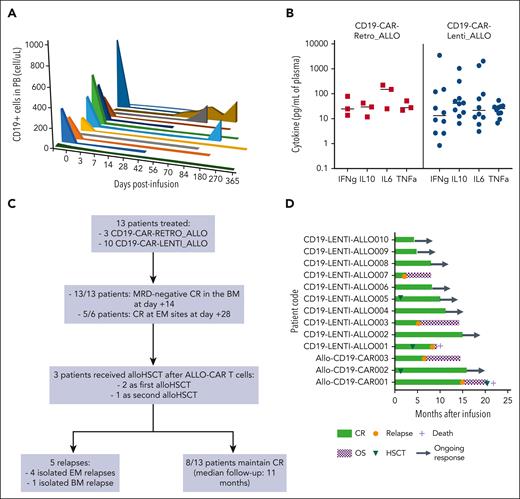
As expected, monitoring of circulating ALLO–CAR-T cells showed a peak of expansion 1 or 2 weeks after infusion of both CD19 CAR_ALLO, as evaluated via both flow cytometry and droplet polymerase chain reaction (Figure 2A-B). ALLO–CAR-T cells were also able to significantly infiltrate the BM (Figure 2C-D). Most in vivo expanded CAR+ T cells were CD8+ (Figure 3A), with a CM or EM phenotype (Figure 3B-E). In terms of activation and exhaustion, CD3+ CAR+ T cells that expanded on day 14 after infusion were characterized by a relevant proportion of CD8+-activated CAR+ T cells in the absence of exhausted CAR+ T cells (supplemental Figure 1). We observed a persistence for >6 months in 5 of 7 patients (71%) who did not receive a subsequent HSCT and had adequate follow-up (Figure 3A). Accordingly, all patients developed B-cell aplasia (BCA), which prolonged >6 months in 6 of 7 patients (86%), up to 15 months in the first patient (Figure 4A). In addition, both patients who received ALLO–CAR-T cells before HSCT showed a significant expansion of CAR-T cells without any evidence of rejection of the allogeneic cells.
Evaluation of expansion, homing to the BM, and persistence of CD19-CAR_ALLO. Expansion and persistence of CD19-CAR_ALLO in the peripheral blood of the patients, evaluated via flow cytometry (A) and droplet PCR (B). Homing to the BM and persistence of CD19-CAR_ALLO, analyzed via flow cytometry (C) and droplet PCR (D). PCR, polymerase chain reaction.
Evaluation of expansion, homing to the BM, and persistence of CD19-CAR_ALLO. Expansion and persistence of CD19-CAR_ALLO in the peripheral blood of the patients, evaluated via flow cytometry (A) and droplet PCR (B). Homing to the BM and persistence of CD19-CAR_ALLO, analyzed via flow cytometry (C) and droplet PCR (D). PCR, polymerase chain reaction.
Characterization of circulating, expanded CAR-T cells. (A) The expanded CAR T-cell CD4/CD8 phenotype evaluated via flow cytometry for both CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO. The memory phenotype, with identification of naive (N), CM, EM, and TEMRA subpopulations, was characterized via flow cytometry for the CD19-CAR–Retro_ALLO CD4+ (B) and CD8+ cells (C), and for the CD19-CAR–Lenti_ALLO CD4+ (D) and CD8+ cells (E).
Characterization of circulating, expanded CAR-T cells. (A) The expanded CAR T-cell CD4/CD8 phenotype evaluated via flow cytometry for both CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO. The memory phenotype, with identification of naive (N), CM, EM, and TEMRA subpopulations, was characterized via flow cytometry for the CD19-CAR–Retro_ALLO CD4+ (B) and CD8+ cells (C), and for the CD19-CAR–Lenti_ALLO CD4+ (D) and CD8+ cells (E).
Evaluation of the BCA and cytokine profile and clinical outcome of the patients. (A) The kinetics of circulating CD19+ cells was monitored via flow cytometry over time in the peripheral blood of all the treated patients. (B) The levels of interferon gamma (IFNg), interleukin-10 (IL-10), IL-6, and tumor necrosis factor α (TNFa) were measured in the plasma of patients after infusion, and the concentrations at peak are shown. (C) Flowchart summarizing the responses and relapses observed in the cohort of patients; (D) Swimmer plot of all the treated patients showing the disease outcome and the patients receiving allo-HSCT. CR, complete remission; OS, overall survival.
Evaluation of the BCA and cytokine profile and clinical outcome of the patients. (A) The kinetics of circulating CD19+ cells was monitored via flow cytometry over time in the peripheral blood of all the treated patients. (B) The levels of interferon gamma (IFNg), interleukin-10 (IL-10), IL-6, and tumor necrosis factor α (TNFa) were measured in the plasma of patients after infusion, and the concentrations at peak are shown. (C) Flowchart summarizing the responses and relapses observed in the cohort of patients; (D) Swimmer plot of all the treated patients showing the disease outcome and the patients receiving allo-HSCT. CR, complete remission; OS, overall survival.
Toxicity
The treatment was well tolerated, with a toxicity profile comparable with that observed in trials with autologous CAR-T cells (Table 2). In particular, all patients developed a grade 3 or 4 peripheral blood cytopenia, usually occurring after lymphodepletion (LD) and persisting after CAR T-cell infusion. In detail, grade 2 neutropenia developed before ALLO–CAR T-cell infusion in 10 of 13 patients and resolved to grade <2 within a median of 27 days (range, 35-46 days); 6 patients received granulocyte colony-stimulating factor, all responding to the stimulation, and 1 patient received a boost of CD34+ cells from the donor. Thrombocytopenia of any grade was present before infusion in 8 of 10 patients and reached grade 4 in 7 of 10 patients; the median duration of thrombocytopenia of any grade was 43.5 days (range, 3-418 days). Finally, grade 1 anemia was present in 12 of 13 patients before LD and reached grade 3 in 3 of 12 patients, 1 of them before infusion of ALLO–CAR-T cells. The median duration of grade 3 anemia was 35 days (range, 9-329 days); no patient developed grade 4 anemia. None of the patients developed any infectious complications.
Main toxicities observed after infusion of ALLO–CAR-T cells
| Toxicity . | Number of patients (%) . |
|---|---|
| CRS | 10 of 13 (77) |
| Grade 1-2 | 10 |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Neutropenia | 13 of 13 (100) |
| Grade 1 or 2 | 0 |
| Grade 3 or 4 | 13 |
| Thrombocytopenia | 10 of 13 (77) |
| Anemia | 12 of 13 (92) |
| BCA | 13 of 13 (100) |
| ICANS | 1 of 13 (8) |
| aGVHD | 1 of 13 (8) |
| Capillary leak syndrome | 1 of 13 (8) |
| Toxicity . | Number of patients (%) . |
|---|---|
| CRS | 10 of 13 (77) |
| Grade 1-2 | 10 |
| Grade 3 | 0 |
| Grade 4 | 0 |
| Neutropenia | 13 of 13 (100) |
| Grade 1 or 2 | 0 |
| Grade 3 or 4 | 13 |
| Thrombocytopenia | 10 of 13 (77) |
| Anemia | 12 of 13 (92) |
| BCA | 13 of 13 (100) |
| ICANS | 1 of 13 (8) |
| aGVHD | 1 of 13 (8) |
| Capillary leak syndrome | 1 of 13 (8) |
In 10 of 13 patients (77%), signs and symptoms of CRS were observed, with a maximum grade 1, mostly characterized by fever and tachycardia. The cytokine monitoring in plasma showed increased levels of proinflammatory cytokines (Figure 4B). One out of 13 patients (8%) developed a transient, self-resolving grade 2 ICANS, with spatial and temporal disorientation and tremors. We recorded only 1 case of aGVHD after ALLO–CAR T-cell infusion. This patient (CD19-LENTI-ALLO010) was treated with ALLO–CAR-T cells 3 months after receiving allo-HSCT from an MFD and developed grade 3 aGVHD with skin and liver involvement, which occurred 1 month after ALLO–CAR T-cell infusion. At the time of GVHD occurrence (day 29), ALLO–CAR-T cells were not detectable in the peripheral blood of the patient (supplemental Figure 2). The patient was treated with a short course of steroids and ruxolitinib, with complete resolution of aGVHD. None of the other patients, including 3 patients infused with ALLO–CAR-T cells from an HLA-haploidentical donor, developed aGVHD. No patient developed signs or symptoms of chronic GVHD. In addition, 1 patient developed capillary leak syndrome and received 3 doses of tocilizumab, with complete resolution. None of the 3 patients treated with CD19-CAR–Retro_ALLO required the activation of iC9 to control toxicity.
Responses and characteristics of the relapses
Characteristics of patient response and relapses are summarized in the flowchart in Figure 4C. All patients obtained CR of the disease with MRD negativity in the BM 14 days after infusion, and 5 of 6 patients had CR of extramedullary disease (bone, liver, and mammary gland), evaluated on day +28; only patient Allo-CD19-CAR002, who was treated for liver disease and >10 bone spots, showed a persistence of 3 spots on day +28 that regressed subsequently (Figure 4D). Three out of 13 patients (23%) received a subsequent allo-HSCT. Two of these patients were treated with ALLO–CAR-T cells before undergoing HSCT for a highly refractory disease and received transplantation only after obtaining MRD– CR. The third patient received a second HSCT from a matched unrelated donor to consolidate the MRD– CR obtained in consideration of the clinical history of multiple relapses and the lack of further therapeutic opportunities (Figure 4D).
With a median follow-up of 12 months (range, 5-21 months), 8 of 13 patients (62%) maintained CR. One patient (Allo-CD19-CAR001) developed a CD19– central nervous system (CNS) relapse 14 months after infusion of CD19-CAR–Retro_ALLO while showing persistent BCA; the remaining 4 relapses were all CD19+. Most of the relapses observed (4/5) involved EM localizations (CNS, bone, pelvis, and pancreas) and were associated with persisting CR in the BM. In particular, besides patient Allo-CD19-CAR001, patient Allo-CD19-CAR003 relapsed with a pelvic lesion and CNS infiltration of the disease; patient CD19-LENTI-ALLO001 relapsed with a bone localization 3 months after receiving HSCT; and patient CD19-LENTI-ALLO003 relapsed with a pancreatic localization of the disease. The only patient (CD19-LENTI-ALLO007) who relapsed with a BM localization received CAR-T cells with minimal disease infiltration in the BM and BCA, which developed after the bridging treatment.
Discussion
Our data show that ALLO–CAR-T cells are capable of controlling highly refractory BCP-ALL, inducing a high rate of MRD– CR that persists in a relevant proportion of patients. Importantly, the treatment was well tolerated, with only 1 patient developing treatment-responsive aGVHD. These results, combined with the possibility of treating patients based on the clinical need, irrespective of the planning of the cell collection and production and the abrogation of the risk of blast contamination in the drug product, suggest that the use of ALLO–CAR-T cells is a promising and valid alternative to autologous CAR-T cells.
Other experiences have been reported to date with allogeneic, donor-derived CAR-T cells in adults, but the strong variability of diagnosis, CAR constructs (second-generation, with either CD28 or 4.1BB or third-generation CD28/CD27), gene delivery strategy (viral platform, either retrovirus or lentivirus, or the Sleeping Beauty transposition), donor selection (mostly matched unrelated donor and MFD), dose of CAR-T cells administered, and administration of previous LD limit the possibility of comparing the results.12-17 Overall, a low rate of GVHD, mostly limited in grade and stage, has been documented, with the exception of Chen et al who reported, with the use of a multitargeting, third-generation construct and haploidentical donors, the occurrence of aGVHD (grade 2-3) in 50% of the patients, a result comparable with their experience using DLI after haploidentical T-cell–replete HSCT.15
In our cohort, the only patient who developed aGVHD was treated early after HSCT, had already developed grade 2 gut aGVHD after transplantation and, being an adult, was at higher risk of developing an allogeneic reaction. Interestingly, despite the treatment with steroids and ruxolitinib, she is still in CR, maintains BCA, and shows a reexpansion of ALLO–CAR-T cells 5 months after infusion. Ghosh et al deeply characterized in vivo the potential of T cells expressing CD28-costimulated CD19 CAR to cause GVHD, showing that the limited GVHD occurrence results from the cumulative CAR and alloreactive T-cell receptor signaling, leading to the exhaustion and eventual deletion of the alloreactive CAR-T cells, whereas the nonalloreactive CAR-T cells retain activity against CD19+ targets.18 Indeed, alloreactive CD28 CAR-T cells show a progressive loss of their effector function and proliferative potential because of the enhanced stimulation endured. However, in contrast with our clinical findings, the same group showed that 4.1BB-costimulated CAR-T cells do not show the same exhaustion and are associated with a more significant GVHD-related mortality in animals, as compared with CD28 CAR-T cells.18 This discrepancy could be associated with structural differences in the constructs, which can mediate different activation and exhaustion profiles of the transduced T cells. In addition, the prolonged ex vivo culture of T cells has already been reported to reduce their alloreactive potential.19,20 Another factor contributing to the limited incidence and severity of GVHD could be related to the relatively low doses of T cells per kg administered, below the threshold dose of 107 T cells per kg, which, in adults, have been suggested to be associated with a clinically significant GVHD in MFD HSCT.21
The most relevant toxicity observed in our cohort was represented by severe cytopenias. This observation is undoubtedly linked to the patient population we selected because 10 of 13 patients had profound cytopenia before treatment, this being the reason for proceeding with ALLO–CAR-T cells. However, the impact of the activation of ALLO–CAR-T cells cannot be excluded and likely contributes to maintaining cytopenia after infusion. In our cohort, CRS developed in 10 of 13 patients (77%) and was mild, reaching a maximum of grade 1, also in patients with high disease burden at the time of infusion. The incidence and severity of CRS were not affected by either the product infused (CD19-CAR–Retro_ALLO vs CD19-CAR–Lenti_ALLO) or the cell dose, as recently reported also by Stefanski et al.22
The low incidence and severity of CRS and ICANS in our cohort of patients could be related to the presence of the 4.1BB costimulatory domain. Riedell et al recently reported, in a real-world analysis in the context of B-cell non-Hodgkin lymphoma, that this costimulatory molecule is associated with a better safety profile as compared with CD28-containing products.23 Moreover, interestingly, in a preclinical model, Arcangeli et al showed that second-generation CD19 CAR-T cells generated from a naive/stem cell memory population of T cells, although displaying a higher expansion capability as compared with CAR-T cells generated from a bulky T-cell population, were characterized by a lower potential to cause toxicities independently from the costimulatory molecule used (CD28 vs 4.1BB).24 This could also account for the low toxicity we observed, considering the high levels of naive CD4+ and CD8+ cells present in the CD19-CAR–Lenti_ALLO products.
To the best of our knowledge, very limited experience with ALLO–CAR-T cells, derived from HSC donors, in the pediatric population has been reported to date. A phase 1 study previously evaluated the feasibility, safety, and antileukemia activity in a pediatric cohort of UCART19, a universal CAR T-cell product characterized by the knockout of the genes encoding the T-cell receptor α chain and the CD52 antigen (PALL trial).25 Among the 7 children treated, 1 developed grade 1 skin aGVHD, and the toxicity profile was comparable with that reported with the use of autologous CAR-T cells. Six out of the 7 patients (86%) obtained CR, and 5 of them received a subsequent allo-HSCT; only 2 of the responders, however, maintained CR. In 2021, Zhang et al. reported their experience using donor-derived, second-generation (containing either CD28 or 4.1BB as costimulatory molecule) CD19 CAR-T cells in 43 participants with B-ALL relapsing after allotransplantation, whose ages ranged from 4 to 60 years.17 A 79% morphological CR and 2 cases of ≤grade 2 aGVHD were reported; however, no details on the outcome of children, as compared with adults, were provided.17
In our cohort, ALLO–CAR-T cells were extremely effective at eradicating highly resistant diseases, including EM localizations, inducing 100% of MRD– CR in the BM and 83% of CR at EM sites, thus offering a chance of cure to patients with failed multiple lines of chemotherapy and immunotherapy, including autologous CD19 CAR-T cells.
One patient experienced a CD19– relapse, and 2 patients experienced a CD19+ relapse; for the remaining 2 patients, we could not perform a biopsy of the extramedullary site of recurrence to evaluate CD19 expression, although both had lost BCA. Importantly, most of the relapses (80%) occurred at EM sites. This observation suggests that, in patients with EM localizations of ALL, a treatment to consolidate the CR obtained with ALLO–CAR-T cells, such as local irradiation whenever possible, might improve the long-term outcome.
Among the 10 patients treated with CD19-CAR–Lenti_ALLO, we observed a higher relapse rate in patients treated at lower doses (the patient treated with 1 × 106 cells per kg, and 1 of 2 patients treated with 2 × 106 cells per kg) than in those treated with 3 × 106 cells per kg (1 of 7 patients). Considering only EM disease, 2 relapses were observed at lower doses, whereas none of the patients treated with CD19-CAR–Lenti_ALLO at 3 × 106 cells per kg experienced a recurrence at EM sites, despite 3 of them having an EM localization before infusion. Notably, changes neither in the bridging strategy nor in the consolidation with a second HSCT were made for the patients treated at the highest dose. Therefore, our data suggest that the cell dose may affect long-term survival, as reported by Stefanski et al.22 Although the low number of patients does not allow for any firm conclusion, if this observation is confirmed in further studies, the strategy to improve sustained disease control at EM sites could also include administration of higher ALLO–CAR T-cell doses.
All patients treated with an isolated BM ALL involvement maintain MRD– CR, including those with a BM infiltration >5%, a well-known adverse prognostic factor for autologous CD19 CAR-T cells.26 Although the follow-up is limited, we observed an encouraging persistence of BCA >6 months in 6 of 7 patients who did not receive a subsequent HSCT; this finding being associated with significantly improved probability of survival after treatment with CAR-T cells.27
Our study is not designed to compare autologous vs allogeneic products because patients were selected using different criteria. Among the 14 patients enrolled in our academic clinical trial (NCT03373071) and treated with 3 × 106 autologous CD19 CAR–Retro per kg, fewer children had relapsed after an allo-HSCT (4/14 [28.5%] vs 11/13 [84.6%] of those who received ALLO–CAR-T cells); no patient had profound cytopenia, and only 2 patients had EM disease. Although the number of patients with high disease burden before treatment is comparable (9/14 [64%] vs 9/13 [69%] for the allogeneic product), none of the patients in the autologous trial had >50% of blasts in the BM (as required per eligibility criteria). Despite these observations suggesting that a less-advanced population was included in the autologous trial, we obtained an 85% MRD– CR on day +28, as compared with 100% in the population treated with allogeneic products. Moreover, despite receiving a consolidation with allo-HSCT in 41.7% of the patients reaching CR, 7 of 14 children (50%) in the autologous trial experienced CD19+ relapse in the BM within 1.5 years after receiving CAR-T cells. Here, with a median follow-up of 12 months (range, 5-22 months), 23% of the patients (3/13) received allo-HSCT, and 38.5% of the patients experienced relapse, mostly at EM sites. Our data on autologous products are in line with those of both the ELIANA clinical trial and the real-world experience with tisagenlecleucel28,29 and suggest, with the aforementioned limitations, that the donor-derived products have an improved disease control as compared with the autologous products.
Recently, the German real-world analysis of pediatric/young adults with relapsed BCP-ALL administered with tisagenlecleucel documented that the survival probabilities of patients with early relapse (<6 months) from allo-HSCT are significantly poorer than those of patients relapsing beyond 6 months.30 This observation is likely related to an incomplete reconstitution of polyclonal T cells and may be overcome thanks to the use of donor-derived CAR-T cells.
Interestingly, none of the 2 patients treated with ALLO–CAR-T cells before allo-HSCT rejected the drug product. It is likely that 3 factors are responsible for this observation: (1) both children were profoundly cytopenic before the treatment; (2) all patients received a Flu/Cy-based LD before infusion and displayed a strong lymphopenia at the time of cell administration; and (3) donors were HLA-identical siblings.
Important and still unsolved questions for optimizing patients’ outcomes include which viral platform (retrovirus vs lentivirus) and manufacturing process (a conventional, manual system vs a semiautomated, closed one) yield a better product. Our study was neither designed nor powered to provide a robust response to these important questions. The manufacturing processes of CD19-CAR–Retro_ALLO and CD19-CAR–Lenti_ALLO have important differences beyond the open method vs automated and retrovirus vs lentivirus, which may have an impact on the characteristics of the final product. In particular, (1) the starting material and final product are both cryopreserved in the first and both fresh in the latter, significantly shortening the waiting window usually required for manufacturing; (2) CD19-CAR–Retro_ALLO is produced from the bulk apheresis collection, whereas CD19-CAR–Lenti_ALLO is generated from a selection of CD4+/CD8+ cells, limiting the heterogeneity of the products and eliminating components known to reduce the quality of the final product, for example, monocytes; and (3) reagents used in the manufacturing are also different between the 2 processes.
In conclusion, our data, although obtained in a limited sample size, suggest that ALLO–CAR-T cells can effectively treat highly refractory BCP-ALL relapsing after allo-HSCT without showing either increased toxicity, as compared with autologous CAR-T cells, or a high incidence of GVHD. If confirmed, these results may also lead to the implementation of transplantation protocols to include administration of ALLO–CAR-T cells in patients with BCP-ALL who received HSCT and with either high pretransplant MRD levels or reemergence of a molecular disease after the allograft to reduce the occurrence of overt relapse, thus improving long-term outcomes. A clinical trial to prospectively evaluate this promising approach will be opened soon.
Acknowledgments
The authors thank Miltenyi Biomedicine for providing the viral supernatant for manufacturing CD19-CAR–Lenti_ALLO cells. The authors also thank Manuel Caruso and the entire Biovec Pharma for providing the retroviral producer cell lines (ie, 293VEC) for the production of retrovirus used for the manufacturing of CAR RETRO-Allo.
The work was supported by research funding from Accelerator award, Cancer Research UK/Associazione Italiana per la Ricerca sul Cancro (AIRC), INCAR project (F.L.); Ministero della Salute (CAR T Italia Project RCR-2019-23669115 [F.L.]; RF-2016-02364388 [F.L.]; RF-2021-12374120 [C.Q.]; CO-2021-12375044 [F.L.]; GR-2021-12372614 [F.d.B.]; grant 5 × 1000 [F.d.B., C.Q., B.D.A.]; Piano Nazionale Complementare, LSH-TA Ecosistema innovativo della Salute [F.L.]); Ministero dell'Università e della Ricerca (Progetti di Ricerca di Interesse Nazionale grant 2017 and 2021 [F.L.]); Piano Nazione di Ripresa e Resilienza, National Center for Gene Therapy and Drugs based on RNA Technology (CUP CN00000041 [F.L.]); AIRC (special grant 5 × 1000 ID 9962 and investigator grant 2018 ID 21724 [F.L.]; MFAG ID 20450 [F.d.B.]; and MFAG ID 25106 [C.Q.]). Innovative Medicines Initiative 2 Joint Undertaking (grant agreement number 116026, T2Evolve); Strategic project 2019 - POR FESR Lazio 2014-2020 "CARSA" (CUP: E82F20000240002 [F.L.]); Lazio-Innova project IMMUNO (CUP code: E82F20000200002 [F.L.]).
Authorship
Contribution: F.d.B. participated in the elaboration of the treatment, managed the patients clinically, analyzed data, and wrote the paper; M.B. and C.R. managed the patients clinically and analyzed data; B.D.A. contributed to the design of the retroviral construct and participated in the immunomonitoring; M.A., G.D.B., E.B., P.M., D.P., and R.C. clinically managed the patients and revised the manuscript; L.H. contributed vital reagents and revised the manuscript; M.G. and S.I. manufactured the drug products and revised the text; G.L.P., E.G., G.L., and S.L. took care of the cell collection and manipulation and revised the paper; V.B., M.S., S.D.C., and L.I. participated in the immunomonitoring of the patients; A.K. referred 1 patient, clinically managed this patient, and revised the manuscript; C.Q. participated in the design of the retroviral construct, supervised the immunomonitoring, analyzed data, contributed to writing the paper, and critically revised the manuscript; F.L. supervised the project and the clinical management of patients, analyzed data, and critically revised the manuscript; and all authors had access to primary clinical trial data.
Confilct-of-interest disclosure: L.H. is an employee of Miltenyi Biomedicine. The remaining authors declare no competing financial interests.
Correspondence: Franco Locatelli, Department of Hematology/Oncology, Cell and Gene Therapy, IRCCS, Bambino Gesù Children’s Hospital, Piazza S. Onofrio, 4, 00165, Rome, Italy; e-mail: franco.locatelli@opbg.net.
References
Author notes
Data are available on request from the corresponding author, Franco Locatelli (franco.locatelli@opbg.net).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal