Key Points
In children with AIC, IMs are associated with a lower splenectomy FFS.
IMs are associated with a higher risk of recurrent or severe bacterial infections and thrombosis.
Abstract
Splenectomy is effective in ∼70% to 80% of pediatric chronic immune thrombocytopenia (cITP) cases, and few data exist about it in autoimmune hemolytic anemia (AIHA) and Evans syndrome (ES). Because of the irreversibility of the procedure and the lack of predictions regarding long-term outcomes, the decision to undertake splenectomy is difficult in children. We report here factors associated with splenectomy outcomes from the OBS’CEREVANCE cohort, which prospectively includes French children with autoimmune cytopenia (AIC) since 2004. The primary outcome was failure-free survival (FFS), defined as the time from splenectomy to the initiation of a second-line treatment (other than steroids and intravenous immunoglobulins) or death. We included 161 patients (cITP, n = 120; AIHA, n = 19; ES, n = 22) with a median (minimum-maximum) follow-up of 6.8 years (1.0-33.3) after splenectomy. AIC subtype was not associated with FFS. We found that immunopathological manifestations (IMs) were strongly associated with unfavorable outcomes. Diagnosis of an IM before splenectomy was associated with a lower FFS (hazard ratio [HR], 0.39; 95% confidence interval [CI], 0.21-0.72, P = .003, adjusted for AIC subtype). Diagnosis of an IM at any timepoint during follow-up was associated with an even lower FFS (HR, 0.22; 95% CI, 0.12-0.39; P = 2.8 × 10−7, adjusted for AIC subtype) as well as with higher risk of recurrent or severe bacterial infections and thrombosis. In conclusion, our results support the search for associated IMs when considering a splenectomy to refine the risk-benefit ratio. After the procedure, monitoring IMs helps to identify patients with higher risk of unfavorable outcomes.
Introduction
Chronic immune thrombocytopenia (cITP) and autoimmune hemolytic anemia (AIHA) are rare diseases in children consisting of autoimmune destruction of platelets and erythrocytes, respectively. They can be associated within an even rarer entity, Evans syndrome (ES). These 3 conditions are referred to here as autoimmune cytopenia (AIC). Associated with AIC, patients can present with various immunopathological manifestations (IMs) such as lymphoproliferation, autoimmune/autoinflammatory organ diseases, and hypogammaglobulinemia.1,2 These IMs can be absent at AIC diagnosis and develop during follow-up.1,2 In a subset of patients with IMs, an underlying condition is diagnosed and the AIC is considered as secondary.3 Systemic lupus erythematosus (SLE) and primary immunodeficiencies (PIDs) are the most frequent diagnosis of secondary AIC in children.1-4 If no underlying condition is identified, AIC is considered primary.
All AICs can evolve toward chronic evolution and need for first- and second-line treatments.5 The spleen is a predilection site of platelet and/or erythrocyte phagocytosis and splenectomy is a classic second-line treatment in adults and children with cITP.6-8 Nevertheless, studies addressing splenectomy efficacy in pediatric AIHA and ES are scarce, consisting of retrospective series with a limited number of patients.9-12 Splenectomy is effective in ∼70% to 80% of pediatric cITP.6,8,13-17 Long-term follow-up suggests sustained responses, but exclusively pediatric data are limited.13,16,17 Despite being one of the most effective cITP treatments, splenectomy use has decreased over time.6,8 This is in part from the emergence of alternative therapies (such as anti-CD20 antibodies and thrombopoietin receptor agonists) and from infectious risk associated with asplenia.6,18 Splenectomy is irreversible and thus questionable, especially in children. Pediatricians lack validated predictive factors to identify children who may benefit from the procedure or who are at risk of treatment failure and/or complications.
Here, we report the long-term splenectomy outcomes of the French nationwide OBS’CEREVANCE cohort for the 3 AICs and the factors associated with splenectomy failure, severe or recurrent bacterial infections, thrombosis, and death.
Material and methods
OBS’CEREVANCE cohort
Since 2004, all French patients with cITP, AIHA, and ES diagnosed before age 18 years have been included in the prospective OBS’CEREVANCE cohort led by the CEREVANCE group.19 Inclusion and exclusion criteria of the cohort are detailed in supplemental Table 1. Some patients underwent genetic analyses, as previously described.2 Written informed consent was obtained from parents and eligible patients. The cohort study was approved by the institutional ethics committee (CPPRB-A; Bordeaux, France) and the database was registered with the national data protection authority (CNIL, 1396823V0).
For patient surveillance and data collection, the CEREVANCE group uses the term IM to include predefined immune manifestations associated with AIC. IMs comprise clinical IM, defined as lymphoproliferation and/or autoimmune/autoinflammatory organ disease, and biological IM, defined as hypogammaglobulinemia, SLE biomarkers, and/or autoimmune lymphoproliferative syndrome (ALPS) biomarkers; detailed definitions are given in supplemental Table 1. SLE diagnosis is based on Systemic Lupus International Collaborating Clinics Classification criteria.20 PID diagnoses are based on international classification,21 and eventual criteria when existing, such as for ALPS.22 The CEREVANCE group recommends scheduling clinical and biological follow-up at least every 6 to 12 months (supplemental Table 2). French recommendations for immunizations before splenectomy include mainly pneumococcal and meningococcal vaccinations but have varied with time and were not precisely recorded in the database.
Patients’ selection
Patients registered in the database on March 3, 2021, were included if they underwent a splenectomy before the age of 18 years and had more than 1 year of follow-up. Exclusion criteria were as follows: (1) AIC known to be secondary to transplantation, SLE, or PID at splenectomy date; and (2) missing data for outcome. Patients with SLE or a PID diagnosed before splenectomy were excluded because the aim of the study was to describe the clinical situation in which no underlying etiology of AIC is known to help the physician in the splenectomy decision.
Outcomes and variables associated
The primary outcome was splenectomy failure-free survival (FFS), defined as the time from splenectomy to the initiation of a second-line treatment (ie, other than steroids and intravenous immunoglobulins) or death from any cause, whichever came first.
Secondary outcomes were severe (grade ≥3 according to Common Terminology Criteria for Adverse Events v5.0) or recurrent (≥2 episodes) bacterial infections, thrombosis, and death.
The following variables were tested for all outcomes: AIC subtype at splenectomy, sex, age at first AIC, rituximab before splenectomy, number of treatments before splenectomy, time from first AIC to splenectomy, age at splenectomy, IM diagnosed before splenectomy, and IM diagnosed at any timepoint during follow-up. New second-line treatment after splenectomy and total number of second-line treatments received (before and after splenectomy) were also tested for severe or recurrent bacterial infections, thrombosis, and death outcomes (for severe or recurrent bacterial infections, only second-line treatments initiated before infection were considered). Severe or recurrent bacterial infections variable was also tested for death outcome.
Statistical analysis
Continuous and categorical variables were compared using nonparametric Wilcoxon-Mann-Whitney and Fisher exact tests, respectively. The splenectomy FFS rate estimates were based on the Kaplan-Meier method and curves reported were compared using log-rank test. The Cox proportional hazards method was used to identify factors associated with splenectomy FFS, thrombosis, and death. Proportionality of hazard was assessed for each variable. Logistic regression was used to analyze factors associated with severe or recurrent bacterial infections. For each outcome investigated, univariate analyses were performed in the whole cohort. Multivariate models were then fitted with all variables with a P value < .20 in univariate analysis as well as AIC subtype at splenectomy, whatever its P value. Two distinct models were fitted for IM diagnosed before splenectomy and IM diagnosed at any timepoint during follow-up. In a sensitivity analysis for the association between IM and splenectomy FFS, clinical and biological IMs were separately tested. In 2 other sensitivity analyses, the effect of splenectomy year on FFS was assessed using 2 approaches: (1) splenectomy year was added as a covariate and (2) the cohort was split in 2 groups based on splenectomy year (<2004 and ≥2004) and each group was analyzed separately.
The association between splenectomy and thrombosis incidence was assessed by using nonsplenectomized patients of the OBS’CEREVANCE cohort as a comparator. A Cox proportional hazards model was fitted with splenectomy and AIC subtype at last follow-up as covariates. To avoid bias from postoperative thrombosis risk carried by surgical procedure, only late thrombosis events (>30 days after splenectomy) were considered for splenectomized patients in this analysis. All tests were 2-sided and a P value < .05 was considered statistically significant. Statistical analyses were performed using R (ver. 4.0, R Development Core Team) and GraphPad Prism (ver. 9.1, GraphPad Software, Inc., San Diego, CA) software.
Role of the funding sources
The funding sources provided financial support to CEREVANCE database and functioning without any involvement in data collection, analysis, interpretation, or publication.
Results
Population
Out of 1736 patients included in OBS’CEREVANCE, 210 (12.1%) underwent a splenectomy and 161 (76.7%) fulfilled the prespecified inclusion criteria from 23 different centers (supplemental Figure 1). Eighty-nine patients (55%) underwent splenectomy in 2004 or after (supplemental Figure 2). AIC subtype at splenectomy was cITP, AIHA, and ES in 120, 19, and 22 cases, respectively (Table 1). For patients with ES, the indication for splenectomy was cITP, AIHA, and both in 7, 3, and 12 cases, respectively. Three patients (2%) underwent emergent splenectomy for bleeding. The median (minimum-maximum) age at splenectomy was 11.2 years (0.8-17.7). The median number of second-line treatments before splenectomy was 1 (0-8). Only 1 grade 4 perioperative hemorrhage related to procedure was reported. Median follow-up after the procedure was 6.8 years (1.0-33.3).
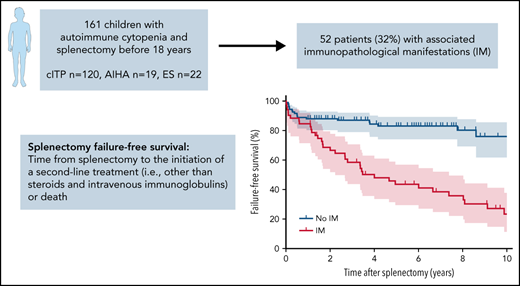
Fifty-two patients (32%) had at least 1 IM, either before or after splenectomy, 35 had both clinical and biological IMs, 8 had clinical IM only, and 9 had biological IM only (supplemental Table 3). The 4 most frequent IMs were antinuclear antibodies (n = 24), lymphoproliferation (n = 20), hypogammaglobulinemia (n = 14), and granulomatous-lymphocytic interstitial lung disease (n = 8). A diagnosis of secondary AIC was established after splenectomy for 16 of the 43 patients with a clinical IM (37%; SLE, n = 8 or PID, n = 8). Eight patients diagnosed with isolated cITP or AIHA at splenectomy developed ES during the follow-up.
Splenectomy FFS
Sixty-one patients (38%) had a splenectomy failure, from the initiation of a second-line treatment in all cases. Four patients (2%; cITP, n = 1; AIHA, n = 1; ES, n = 2) required a second-line treatment within 30 days after splenectomy. For 7 patients (4%; cITP, n = 4; ES, n = 3), the splenectomy failure occurred more than 10 years after the procedure (longest delay, 28 years). Overall, 4 patients (2%) presented a total of 7 severe hemorrhage episodes during follow-up (median delay to first episode, 7.2 years [2.2-30.2]): intracranial hemorrhage (n = 3, leading to death in 1 patient), gastrointestinal bleeding (n = 3 episodes in the same patient), and hemoperitoneum (n = 1 with a suspected underlying endometriosis).
Splenectomy FFS rates (95% confidence interval [CI]) at 1, 2, and 5 years were 88.3% (81.1-92.9), 84.0% (76.1-89.5), and 76.5% (67.3-83.5) for cITP; 80.0% (55.1-92.0), 75.0% (50.0-88.8), and 59.6% (35.1-77.4) for AIHA; and 85.7% (62.0-95.2), 80.7% (56.3-92.3), and 65.5% (40.9-81.9) for ES, respectively (Figure 1A-C). After splenectomy failure, the median number of second-line treatments was 2 (1-10).
Outcomes after splenectomy across the autoimmune cytopenias. Splenectomy failure-free survival curves and 95 CIs for 10 years of follow-up in patients with (A) cITP, (B) AIHA, and (C) ES.
Outcomes after splenectomy across the autoimmune cytopenias. Splenectomy failure-free survival curves and 95 CIs for 10 years of follow-up in patients with (A) cITP, (B) AIHA, and (C) ES.
Factors associated with FFS
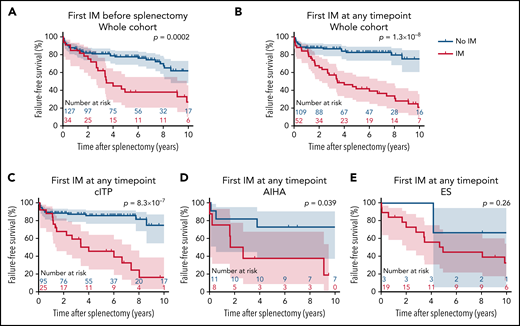
In multivariate analysis, IMs diagnosed before splenectomy were associated with a lower FFS (hazard ratio [HR], 0.39; 95% CI, 0.21-0.72; P = .003, adjusted for AIC subtype; Table 2). IMs diagnosed at any timepoint during follow-up were more strongly associated with a lower FFS (HR, 0.22; 95% CI, 0.12-0.39; P = 2.8 × 10−7, adjusted for AIC subtype). AIC subtype at splenectomy was not significantly associated with FFS.
Patients with and without IMs diagnosed at any timepoint during follow-up had an FFS rate (95% CI) of 66.7% (51.9-77.8) and 88.1% (80.3-92.9) at 2 years, and 43.7% (29.6-56.9) and 83.2% (74.1-89.3) at 5 years after splenectomy, respectively (Figure 2A-B).
Outcomes after splenectomy according to immunopathological manifestations (IMs). (A,B) Splenectomy failure-free survival curves and 95 CIs concerning the whole cohort according to the diagnosis of IM (A) before splenectomy or (B) at any timepoint during follow-up. (C-E) Splenectomy failure-free survival curves and 95 CIs according to the diagnosis of IM at any timepoint during follow-up in patients with (C) cITP, (D) AIHA), and (E) ES. Curves were compared using log-rank test; therefore, P values can be slightly different than in univariate Cox proportional hazards models.
Outcomes after splenectomy according to immunopathological manifestations (IMs). (A,B) Splenectomy failure-free survival curves and 95 CIs concerning the whole cohort according to the diagnosis of IM (A) before splenectomy or (B) at any timepoint during follow-up. (C-E) Splenectomy failure-free survival curves and 95 CIs according to the diagnosis of IM at any timepoint during follow-up in patients with (C) cITP, (D) AIHA), and (E) ES. Curves were compared using log-rank test; therefore, P values can be slightly different than in univariate Cox proportional hazards models.
Considering each AIC subtype separately, IMs diagnosed at any timepoint during follow-up were associated with a lower FFS in cITP and AIHA and had a statistically nonsignificant trend in ES (Figure 2C-E).
As a sensitivity analysis, IMs were then divided in 2 categories: clinical IM and biological IM. Patients with clinical IM only had a lower FFS compared with patients without any IM (HR, 0.23; 95% CI, 0.07-0.77; P = .02, adjusted for AIC subtype). As well, patients with biological IM only had a lower FFS compared with patients without any IM (HR, 0.27; 95% CI, 0.10-0.73; P = .01, adjusted for AIC subtype).
Finally, the effect of splenectomy year was investigated to consider the long timespan covered by the cohort. In a first analysis, splenectomy year was incorporated as covariate in the model. In a second analysis, the cohort was split in 2 groups based on splenectomy year (before 2004 and 2004 or after). In each case, IM was independently associated with a lower FFS (supplemental Table 4).
Severe or recurrent bacterial infections
Nineteen patients (12%) presented recurrent or severe bacterial infections, including 14 (9%) with grade ≥ 3 infection. Infection types were pneumonia (n = 15, including 2 with septic shock), pneumococcal meningitis (n = 2), Staphylococcus aureus bacteremia (n = 2), pneumococcal septic shock (n = 1), angiocholitis (n = 1), and gram-negative bacteriemia (n = 1). A microorganism was identified in 7 cases and was an encapsuled germ in 3 patients.
Median age at splenectomy of patients with recurrent or severe bacterial infection was 9.4 years (2.0-17.7), and infections occurred after a median delay of 3.3 years (8 days-11.4 years) after procedure. Among patients with severe or recurrent bacterial infection, 15 (79%) received new second-line treatment and 15 (79%) had IM diagnosed before infection. Four (21%) had a PID diagnosed at last follow-up (ALPS [n = 2], CTLA4 deficiency [n = 1], RAS-associated autoimmune leukoproliferative disorder [n = 1]).
In multivariate analyses, IMs diagnosed before splenectomy, IMs diagnosed at any timepoint during follow-up and new second-line treatment after splenectomy were associated with a higher risk of severe or recurrent bacterial infections (Table 3).
The infection rate was 10-fold higher in the subgroup with IM and/or new second-line treatment after splenectomy compared with those without IM nor new second-line treatment after splenectomy: 17/76 (22%) vs 2/85 (2% [including 1 pneumococcal septic shock caused by nonvaccine serotype], P = .0001). Excluding the 13 pneumonias without septic shock from the infections considered, the infection rate was ninefold higher in the subgroup with IM and/or new second-line treatment after splenectomy compared with those without IM nor splenectomy failure: 8/76 (11%) vs 1/85 (1%, P = .01).
Thrombosis
Eleven patients (7%) were diagnosed with a thrombosis after splenectomy. Three patients (2%) presented postoperative thrombosis (diagnosed < 30 days after procedure), including 2 portal vein thrombosis and 1 cerebral venous thrombosis (supplemental Table 5).
Nine patients (6%) presented a total of 12 late thrombosis (diagnosed > 30 days after procedure), including deep vein thrombosis (n = 8), pulmonary embolism (n = 1), cerebral venous thrombosis (n = 2), and pulmonary hypertension (n = 1). In 5 patients, a potential additional pro-thrombotic factor was identified, the most frequent was corticosteroid-induced hypertension (n = 3). One patient was diagnosed with an antiphospholipid syndrome upon thrombosis.
In multivariate analysis, AIC subtype at splenectomy and IMs diagnosed at any timepoint during follow-up were independently associated with thrombosis: HR, 0.11; 95% CI, 0.02-0.65; P = .015 for cITP; HR, 0.21; 95% CI, 0.05-0.90; P = .035 for ES; and HR, 10.36; 95% CI, 1.21-88.41; P = .033 for IMs diagnosed at any timepoint during follow-up (supplemental Table 6).
To assess whether splenectomy by itself augments the risk of thrombosis in children with AIC, the entire OBS’CEREVANCE cohort was analyzed, including nonsplenectomized patients (n = 1523 children with available data, of which 11 had a thrombosis). In multivariate analysis, splenectomy was associated with a higher risk of thrombosis (HR, 4.70; 95% CI, 1.76-12.53; P = .002), whereas cITP was associated with a lower risk (HR, 0.12; 95% CI, 0.03-0.46; P = .002).
Survival
Seven (4%) patients died in the follow-up (supplemental Table 7). Median age at splenectomy and at death was 8.3 (4.9-12.5) and 18.6 (12.7-28.1) years, respectively. The median delay between splenectomy and death was 11.6 years (4.4-21.2). The cause of death was sepsis (n = 5), cerebral hemorrhage (n = 1), and RAS-associated autoimmune leukoproliferative disorder (n = 1). All deceased patients were diagnosed with ES pre- or postsplenectomy and had received second-line treatments after splenectomy. All had IM excepted the 1 who died with hemorrhage. Four had presented several pneumonias.
In univariate analysis, recurrent or severe bacterial infections were marginally associated with death (HR, 4.50; 95% CI, 1.00-20.46; P = .0495; supplemental Table 8) but no factor was found to be statistically significant in multivariate analysis.
Discussion
Based on a postsplenectomy follow-up of 1375 patients-years and including the 3 AIC subtypes, these data confirmed the favorable results of splenectomy in pediatric cITP and showed that it may also be effective in AIHA and ES. This study allows us to establish IM as a major factor for several unfavorable outcomes: FFS, recurrent or severe bacterial infections, and thrombosis.
Since the first report in 1916, splenectomy has been widely used in cITP.6 The largest studies come from adult patients, and a recent meta-analysis found a response rate of ∼70%.7,16 Exclusively pediatric and mixed adolescent-adult studies reported similar results.6,13-17 However, not all the adult patients’ experiences can be transposed to children. Despite similar disease mechanisms, potential underlying causes of secondary AIC differ between these 2 populations. Pediatric secondary AICs are mainly the result of PID and SLE, whereas malignancies are uncommon.4 As a result, disease courses may differ,1 and some treatments may have different results in children and in adults.
cITP failure rate observed in our cohort is similar to previously published reports, although we used patient-centered criteria for splenectomy failure.6,13-15,17 The smaller number of patients with AIHA and ES in our study prevent definitive conclusions for these AICs. Our results suggest, however, that splenectomy outcome is relatively comparable to cITP and that the presence of IM is a stronger risk factor for splenectomy failure than AIC subtype. However, splenectomy decision should be carefully balanced in patients with ES. These patients present an age-increasing prevalence of IM1 and frequent underlying PID.2 In addition, all deceased patients within our study were diagnosed with ES.
Our study identified a broad range of IM with prevalence that differed between patients splenectomized for cITP, AIHA, and ES: 22%, 42%, and 86%, respectively. Despite IM in the context of AIC being suggestive of secondary AIC (ie, SLE and PID),2,3,23 70% of patients with IM reported here had no underlying diagnosis identified at last follow-up. Thus, IM is a common feature of heterogenous individual conditions, and our data suggest that it is an effective surrogate marker for unfavorable splenectomy outcomes. Even without a definite diagnosis of PID or SLE, the presence of IM should be integrated for splenectomy decision. The impact on splenectomy outcomes probably differs across the individual IMs and possibly between clinical and biological IM categories. Larger studies are needed to precisely determine the effect of each IM and combinations. In addition to IM research, the CEREVANCE group also suggest genetic analyses to search for a PID before splenectomy for an AIC (supplemental Table 2).
Infection is the main concern in splenectomized patients, especially for children younger than age 6 years. The risk of overwhelming postsplenectomy infection (OPSI) because of encapsulated bacteria exists lifelong in every patient.24 It may nevertheless be affected by underlying conditions. For instance, patients with ALPS have been shown to carry a particularly high risk of OPSI.25 Our data suggest that most postsplenectomy severe bacterial infections occurred in patients with an additional risk factor, with new second-line treatment and/or IM both being independently associated. Although OPSI should be a concern for every splenectomized patient, these factors may be considered a marker of increased infectious risk that may not be preventable by standard measures (ie, vaccinations and oral penicillin). Of note, despite French pediatricians following national and international guidelines to prevent OPSI, we could not confirm that immunization status and antibiotic prophylaxis were adequate at infection occurrence. Despite this limitation, we suggest an intensified follow-up for patients with IM and/or subsequent second-line treatment. Based on individual clinical condition, additional anti-infectious approaches, such as intensified prophylactic antibiotics or immunoglobulin replacements, could be considered in some of these patients.
Splenectomy has been associated with an increased thrombosis risk in adult patients, specifically in patients with cITP, who spontaneously bear an increased risk.18,24,26,27 We confirm here that thrombosis risk is increased in children after splenectomy compared with nonsplenectomized children with AIC. Additionally, we found that among children with AIC, those with cITP had a lower risk of thrombosis, independent of splenectomy. Patients with AIHA or ES in the entire OBS’CEREVANCE cohort or only those with AIHA in the splenectomy group seem to be at higher risk. Further data are needed to detail this point and to elucidate the mechanism of this previously unknown risk. Chronic hemolysis may contribute to this increased risk. Independent of AIC subtype, our data also suggest that thrombosis is another unfavorable postsplenectomy outcome associated with IM.
Death in our study occurred exclusively in patients with several common features: (1) ES, diagnosed either before or after splenectomy, which is known to be associated with a much higher risk of death than isolated cITP and AIHA1; (2) new second-line treatment; and (3) IMs, present in all nonhemorrhagic deaths. As in nonsplenectomized patients with ES,1 infection was the main cause of death. Therefore, broader infection prevention and close follow-up should be considered in these patients, as discussed previously.
In conclusion, IM should be investigated and integrated when evaluating risks and benefits of splenectomy in patients with AIC. In splenectomized patients, the presence of IM indicated higher risk for second-line treatment, severe or recurrent bacterial infections, and thrombosis. Therefore, this subgroup requires careful follow-up, especially in the context of transition to adult medical departments. Additional infection prophylaxis warrants consideration and further evaluation in these patients. Given the increasing prevalence of IM with age in ES, splenectomy indication is still a matter of discussion in this population. Finally, systematic genetic analysis looking for a PID should be discussed before splenectomy for an AIC.
Acknowledgments
The authors thank all the patients, families, medical, and para-medical teams involved in the CEREVANCE prospective cohort study from 2004 onwards. They especially thank Alain Fischer (AP-HP Necker), Stéphane Blanche (AP-HP Necker), Yves Bertrand (IHOP Lyon), Gérard Michel (AP-HM Marseille), Paul Saultier (AP-HM Marseille), and Brigitte Nelken (Lille). They thank Françoise Méchinaud for English review and corrections. The authors also thank the following pediatricians and their teams in charge of the pediatric patients: N. Garnier (IHOP Lyon, n = 16), T. Leblanc (AP-HP R. Debré, n = 16), G. Leverger (AP-HP A. Trousseau, n = 15), V. Barlogis (AP-HM Marseille, n = 13), W. Abou Chahla (Lille, n = 12), N. Aladjidi (Bordeaux, n = 11), M. Pasquet (Toulouse, n = 11), S. Bayart (Rennes, n = 9), D. Moshous (AP-HP Necker, n = 8), N. Cheikh (Besançon, n = 7), C. Paillard (Strasbourg, n = 7), D. Plantaz (Grenoble, n = 6), E. Jeziorski (Montpellier, n = 4), C. Thomas (Nantes, n = 4), C. Guitton (AP-HP Bicêtre, n = 4), M. Deparis (Caen, n = 3), A. Marie-Cardine (Rouen, n = 3), J-L. Stéphan (Saint-Etienne, n = 3), I. Pellier (Angers, n = 2), E. Dore (Clermont-Ferrand, n = 2), J. Benadiba (Nice, n = 2), C. Pulchart (Reims, n = 2), and C. Briandet (Dijon, n = 1).
This work was supported from 2004 by the French Ministry of Health (Programme Hospitalier de Recherche Clinique 2005, Rare Disease Plan 2007 and 2017), the Association Bordelaise pour l’Avancement des Sciences Pédiatriques research charity, the Association pour la Recherche et les Maladies Hématologiques de l’Enfant research charity, the Association Française du Syndrome d’Evans, the O-CYTO patients’ association, and partially by GlaxoSmithKline, AMGEN, and Novartis. T.P. is a recipient of a Charles Bruneau fellowship award and merit scholarship program for foreign students from the Ministry of Education and Higher Education of Quebec.
Authorship
Contribution: T.P., N.A., H.F., C.D., G.L., and T.L. designed the study, analyzed the data, and drafted the paper; T.P. and H.F. performed statistical analyses; T.P., N.A., and H.F. had access to and verified the primary data; and S.H., N.G., M.F., W.A.C., S.D., M.P., S.B., D.M., N.C., C. Paillard, D.P., E.J. C.T., C.G., M.D., A.M.C., J.-L.S., I.P., E.D., J.B., C. Pluchart, C.B., and V.B. participated in patient recruitment, prospective data collection, and data interpretation and revised the manuscript for critical content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Aladjidi, Unité d’hématologie pédiatrique, Centre de référence national des cytopénies auto-immunes de l’enfant CEREVANCE, Hôpital des Enfants, Hôpital Pellegrin, Place Amélie Raba Léon, 33000 Bordeaux, France; e-mail: nathalie.aladjidi@chu-bordeaux.fr; and Thierry Leblanc, Service d'hémato-immunologie, Pôle de pédiatrie médicale, CHU Paris - Hôpital Robert-Debré, 48 boulevard Sérurier, 75019 Paris, France; e-mail: thierry.leblanc@aphp.fr.
Deidentified individual data are available from a corresponding author on request upon a signed data access agreement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
T.P. and N.A. are joint first authors.
G.L. and T.L. are joint last authors.