In 1969, Li and Fraumeni described 4 families characterized by the occurrence of soft-tissue sarcomas in children and cancers of the breast and other organs in their parents and relatives.1 Twenty-one years later, the germ line transmission of a mutant TP53 gene was reported in families with Li-Fraumeni syndrome, indicating that the inherited TP53 mutation predisposed the members of these families to various malignancies.2,3
The landmark paper of Li and Fraumeni represents the beginning of the “cancer predisposition revolution,” a field that has expanded enormously in the last decades.4 Specifically, the use of massively parallel DNA sequencing has led to the identification of individuals with germ line genetic predisposition to hematologic malignancies.5 Of note, these predisposition disorders already represent distinct chapters in the classification of tumors of hematopoietic and lymphoid tissues.6,7
Blood review series on germ line predisposition to hematologic malignancies
The review series in this issue of Blood describes the latest advances in our understanding of the following predisposition disorders:
Christopher R. Reilly and Akiko Shimamura, "Predisposition to myeloid malignancies in Shwachman-Diamond syndrome: biological insights and clinical advances"
Katherine R. Calvo and Dennis D. Hickstein, "The spectrum of GATA2 deficiency syndrome"
Claire C. Homan, Hamish S. Scott, and Anna L. Brown, "Hereditary platelet disorders associated with germ line variants in RUNX1, ETV6, and ANKRD26"
Hideki Makishima, Teresa V. Bowman, and Lucy A. Godley, “DDX41-associated susceptibility to myeloid neoplasms”
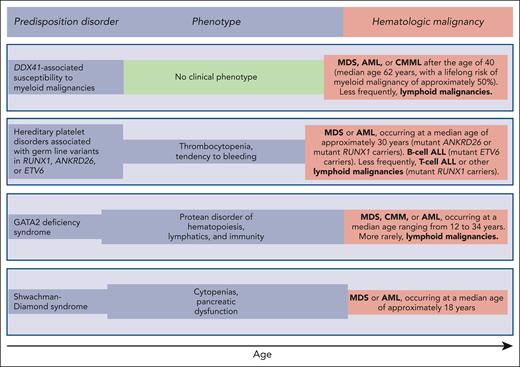
Reilly and Shimamura discuss the biology and clinical features of the Shwachman-Diamond syndrome (SDS). This condition is associated with biallelic germ line mutations in the SBDS gene and is generally diagnosed in early childhood when patients develop bone marrow failure and exocrine pancreatic dysfunction. Patients with SDS have a high risk of acquiring somatic mutations in TP53 and progressing to myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) in adolescence or early adulthood (Figure 1). These myeloid malignancies are typically associated with a TP53 multihit state, that is, biallelic inactivation of both parental alleles of this gene.8 The prognosis of patients with SDS and overt MDS or AML is extremely poor, even when the subjects undergo allogeneic stem cell transplantation.9,10 Reilly and Shimamura present evidence supporting hematologic surveillance that includes genomic profiling for risk stratification of patients with SDS. The aim of this monitoring is to identify early those patients who can benefit from allogeneic stem cell transplantation, ideally before a TP53 multihit state develops.
Germ line predisposition disorders analyzed in the review series in the current issue of Blood. The phenotype may be highly variable, even within the same family; in some subjects, it may be absent or very mild, going unnoticed. Of note, patients with DDX41-associated susceptibility to myeloid malignancies have no phenotype before the development of a hematologic malignancy, which typically occurs in the sixth and seventh decades of life; that is, at an age when sporadic hematologic malignancies (not associated with any predisposition disorder) occur.
Germ line predisposition disorders analyzed in the review series in the current issue of Blood. The phenotype may be highly variable, even within the same family; in some subjects, it may be absent or very mild, going unnoticed. Of note, patients with DDX41-associated susceptibility to myeloid malignancies have no phenotype before the development of a hematologic malignancy, which typically occurs in the sixth and seventh decades of life; that is, at an age when sporadic hematologic malignancies (not associated with any predisposition disorder) occur.
Hickstein and Calvo coauthored the original report describing 57 patients with GATA2 deficiency, a condition they defined as “a protean disorder of hematopoiesis, lymphatics, and immunity” (Figure 1).11 In the aforementioned review, they underline that GATA2 is crucial for the proliferation and maintenance of hematopoietic stem cells. In turn, inherited GATA2 deficiency is associated with severe deficiency of B-lymphocytes, monocytes, NK cells, and dendritic cells, leading to cytopenias of these lineages and subsequent infections. The inheritance pattern of GATA2 deficiency is autosomal dominant. The first manifestations of the disease typically occur during late adolescence or early adulthood. The acquisition of cytogenetic abnormalities (trisomy 8 or monosomy 7) or somatic gene mutations (primarily in ASXL1 or STAG2) may cause progression to a myeloid malignancy, more commonly MDS, and less frequently chronic myelomonocytic leukemia or AML (Figure 1). Hickstein and Calvo underline that myeloid malignancy may represent the primary presentation of disease, mainly because syndromic features may be mild and go unnoticed. The authors have previously shown that allogeneic transplantation in GATA2 deficiency reverses the hematologic disease phenotype.12 However, because of the highly variable clinical course of GATA2 deficiency, they do not recommend pre-emptive transplantation but rather proper surveillance and expectant management.
Homan et al discuss hereditary platelet disorders characterized by thrombocytopenia and/or platelet dysfunction and associated with heterozygous germ line mutations in RUNX1, ANKRD26, or ETV6. Overall, these patients had mild to moderate thrombocytopenia with dysmegakaryopoiesis and a bleeding tendency. In RUNX1-related hereditary platelet disorder, progression to a myeloid neoplasm is typically associated with the acquisition of a somatic mutation in the second RUNX1 allele; however, somatic lesions may also occur in other genes, such as PHF6 and BCOR. The median age of onset of myeloid malignancy is ∼30 years (Figure 1), with a cumulative incidence of 43% by 50 years, and a few patients develop childhood T-cell ALL. Patients with ETV6-related hereditary platelet disorder are at a high risk of developing ALL, commonly B-cell ALL. Homan et al concluded that international cooperation and ongoing technology development are needed to increase the speed and accuracy of diagnosis and develop new therapies for these patients. Accordingly, the authors established the RUNX1 database (RUNX1db).13
Makishima et al examined DDX41-associated susceptibility to myeloid neoplasms. This condition is associated with heterozygous germ line mutations in the DDX41 gene and has no clinical phenotype before progression.14 Acquisition of a somatic mutation in the second DDX41 allele typically leads to MDS or AML, most commonly in the sixth or seventh decade of life, that is, at an age typical of sporadic disease (Figure 1). The lifelong risk of developing a myeloid malignancy is ∼50% in mutant DDX41 carriers, and men progress more frequently than females. Patients with DDX41-mutant MDS have a high risk of rapidly progressing to AML, and this risk is confined to patients carrying truncating variants.14 This feature predicts leukemic transformation much better than both the revised and molecular International Prognostic Scoring System (IPSS-R and IPSS-M).15,16 Because virtually all truncating variants are germ line, the identification of a truncating DDX41 allele on genomic profiling to detect somatic mutations should prompt germ line genetic testing. Finally, Makishima et al underline that patients with deleterious germ line DDX41 variants frequently develop severe acute graft-versus-host disease after allogeneic stem cell transplantation, unless they receive posttransplant cyclophosphamide.17 This observation may suggest a proinflammatory milieu that stimulates donor-derived T-cells.
Time to consider germ line genetic testing in the diagnostic workup of patients with myeloid malignancies
Two recent articles in this journal have analyzed how genomic testing should complement clinical diagnosis in hematologic malignancies.18,19 With respect to germ line predisposition, Duncavage et al concluded that this risk should be considered for all patients diagnosed with a myeloid neoplasm regardless of age.18 In a recent study, pathogenic germ line variants were identified in 28 of 404 (7%) patients with MDS of all ages treated with allogeneic stem cell transplantation from a family donor.20 Because variants were called as those detectable in both the transplanted patient and related donor, the 7% overall frequency of deleterious germ line variants is clearly an underestimate. Based on the significant frequency of germ line variants in these patients, the authors concluded that germ line genetic testing is recommended for all patients with MDS.
The conventional diagnostic workup of patients with unexplained cytopenia includes bone marrow aspiration to detect morphologic dysplasia and blasts, bone marrow biopsy to assess marrow cellularity and fibrosis, and chromosome banding analysis to detect nonrandom chromosomal abnormalities.21 Over the past few years, gene panel sequencing to detect somatic mutations has become a routine procedure in most academic institutions in Western countries.22Figure 2 highlights the potential benefits of including germ line genetic testing in the diagnostic workup of patients with MDS, specifically considering the predisposition disorder of the current review series. To make this approach feasible, ad hoc infrastructures should be built into our health system.22
Schematic representation of the potential benefits of including germ line genetic testing in the diagnostic workup of patients with unexplained blood cytopenia, the essential diagnostic criterion for MDS.
Schematic representation of the potential benefits of including germ line genetic testing in the diagnostic workup of patients with unexplained blood cytopenia, the essential diagnostic criterion for MDS.
Conflict-of-interest disclosure: The author declares no competing financial interests.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal