Abstract
Background: The prevalence of monoclonal gammopathy of undetermined significance (MGUS) in a population at high risk of developing multiple myeloma (MM) was recently described in a US screening study. In a high-risk population, MGUS prevalence was measured as 6% by serum protein electrophoresis with immunofixation (SPEP/IFX) and 13% by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Screening by MALDI-TOF MS allowed for the detection of monoclonal proteins (M-proteins) below the threshold of detection of gel-based assays, that was referred to as Monoclonal Gammopathy of Indeterminate Potential (MGIP). MGIP was found in 25% of the screened cohort and was significantly associated with aging. Other studies indicated that MGUS and MGIP detected by MS may be transient and their detection may not be critical for long term outcomes. Therefore, we aimed to determine the fate of M-proteins detected by MS in a US population-based study.
Methods: High-risk individuals enrolled in the study include those who self-identify as Black or have a family history of hematologic malignancies or a precursor condition to MM. Samples that tested positive for MGUS (M-protein >= 0.2 g/L) by MALDI-TOF MS were confirmed using clinically approved assays and results were returned to participants. All participants who had an M-protein detected at baseline by MALDI-TOF MS were invited to submit follow-up samples to investigate the fate of the M-proteins detected at the MGIP and MGUS levels. Serum was analyzed using the Binding Site Group's EXENT® assay based on MALDI-TOF MS for the identification and quantification of M-proteins. Persistence of the M-protein detected at baseline was defined as the presence of the same isotype M-protein on a serial sample and within +/-5 mass to charge ratio on mass spectra. All serial samples where the M-protein was not detected by MALDI-TOF MS were reflex tested to liquid chromatography (LC) MS.
Results: We analyzed 262 multi-timepoint samples collected from 102 participants who tested positive for an M-protein, with a median follow-up of 336 days (Range 36 - 1,240; IQR 141 - 737). The median age at screening was 60.8 years, 65 (64%) participants were female, 9 (9%) self-identified as Black, and 93 (91%) were non-Black and reported a family history of hematologic malignancy.
At baseline, 66 (65%) participants had MGIP (M-protein <0.2g/L) and 36 (35%) had MGUS (M-protein >=0.2/L) measured by MALDI-TOF MS. All participants with MGIP were negative at baseline by SPEP/IFX, while among those with MGUS at baseline, 25/36 (69%) participants had an M-protein detected by SPEP/IFX. At baseline, the distribution of isotypes was 21% IgG, 17% IgA, and 62% IgM for MGIP and 69% IgG, 17% IgA, and 14% IgM for MGUS.
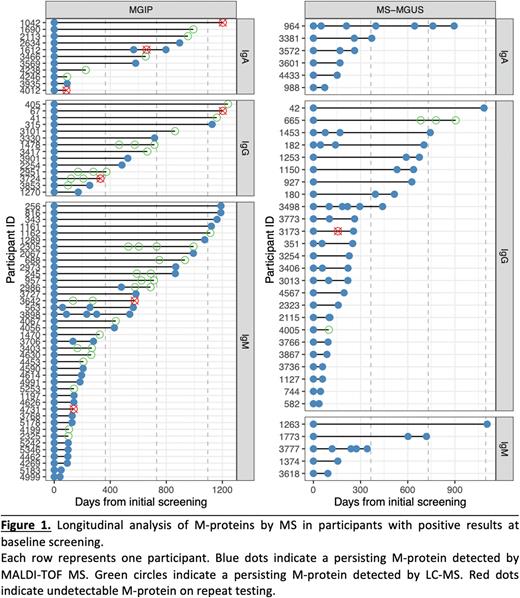
For all 102 cases, their most recent serial samples were tested by MALDI-TOF MS or LC-MS. 60/66 (91%) of the MGIP cases were re-confirmed on serial sampling, with a median follow-up time of 504 days (Range 41 - 1,240; IQR 149 - 888). As for MGUS, 36/36 (100%) of cases detected at baseline persisted on serial sampling, with a median follow-up time of 239 days (Range 36 - 1105; IQR 103 - 628) (Figure 1). Time from baseline to serial sampling was not associated with persistence. In 12 participants so far, M-proteins detected by MS were seen to persist for more than 3 years after baseline testing.
In 5 of the MGIP cases, M-protein concentration measured by MALDI-TOF MS increased from MGIP level to above the MGUS threshold. These participants had a median baseline M-protein concentration of 0.14 g/L (Range 0.13 - 0.17 g/L; IQR 0.13 - 0.14), which was in the upper MGIP concentration level, and had a median follow-up time of 532 days (Range 171 - 1,127; IQR 302 - 570). Of these, 3 persisted as IgG, 1 IgA, and 1 IgM isotypes.
Conclusion: We present the first longitudinal analysis of M-proteins detected by MS, including those below the threshold of detection of gel-based assays, in a US population-based screening study. Results confirm the persistence of 91% of MGIP and 100% of MGUS cases detected by MS. Some cases showed increasing M-protein concentration, crossing the threshold of detection by gel-based assays. Additional studies are underway to further validate our findings in a larger cohort with longer follow-up time. Testing serial samples with increasing M-protein concentration by gel-based assays will help better understand the significance of early detection of MGIP cases by MS.
Disclosures
Sakrikar:The Binding Site Group Ltd: Current Employment. Troske:The Binding Site Group Ltd: Current Employment. Barnidge:The Binding Site Group Ltd: Current Employment. Perkins:The Binding Site Group Ltd: Current Employment. Harding:The Binding Site: Current Employment, Membership on an entity's Board of Directors or advisory committees. Getz:MSIDetect: Patents & Royalties; MSMutSig: Patents & Royalties; Pharmacyclics: Research Funding; MSMuTect: Patents & Royalties; SignatureAnalyzer-GPU: Patents & Royalties; IBM: Research Funding; Scorpion Therapeutics: Consultancy, Current equity holder in publicly-traded company, Other: Founder; POLYSOLVER: Patents & Royalties. Ghobrial:Adaptive: Honoraria; Huron Consulting: Honoraria; Veeva Systems: Honoraria; Menarini Silicon Biosystems: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Oncopeptides: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; The Binding Site: Honoraria; Takeda: Honoraria; Aptitude Health: Honoraria; AbbVie: Honoraria; Bristol Myers Squibb: Honoraria; Sognef: Honoraria; Vor Biopharma: Honoraria; Novartis: Research Funding; Celgene: Research Funding; Window Therapeutics: Other: Advisory board participation.
Author notes
Asterisk with author names denotes non-ASH members.