Abstract
Inherited or de novo germ line heterozygous mutations in the gene encoding the transcription factor GATA2 lead to its deficiency. This results in a constellation of clinical features including nontuberculous mycobacterial, bacterial, fungal, and human papillomavirus infections, lymphedema, pulmonary alveolar proteinosis, and myelodysplasia. The onset, or even the presence, of disease is highly variable, even in kindreds with the identical mutation in GATA2. The clinical manifestations result from the loss of a multilineage progenitor that gives rise to B lymphocytes, monocytes, natural killer cells, and dendritic cells, leading to cytopenias of these lineages and subsequent infections. The bone marrow failure is typically characterized by hypocellularity. Dysplasia may either be absent or subtle but typically evolves into multilineage dysplasia with prominent dysmegakaryopoiesis, followed in some instances by progression to myeloid malignancies, specifically myelodysplastic syndrome, acute myelogenous leukemia, and chronic myelomonocytic leukemia. The latter 3 malignancies often occur in the setting of monosomy 7, trisomy 8, and acquired mutations in ASXL1 or in STAG2. Importantly, myeloid malignancy may represent the primary presentation of disease without recognition of other syndromic features. Allogeneic hematopoietic stem cell transplantation (HSCT) results in reversal of the phenotype. There remain important unanswered questions in GATA2 deficiency, including the following: (1) Why do some family members remain asymptomatic despite harboring deleterious mutations in GATA2? (2) What are the genetic changes that lead to myeloid progression? (3) What causes the apparent genetic anticipation? (4) What is the role of preemptive HSCT?
Introduction to GATA2 deficiency
In 2010 a unique disease syndrome was described with features of both an immunodeficiency disease and a myelodysplastic syndrome.1 Subsequently nicknamed MonoMAC by our group, this syndrome describes individuals with a severe deficiency of monocytes (Mono) in the peripheral blood and the propensity to develop Mycobacterium avium complex (MAC) infections. At the same time, 3 similar syndromes were described: dendritic cell, monocyte, B and natural killer (NK) lymphoid deficiency; Emberger syndrome (primary lymphedema with myelodysplasia); and familial myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).2-4 In 2011, all 4 diseases were shown to result from germ line mutations in the GATA2 gene and to represent protean manifestations of the same disease, subsequently termed GATA2 deficiency.4-7
The important differences between patients with GATA2 deficiency and patients with previously described inborn errors of immunity (IEI) are that the patients with GATA2 deficiency typically have (1) disease onset beyond infancy, frequently in late adolescence or early adulthood, of life-threatening opportunistic infections with nontuberculous mycobacterial infections (NTM), fungal infections, and human papillomavirus (HPV) infections; (2) a peripheral blood leukocyte subset profile with the presence of T lymphocytes, but a severe deficiency of B lymphocytes, NK cells, and monocytes; (3) progression to MDS, chronic myelomonocytic leukemia (CMML), and AML; and (4) an autosomal dominant inheritance pattern with mutations in one allele of GATA2. This contrasts with most genetic IEI which manifest in infancy, rarely have both disseminated MAC and disseminated fungal infections, lack T lymphocytes, and do not progress to MDS or AML.
The natural history of GATA2 deficiency is highly variable, even in individuals within the same family who harbor the identical mutation in the GATA2 gene. Individuals who become symptomatic with GATA2 deficiency, typically present in their late teens or early twenties, and ultimately succumb to either disseminated infections, MDS, or AML within 2 to 20 years. It appears that the loss of monocytes and NK cells accounts for the propensity to develop opportunistic infections with MAC, fungal organisms, including Histoplasma and Aspergillus, and HPV. Prophylactic azithromycin is prescribed to prevent disseminated mycobacterial infections. The onset of MDS is also quite variable, but when it develops, it usually develops in the late teens to early twenties. Once clinical manifestations begin, relapses with infections tend to recur leading to progressive disability. Many patients also develop pulmonary alveolar proteinosis over time, although this tends to be a late manifestation of disease.8 Epstein-Barr virus and HPV associated cancers, other solid tumors, and rarely B-cell acute lymphoblastic leukemia, are reported in GATA2 deficiency, although these are considerably less frequent than myeloid malignancies.9,10
Both hereditary and de novo germ line cases of GATA2 mutation occur in this syndrome. The de novo mutations in GATA2 constitute nearly one-half in the patients with GATA2 deficiency at our center. A variant allele frequency in the vicinity of 50% is the tip-off that the mutation may be germ line rather than being somatic. Germ line mutations can be confirmed by sequencing DNA from cultured fibroblasts of a skin biopsy and demonstrating the mutation in both hematopoietic and nonhematopoietic tissue. Germ line mutations are classified as de novo in the proband when DNA sequencing of the parents demonstrates both having wild-type GATA2 sequence. To date, over 150 distinct mutations in GATA2 have been described in patients, including recurrent missense mutations affecting the zinc finger-2 domain (eg, T354M, R398W, and R396W).11 These mutations implicate haploinsufficiency as a mechanism of action because the remaining GATA2 allele is invariably wild-type. Recurrent mutations may also involve an intronic cis-element enhancer that is critical for regulating hematopoiesis and vascular integrity.12,13 The human enhancer element has been referred to as residing in either intron 5 or intron 4, depending on the reference transcript used in the study, resulting in some confusion in the literature, although the hotspot region is the same.12,14 Mutations involving noncoding regions may be missed by whole exome sequencing or panels that do not cover the whole gene. Importantly, synonymous mutations have recently been identified that lead to selective loss of mutant RNA and can be inadvertently filtered out in some next-generation sequencing analyses pipelines.15,16 New novel germ line mutations continue to be reported.17,18 Hence, if suspicion for germ line GATA2 mutation is high, and a mutation is not identified by either whole exome sequencing or other sequencing platforms, a detailed evaluation of the gene including noncoding regions and those affecting RNA splicing is recommended.
The concept of transcription factor haploinsufficiency resulting in a clinical phenotype is derived from the importance of gradients of transcription factors or “dose” in developmental biology, a concept particularly well-described in Drosophila organogenesis.19 Mutations inactivating 1 allele result in a reduction by approximately one-half in the protein level of the encoded transcription factor. One-half normal levels of many transcription factors, such as GATA2, are simply not biologically sufficient. Why one-half normal levels of transcription factors, such as GATA2, result in a multisystem syndrome may seem puzzling on first approximation. However, transcription factors bind to regulatory sequences controlling gene expression. In target genes in which there are multiple transcription factor binding sites, initiation of gene transcription may require occupancy of most, if not all, of the binding sites. By inference, the more the regulatory sequence binding sites for the transcription factor, the more sensitive gene expression will be to transcription factor concentration.20
Transcription factor haploinsufficiency can also underlie the variable clinical manifestations of the disease phenotype of family members with the identical mutation in the transcription factor, a phenomenon observed in GATA2 deficiency. This variability may be due to differences in either modifying background genes, variable expression in the wild-type allele, or polymorphisms in DNA sequences flanking the transcription factor binding because transcription factors typically function in complexes.
Role of GATA2 in hematopoiesis
GATA2 is highly expressed in immature hematopoietic cells, and its expression decreases with blood cell maturation. GATA2 is crucial for the proliferation and maintenance of HSC.21,22 Impaired GATA2 expression induces MDS and myeloproliferative syndrome in hypomorphic Gata2-/- mice; thus, generating in vivo a disease resembling CMML in humans.23 Progenitors from the mutant mice display myeloid lineage-biased proliferation and differentiation. Mice harboring mutations in the intronic enhancer element in GATA2 also show deficits in hematopoietic stem or progenitor activity, increase in apoptosis, reduction in long-term self-renewal capability, loss of vascular activity, and disruption of the endothelial cell transcriptome.13,24 Conditional inactivation of Gata2 in transgenic mice embryos leads to defective lymphatic vascular development, edema, anemia, and hemorrhage.25
GATA2 expression appears to be exquisitely regulated because overexpression of GATA2 in AML is associated with a poor prognosis.26 Similarly, somatic alterations resulting in GATA2 overexpression have been described in sporadic MDS or AML.27,28
Patients with GATA2 deficiency frequently present with hypocellular bone marrow (BM) and features of BM failure29 with progressive loss of cell lineages over time.30 Single-cell whole-transcriptomic sequencing of CD34+ cells isolated from the marrow of 8 patients with MDS and GATA2 deficiency demonstrates deficiency in lymphoid or myeloid progenitors and dysregulation in gene expression in hematopoietic stem and progenitor cells related to apoptosis and cell cycle with increased expression of erythroid or megakaryocyte priming genes.31 Induced pluripotent stem cells derived from patients with GATA2 deficiency display impaired maturation toward hematopoietic lineages.32 Epstein-Barr virus–transformed cell lines derived from patients with GATA2 deficiency show increased expression of mir-181c, which is regulated by GATA2 and represses MCL1 translation leading to decreased cell survival.33
Hematologic manifestations are a hallmark of GATA2 deficiency. Flow cytometric analysis of marrow in many patients with GATA2 deficiency shows evidence of the underlying immunodeficiency from a disproportionate decrease to absence of monocytes, B cells, B-cell precursors, NK cells and/or plasmacytoid dendritic cells.34 The loss of these populations can be detected using routine clinical flow cytometry antibody panels designed to assess monocyte and immune cell populations. The disproportionate loss of monocytes, B cells, B-cell precursors, NK cells and/or plasmacytoid dendritic cells in the marrow of an untreated patient presenting with BM failure should raise the possibility of GATA2 deficiency in the differential diagnosis with consideration for germ line testing. In contrast to GATA2 deficiency, idiopathic aplastic anemia typically shows preservation of immune populations reflecting the immunologic basis of disease and response to immunosuppression, which is contraindicated in GATA2 deficiency. Notably, the loss of the immune populations seen in GATA2 deficiency is not specific and can sometimes be seen in the marrow of patients treated with chemotherapy for cancer or other diseases. Additionally, not all patients with GATA2 deficiency have significant immunodeficiency; instead, some may present with myeloid malignancy without evidence of either immunodeficiency or loss of immune cell populations. Thrombocytopenia is considerably less common, tending to be a late and more ominous development. Megakaryocytic dysplasia is present in nearly all cases of MDS in GATA2 deficiency35 with separation of nuclear lobes in the megakaryocytes (MKs), mononuclear MKs, and microMKs. An inverted myeloid to erythroid ratio with erythroid predominance and dyserythropoiesis is common with binucleation, nuclear budding, and megaloblastic changes. Hypogranular and pelgeroid neutrophils are a common manifestation of dysmyelopoiesis. Immunophenotypic evidence of dysplasia is frequently apparent, including either loss of CD38 or aberrant expression of CD7 on myeloblasts. Despite the loss of monocytes, macrophages are abundant in the marrow as seen with immunohistochemistry of CD68 or CD163. Similarly, despite the loss of B cells, plasma cells are usually abundant and are polyclonal. Immunoglobulin levels are either typically normal or may be increased.
Evaluation of BM in GATA2 deficiency can be helpful in multiple situations, including assessment of GATA2 variants of unknown significance and evaluation of individuals for potential hematopoietic stem cell transplantation (HSCT). For GATA2 variants of unknown significance we assess BM to look for evidence of either loss of monocytes, B cells, NK cells, and plasmacytoid dendritic cells, dysplasia, or cytogenetic abnormalities. BM histopathology can also be a tipping point for HSCT if there are excess blasts with or without cytogenetic abnormalities.
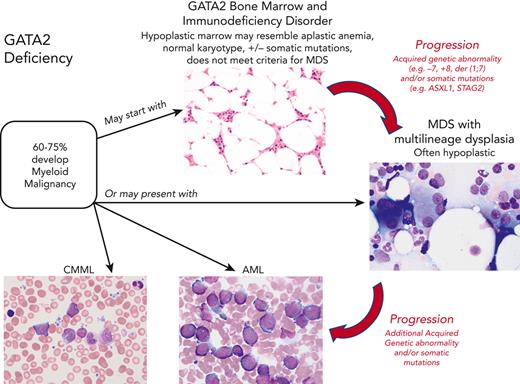
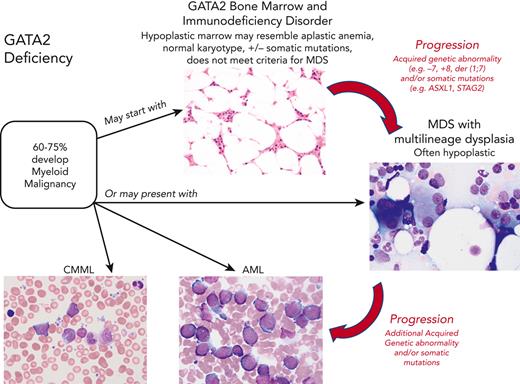
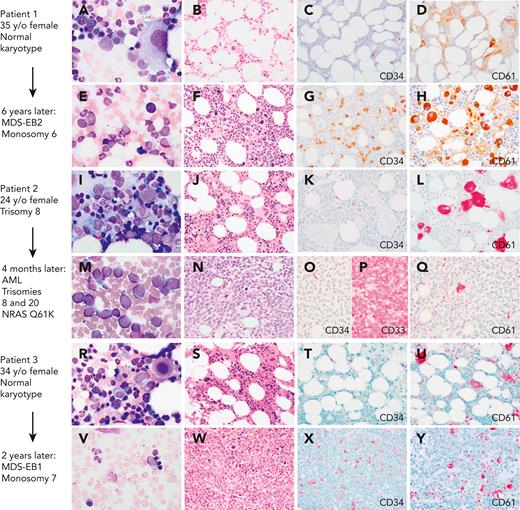
In pediatric GATA2 deficiency–associated MDS, the loss of B cells and B-cell precursors is the most common flow cytometry finding.36 In contrast to GATA2 deficiency in adults, who commonly display monocytopenia, pediatric patients with GATA2 with MDS may have monocytosis.11 The BM with GATA2 haploinsufficiency would be expected to display reduced expansion potential when placed under stress, which leads to progressive loss of progenitors. The subsequent hypocellularity then would provide an environment in which more select clones, such as those harboring chromosomal changes or somatic mutations, would become dominant. Serial BM exams from a subset of patients have shown progression from a hypocellular BM with normal cytogenetics and mild or minimal dysplasia to a hypercellular BM with multilineage dysplasia and increased blasts, and new cytogenetic changes. This progression to high-grade MDS or AML occurs over months to several years (Figure 1).
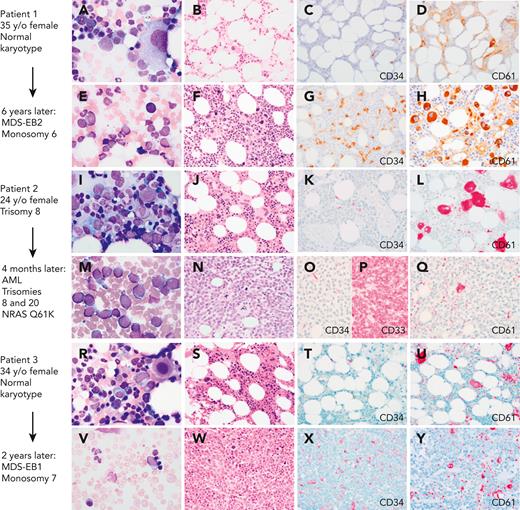
Myeloid progression in GATA2 deficiency. (A-D) A 35-year-old (35 y/o) female with neutropenia, normal hemoglobin and platelet count, and very low monocytes, B cells, and NK cells count. (A) BM aspirate showed dysplastic MKs with minimal morphologic dysplasia in other lineages and no increase in blasts. (B) The biopsy was hypocellular with trilineage hematopoiesis, (C) low number of CD34+ cells, (D) few atypical MKs highlighted by CD61, and normal cytogenetics. (E-F) Six years later, she presented with severe anemia with 16% circulating blasts. (E)The marrow aspirate showed increased scattered blasts. (F) The marrow biopsy was hypercellular with (G) 10% CD34+ blasts, (H) increased CD61+ dysplastic MKs and increased reticulin fibrosis (not shown) indicative of MDS with excess blasts. Cytogenetics showed presence of monosomy 6. A germ line GATA2 mutation was subsequently identified (T354M). (I-L) A 24-year-old female with a prior diagnosis of aplastic anemia in adolescence presented with moderate pancytopenia and very low monocytes, B cells, and NK cells count. (I) The marrow aspirate and (J) BM biopsy showed normocellular marrow with trilineage hematopoiesis, (K) no increase in CD34 positive blasts, and (L) moderate dysmegakaryopoiesis. Cytogenetics revealed trisomy 8. Germ line GATA2 mutation was identified (L375V). (M-P) Four months later she presented with a platelet count of 10 × 103/μL. (M) The BM aspirate showed increased blasts. (N) The marrow biopsy was markedly hypercellular with (O) sheets of blasts that were negative for CD34 and (P) positive for CD33, and (Q) markedly decreased dysplastic MKs. Flow cytometry analysis of the marrow aspirate (not shown) identified 85% monoblasts that expressed CD56, CD64, CD36, and CD123 with minimal expression of CD14 indicative of acute monoblastic leukemia. Cytogenetics showed new trisomy 20 plus trisomy 8 in 65% of metaphases. (R-U) A 34-year-old female with mild pancytopenia, low monocytes, B cells, and NK cells count. (R)The marrow aspirate showed trilineage hematopoiesis with a subset of mononuclear MKs. (S) The BM biopsy was hypocellular for age with trilineage hematopoiesis with (T) no increase in CD34+ blasts, (U) mild megakaryocytic atypia, and trisomy 8. (V-Y) Two years later she presented with circulating blasts. (V) The marrow aspirate was paucicellular with scattered blasts. (W) The BM biopsy was markedly hypercellular with (X) 8% CD34+ myeloblasts confirmed by flow cytometry, and (Y) dysplastic megakaryopoiesis with microMKs. Cytogenetics showed new monosomy 7. Germ line GATA2 mutation was identified (N371K). Marrow aspirates were stained with Wright-Giemsa stain (1000×). BM biopsies were stained with hematoxylin and eosin or immunohistochemistry (IHC) as indicated (500×). Images were taken using an Olympus BX41 microscope equipped with a DP74 camera using Olympus cellSens software. EB1, excess blasts.
Myeloid progression in GATA2 deficiency. (A-D) A 35-year-old (35 y/o) female with neutropenia, normal hemoglobin and platelet count, and very low monocytes, B cells, and NK cells count. (A) BM aspirate showed dysplastic MKs with minimal morphologic dysplasia in other lineages and no increase in blasts. (B) The biopsy was hypocellular with trilineage hematopoiesis, (C) low number of CD34+ cells, (D) few atypical MKs highlighted by CD61, and normal cytogenetics. (E-F) Six years later, she presented with severe anemia with 16% circulating blasts. (E)The marrow aspirate showed increased scattered blasts. (F) The marrow biopsy was hypercellular with (G) 10% CD34+ blasts, (H) increased CD61+ dysplastic MKs and increased reticulin fibrosis (not shown) indicative of MDS with excess blasts. Cytogenetics showed presence of monosomy 6. A germ line GATA2 mutation was subsequently identified (T354M). (I-L) A 24-year-old female with a prior diagnosis of aplastic anemia in adolescence presented with moderate pancytopenia and very low monocytes, B cells, and NK cells count. (I) The marrow aspirate and (J) BM biopsy showed normocellular marrow with trilineage hematopoiesis, (K) no increase in CD34 positive blasts, and (L) moderate dysmegakaryopoiesis. Cytogenetics revealed trisomy 8. Germ line GATA2 mutation was identified (L375V). (M-P) Four months later she presented with a platelet count of 10 × 103/μL. (M) The BM aspirate showed increased blasts. (N) The marrow biopsy was markedly hypercellular with (O) sheets of blasts that were negative for CD34 and (P) positive for CD33, and (Q) markedly decreased dysplastic MKs. Flow cytometry analysis of the marrow aspirate (not shown) identified 85% monoblasts that expressed CD56, CD64, CD36, and CD123 with minimal expression of CD14 indicative of acute monoblastic leukemia. Cytogenetics showed new trisomy 20 plus trisomy 8 in 65% of metaphases. (R-U) A 34-year-old female with mild pancytopenia, low monocytes, B cells, and NK cells count. (R)The marrow aspirate showed trilineage hematopoiesis with a subset of mononuclear MKs. (S) The BM biopsy was hypocellular for age with trilineage hematopoiesis with (T) no increase in CD34+ blasts, (U) mild megakaryocytic atypia, and trisomy 8. (V-Y) Two years later she presented with circulating blasts. (V) The marrow aspirate was paucicellular with scattered blasts. (W) The BM biopsy was markedly hypercellular with (X) 8% CD34+ myeloblasts confirmed by flow cytometry, and (Y) dysplastic megakaryopoiesis with microMKs. Cytogenetics showed new monosomy 7. Germ line GATA2 mutation was identified (N371K). Marrow aspirates were stained with Wright-Giemsa stain (1000×). BM biopsies were stained with hematoxylin and eosin or immunohistochemistry (IHC) as indicated (500×). Images were taken using an Olympus BX41 microscope equipped with a DP74 camera using Olympus cellSens software. EB1, excess blasts.
Evaluation of healthy family members of probands, who harbor GATA2 mutations, indicated that the majority have normal peripheral blood counts, including monocytes, NK cells, and B lymphocytes, with normal trilineage hematopoiesis in the BM and normal cytogenetics. However, several family members have had an unexpected, emerging myeloid malignancy (Figure 2). These experiences have led us to conduct ongoing surveillance of asymptomatic GATA2 mutation-positive relatives. In mutation-positive family members, we recommend a baseline BM with cytogenetics followed by a complete blood count every 6 months and a BM with cytogenetics if neutropenia, anemia, or thrombocytopenia is detected. To emphasize this point, we recently screened an asymptomatic 34-year-old woman from a large GATA2 kindred with multiple cases of myeloid malignancy. She had normal peripheral blood counts. However, her BM revealed presence of 10% myeloblasts and trisomy 8. She underwent a successful unrelated donor HSCT.
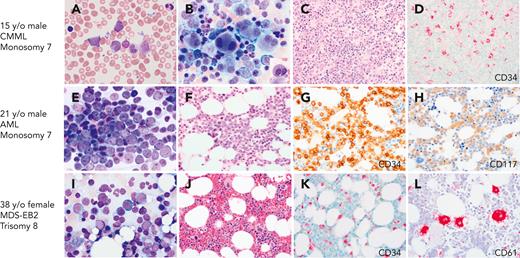
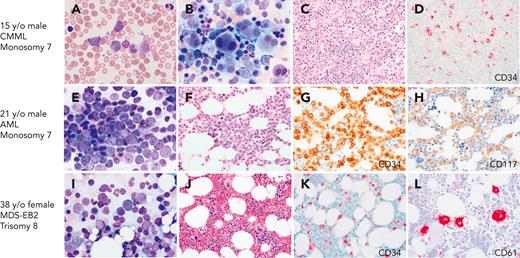
BMs with CMML, AML, and MDS with excess blasts on initial presentation with GATA2 deficiency. (A-D) A 17-year-old healthy male with family history of GATA2 deficiency (c.1017+572C>T) and AML (mother), MDS (aunt), and CMML (grandfather), presented for his initial evaluation. White blood cell counts of 15.6 × 103/μL. (A) Peripheral blood smear with leukocytosis and circulating blasts (6%), monocytosis (1.5 × 103/μL), and left-shifted granulocytes. (B) BM aspirate showing marked megakaryocytic dysplasia with dysplastic myelopoiesis (inset). (C) BM core biopsy was markedly hypercellular. (D) CD34 IHC on the marrow biopsy showed increased blasts. Cytogenetic analysis revealed monosomy 7, and myeloid next-generation sequencing analysis detected ASXL1, SETBP1, and U2AF1 mutations. Findings indicative of CMML. (E-H) A 21-year-old male presented with fatigue and pancytopenia with no history of infections or family history of myeloid malignancy. (E) BM aspirate with sheets of blasts confirmed by flow cytometry as myeloblasts. (F) BM core biopsy was hypocellular for age. (G) CD34 IHC on the marrow biopsy showed marked increase in blasts >50%. (H) CD117 IHC highlighting the blasts. Monosomy 7 detected on cytogenetic analysis. The patient was diagnosed with AML and subsequently found to have a germ line mutation in GATA2 (T354M). (I-L) A 39-year-old female who was a healthy relative of a proband with GATA2 deficiency (R398W), was recently found to be positive for the mutation and presented for evaluation. Peripheral blood counts were normal to slightly decreased. (I) BM aspirate with increased blasts (∼10%). (J) BM core biopsy was normocellular. (K) CD34 IHC showing increased blasts (∼10%). (L) CD61 IHC staining MKs with a few small forms. The patient was diagnosed with MDS with excess blasts. All peripheral blood and BM aspirates were stained with Wright-Giemsa stain at 1000×. Core biopsies were stained with H&E or IHC as indicated. Images were taken using an Olympus BX41 microscope equipped with a DP74 camera using Olympus cellSens software.
BMs with CMML, AML, and MDS with excess blasts on initial presentation with GATA2 deficiency. (A-D) A 17-year-old healthy male with family history of GATA2 deficiency (c.1017+572C>T) and AML (mother), MDS (aunt), and CMML (grandfather), presented for his initial evaluation. White blood cell counts of 15.6 × 103/μL. (A) Peripheral blood smear with leukocytosis and circulating blasts (6%), monocytosis (1.5 × 103/μL), and left-shifted granulocytes. (B) BM aspirate showing marked megakaryocytic dysplasia with dysplastic myelopoiesis (inset). (C) BM core biopsy was markedly hypercellular. (D) CD34 IHC on the marrow biopsy showed increased blasts. Cytogenetic analysis revealed monosomy 7, and myeloid next-generation sequencing analysis detected ASXL1, SETBP1, and U2AF1 mutations. Findings indicative of CMML. (E-H) A 21-year-old male presented with fatigue and pancytopenia with no history of infections or family history of myeloid malignancy. (E) BM aspirate with sheets of blasts confirmed by flow cytometry as myeloblasts. (F) BM core biopsy was hypocellular for age. (G) CD34 IHC on the marrow biopsy showed marked increase in blasts >50%. (H) CD117 IHC highlighting the blasts. Monosomy 7 detected on cytogenetic analysis. The patient was diagnosed with AML and subsequently found to have a germ line mutation in GATA2 (T354M). (I-L) A 39-year-old female who was a healthy relative of a proband with GATA2 deficiency (R398W), was recently found to be positive for the mutation and presented for evaluation. Peripheral blood counts were normal to slightly decreased. (I) BM aspirate with increased blasts (∼10%). (J) BM core biopsy was normocellular. (K) CD34 IHC showing increased blasts (∼10%). (L) CD61 IHC staining MKs with a few small forms. The patient was diagnosed with MDS with excess blasts. All peripheral blood and BM aspirates were stained with Wright-Giemsa stain at 1000×. Core biopsies were stained with H&E or IHC as indicated. Images were taken using an Olympus BX41 microscope equipped with a DP74 camera using Olympus cellSens software.
MDS in GATA2 deficiency
Since the 2016 revision of the WHO classification of myeloid neoplasms and acute leukemias, germ line predisposition has constituted a distinct clinical entity in the category of “myeloid neoplasm with germ line predisposition and other organ dysfunction.” MDS in GATA2 deficiency most often meets the criteria for MDS with either multilineage dysplasia or refractory cytopenia of childhood. There are distinct differences between sporadic MDS and MDS with germ line predisposition. Sporadic MDS are a heterogenous group of myeloid stem cell diseases characterized by dysplasia and peripheral cytopenias, in which the overall survival and risk of leukemia transformation are highly variable.37,38 The incidence of MDS as well as AML dramatically increase with advanced age, particularly among individuals ≥60 years of age. In contrast, the median age of onset of MDS in GATA2 deficiency varies from 12 to 34 years based on the study.10,11 Also, compared with sporadic MDS in adults, which is associated with a hypercellular marrow, MDS in the setting of GATA2 deficiency in both children and adults is usually hypoplastic35 (Figure 1). Pediatric MDS typically presents with a hypoplastic bone marrow, and a much greater proportion result from germ line predisposition, including GATA2 deficiency. Taken together, >50% of individuals with GATA2 deficiency develop megakaryocytic dysplasia and MDS, often with increased fibrosis.5,35,39 In some GATA2 families the myeloid malignancy has occurred at an earlier age with each successive generation, suggesting a potential role for anticipation, a feature requiring further investigation.
Children and adolescents with MDS constitute an important group, in which the incidence of germ line GATA2 mutations is disproportionately high.11 Wlodarski et al, evaluated a group of 426 children and adolescents with primary MDS and 82 cases of secondary MDS in 2 prospective European studies of MDS in childhood conducted in Germany over 15 years.11 Germ line GATA2 mutations accounted for 15% of advanced and 7% of all primary MDS cases. Mutation-positive individuals were more likely to present with monosomy 7. This study identified germ line GATA2 mutations as the most common germ line defect predisposing to pediatric MDS. The majority of the pediatric cases in this study were associated with de novo germ line mutations. In addition, many pediatric patients lack a history of infections or recognized immunodeficiency, indicating that MDS or AML can be the presenting manifestation of disease (Figure 2).40
Cytogenetics in GATA2 deficiency
The acquisition of new cytogenetic changes and somatic mutations frequently lead to hyperproliferation and overt malignant myeloid progression in the setting of GATA2 deficiency.41,42 We studied 106 patients and their family members with GATA2 deficiency to characterize acquired cytogenetic changes predisposing to myeloid progression.42 In this heterogenous group, 44% had MDS, 37% had GATA2 BM immunodeficiency disorder with hypocellular marrow lacking overt dysplasia, and 13% had no BM changes. Cytogenetic changes were present in 43% with trisomy 8 (∼23%) being the most common change, followed by monosomy 7 (∼12%). In individuals with GATA2 deficiency and cytogenetic abnormalities, we have invariably seen some evidence of marrow dysplasia, albeit subtle, and only in the megakaryocytic lineage. The implications of trisomy 8 in GATA2 deficiency in particular are unclear in that we have observed a patient with trisomy 8 segue into AML in a 4-month period. In contrast, we recently carried out an unrelated donor HSCT on a 47-year-old woman with a documented 26-year history of MDS with trisomy 8. Other patients had trisomy 8 for a period of time, followed by acquisition of monosomy 7 and progressive MDS.
In an intriguing observation, der(1;7)(q10;p10), an unbalanced, whole-arm chromosomal translocation resulting in trisomy 1q and deletion 7q, was shown to be considerably enriched in a cohort (n = 25) of patients with GATA2 deficiency.43 It would appear that trisomy 1q with der(1;7)(q10;p10) would be an adverse prognostic indicator because MDM4, an inhibitor of p53, is located on 1q, and trisomy 1q would result in overexpression of MDM4 and suppression of p53. Upon screening 15 cases of AML with complex karyotype and nonmutated p53, increased levels of MDM4 were detected.44 However, because 21 of the 22 patients with GATA2 deficiency with this cytogenetic abnormality who underwent HSCT are alive, this indicates that the der(1;7)(q10;p10) did not confer an unfavorable prognosis.
Somatic mutations in collaborating genes in GATA2 deficiency
We investigated the somatic mutations in genes associated with myeloid progression in the same 106 individuals harboring mutations in GATA2. Somatic mutations in ASXL1 and STAG2 were present in ∼20% and ∼25%, respectively, of individuals with germ line GATA2 mutations, although mutations in ASXL1 and STAG2 were rarely found concomitantly.42 These 2 somatic mutations were similarly found in individuals with GATA2 BM immunodeficiency disorder and MDS, suggesting that clonal hematopoiesis develops early in GATA2 deficiency, before the onset of MDS. Of note, mutations in both ASXL1 and STAG2 conferred a lower survival probability. Many of the commonly mutated genes associated with sporadic myeloid malignancy, including TET2, IDH1/2, and splicing factor genes, were conspicuously absent in the individuals harboring GATA2 mutations, demonstrating that the genetic landscape of MDS in GATA2 deficiency is different from sporadic MDS. Somatic mutations in chromatin-related genes, such as ASXL1, and cohesin genes, such as STAG2, were associated with malignant progression in the setting of GATA2 deficiency.
Mutations in cohesin genes such as STAG2 are particularly problematic in GATA2 deficiency because the cohesion complex plays a central role in mitosis, in which it promotes sister chromatid cohesion. The cohesion complex also plays a critical role in gene expression by regulating chromatin “looping” bringing distal regulatory elements in proximity to proximal regulatory sequences.45 Although complete loss of STAG2 would be expected to have a lethal effect on mitosis, particularly in males because STAG2 is X-linked, its loss can be compensated by its paralog STAG1.46 Cohesin haploinsufficiency enhances hematopoietic stem cell and progenitor cell self-renewal and may contribute to malignant progression in GATA2 deficiency.
Dameshek’s riddle
Patients with GATA2 deficiency, who develop cytopenias, typically transition from a normocellular BM to a hypocellular BM with or without overt dysplasia. In this regard, GATA2 deficiency can masquerade as aplastic anemia.34 Subsequently, a hypercellular marrow may emerge, coinciding with transformation to high-grade MDS or AML with increased blasts. Many years may intervene between these stages with some patients never progressing to a hyperproliferative state.
This type of marrow transition was described >50 years ago by William Dameshek in another context. In 1967, he wrote an editorial in this journal titled: “Riddle: what do aplastic anemia, paroxysmal nocturnal hemoglobinemia (PNH), and hypoplastic leukemia have in common?”47 In these diseases he posits that an initial insult to the BM, an exogenous insult in his case or an intrinsic defect in the case of germ line GATA2 deficiency, leads to “hypoproliferation” or BM failure. This is followed by “repair” in his lexicon, “second hits” in the form of new cytogenetic abnormalities, or a mutation in a gene involved in hematopoiesis in GATA2 deficiency leading to hyperproliferation, clonal hematopoiesis, and myeloid transformation.48
Clonal progression to AML and CMML in GATA2 deficiency
Clonal hematopoiesis is an early feature in GATA2 deficiency and can be seen without overt changes in the BM, such as GATA2 BM immunodeficiency disorder, which is characterized by having hypocellular marrow without overt dysplasia and not meeting criteria for MDS.34,42,49 Progression to MDS is common, whereas progression to AML and CMML is less common, especially with close surveillance of individuals with GATA2 haploinsufficy.10,14 However, overt myeloid malignancy can represent the initial presentation of GATA2 deficiency (Figure 2). The relationship between infection and clonal hematopoiesis progression remains speculative.
AML in GATA2 deficiency may arise in the setting of preceding MDS, or patients may present with AML or CMML (Figure 2). Blasts frequently have a classic myeloid phenotype with expression of CD34, CD33, CD117, and CD13. Monocytic differentiation of blasts that are CD34- and CD64+ is also common with either acute monoblastic leukemia,50 or acute myelomonocytic leukemia. Emergence of monocytes in a patient with longstanding monocytopenia may herald an evolving leukemia.
CMML constitutes a form of MDS or myeloproliferative neoplasms, more commonly seen in older patients.51 The pediatric analogue juvenile myelomoncytic leukemia and some adult CMML can be seen with rasopathies.52 However, CMML can develop in teenagers and young adults with germ line GATA2 mutations.53 In this situation the CMML takes a proliferative form with leukocytosis and circulating blasts, frequently accompanied by cytogenetic abnormalities and somatic mutations in ASXL1 or SETBP1. The outcomes for adults with CMML, especially those with unfavorable cytogenetics, are poor with a 5-year overall survival of <5%.54,55 MDS or myeloproliferative neoplasms with absent monocytes is also seen in GATA2 deficiency. A small subset of patients with GATA2 deficiency have eosinophilia in the marrow and peripheral blood with absence of parasitic infections or rearrangements involving PDGFRA or other cytogenetic aberrations associated with myeloid or lymphoid neoplasms with eosinophilia.
Allogeneic HSCT represents the only definitive therapy for CMML, however the outcomes with HSCT remain suboptimal because of the advanced age of the patients and accompanying comorbidities.55 In contrast, patients harboring germ line GATA2 mutations, who develop CMML, appear to have a more favorable outcome largely because of less comorbidities and transplant-related complications. We recently described a 17-year-old male with a germ line GATA2 mutation who presented with a proliferative CMML with circulating blasts, monosomy 7 in 20 of 20 metaphases, and somatic mutations in SETBP1, U2AF1, and ASXL1; he was successfully bridged to haploidentical transplant with 2 cycles of venetoclax/decitabine.53
The curious cases of B-cell ALL and early T-cell ALL in GATA2 deficiency
The relationship between GATA2 deficiency and the development of lymphoid malignancies highlight the role of GATA2 in very early hematopoiesis. Two lymphoid leukemias have been described that arise in a background of GATA2 deficiency. A B-cell ALL in an 11-year-old female with GATA2 deficiency and monosomy 7 was described.56 More intriguing, an early T-cell precursor ALL was described in an 8-year-old child with GATA2 deficiency.57 Early T-cell precursor ALL lymphoblasts have multilineage pluripotency as suggested by the coexpression of myeloid and stem cell markers. Early T-cell precursor ALL cells also have coexpression of gene mutations found in AML (FLT3, RAS, and DNMT3A) and gene mutations found in lymphoid malignancies. A substantial number of early T-cell precursors in the thymus at the DN1 and DN2 stage before T-cell receptor rearrangement have myeloid potential. These cells are recent thymic immigrants from the BM to the thymus, and they are derived from HSCs. These cells are committed to the T-cell lineage but with limited early T-cell differentiation. Thus, a hematopoietic progenitor with GATA2 deficiency is at the nexus of development and is poised to develop into myeloid or lymphoid leukemogenesis depending upon the additional hits.58-60
Allogeneic HSCT in GATA2 deficiency
Allogeneic HSCT has been used successfully to treat both genetic IEI and MDS or AML, the 2 characteristics of the patients with germ line mutations in GATA2, and therefore represents a definitive therapy for reversal of the clinical course in these patients. However, the indications for HSCT in GATA2 deficiency remain challenging because of the variability in disease progression. Infectious complications, notably disseminated NTM infections and advanced HPV infections, are the main drivers to HSCT in ∼50% of patients with GATA2 deficiency.61,62 The NTM infections are frequently recurrent, leading to impaired pulmonary function. HPV infections are also progressive, leading to high-grade cervical and rectal lesions that lead to squamous cell carcinoma.61-63 Allogeneic HSCT is highly effective in preventing recurrent NTM infections. We have yet to see a recrudescence of NTM infections after HSCT. The outcomes with HPV infections and HSCT are more variable with ∼50% having resolution of HPV disease.62 BM failure with cytopenias, along with myeloid progression, lead to HSCT in ∼50% of the remaining patients.
Two features of GATA2 deficiency make the decision regarding HSCT especially difficult. The first is the highly variable clinical course of the disease, even in the same family with the same mutation, in which some members develop myeloid progression early in life, whereas others remain asymptomatic for their entire life. In 1 small study, variability in penetrance was associated with monoallelic expression of the mutated GATA2 allele in symptomatic individuals, whereas asymptomatic family members demonstrated biallelic expression.64 Malignant transformation in seemingly stable patients can be abrupt.
Type of HSCT in GATA2 deficiency
In our initial cohort of 14 patients, in which we described the use of allogeneic HSCT, a number of the patients had not been diagnosed with GATA2 deficiency at the time of transplant because the disease was yet to be identified.61 All these patients had serious comorbidities, therefore we used a nonmyeloablative regimen.61 Although 8 of the 14 patients had complete reversal of the GATA2 phenotype, the need for pretransplant chemotherapy, 1 graft rejection, and 1 relapse, together supported the use of a higher dose conditioning regimen in this group of patients.
In 2 subsequent studies we described the results after HSCT in a total of 59 patients using a busulfan-based myeloablative regimen.65,66 The overall survival and event-free survival were 85.1% and 82.1%, respectively. Surprisingly, all 20 of the haploidentical related donor recipients were alive and disease-free at the time of the report, although 1 subsequently relapsed and died. Notably, the use of posttransplant cyclophosphamide in the matched-related donor and unrelated donor transplant recipients resulted in no cases of grade III to IV acute graft-versus-host disease.
We addressed this question of the optimal type of conditioning regimen for GATA2 deficiency in an Inside Blood commentary in this journal in 2018 following a report from the United Kingdom in which an in vitro T-cell depleted reduced intensity regimen was used successfully for 4 patients undergoing HSCT for GATA2 deficiency.67,68 Analyzing the data from United Kingdom together with our previous data using a nonmyeloablative regimen, in which patients with GATA2 deficiency have a hypocellular BM with normal cytogenetics or trisomy 8, it was found that a reduced intensity or nonmyeloablative regimen results in reliable engraftment because the GATA2 deficient BM is at a proliferative disadvantage.48,67 However, with clonal progression leading to a normocellular or hypercellular BM and unfavorable cytogenetic changes in which the myeloid clone has a proliferative advantage, a myeloablative regimen results in more reliable engraftment and relapse prevention.65
Ensuring that a related donor does not have a GATA2 mutation is critical in HSCT for GATA2 deficiency. We have reported deleterious outcomes in patients with unrecognized germ line GATA2 mutations who received an HSCT using a related donor harboring the same GATA2 mutation.69,70 This suggests that although a healthy asymptomatic carrier may not have detectable hematopoietic disease, the HSCs are abnormal and have the propensity for malignant transformation and/or lack robust lineage repopulation capacity. Variable penetrance and expressivity of germ line mutations may mask an underlying family history of disease, underscoring the need for consideration of potential germ line mutations in younger patients with myeloid malignancies undergoing HSCT using a related donor.
Preemptive HSCT has been proposed for diseases such as GATA2 deficiency, however the highly variable clinical course supports expectant management, provided proper surveillance is carried out.
Acknowledgments
This work was supported (in part) by the Intramural Research program of the National Institutes of Health (NIH) including the National Cancer Institute, National Institutes of Health (Hematopoietic Stem Cell Transplant for GATA2 Deficiency, 1 ZIA BC010870 Hickstein, Dennis – NCI), in part by the Division of Intramural Research, and (in part) under Contract No. HHSN261200800001E, and Contract No. 75N910D00024, Task Order No. 75N91019F00131, and the NIH Clinical Center RASCL award (K.R.C.).
The content of this publication neither necessarily reflects the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations implying endorsement by the US government.
Authorship
Contribution: K.R.C. and D.D.H. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dennis D. Hickstein, Immune Deficiency-Cellular Therapy Program, Bldg 10-CRC, Room 3-3142, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: hicksted@mail.nih.gov.