In this issue of Blood, Müller et al demonstrate the efficacy of a new combinatorial strategy for targeting pediatric T-cell acute lymphoblastic leukemia (T-ALL).1
Survival rates in pediatric T-ALL, which makes up approximately 10% to 15% of pediatric ALLs, have improved steadily over the last decades.2 Survival in adult patients has also improved but is significantly lower than that in children. In the setting of relapsed disease, survival in pediatric and adult T-ALLs remains poor, because salvage therapies are ineffective for most patients. New treatment strategies for this population are badly needed.
Immunotherapy for T-ALL has lagged behind that for B-cell ALL for several reasons, including the fundamental heterogeneity of T-ALL blasts. Moreover, antigens that are widely present on normal T cells risk significant toxicity with immune therapies. CD38 has previously been identified as an ideal target for immunotherapy in T-ALL. It has high surface expression on T-ALL blasts, and expression is durable during chemotherapy and after relapse.3-5 CD38 is expressed on activated T cells and terminally differentiated B cells but is only expressed at low levels on normal lymphoid and myeloid cells. Expression is absent to low on most healthy tissues. The use of the CD38-targeting antibody daratumumab in combination with chemotherapy was investigated for relapsed pediatric T-ALL in a recently completed early-phase trial (registered at www.clinicaltrials.gov as #NCT03384654). The results of the trial will be presented at the American Society of Clinical Oncology Annual Meeting and European Hematology Association Annual Meeting in 2022. Preliminary results seem encouraging, with an overall response rate (complete remission and complete remission with incomplete count recovery) of 83.3% in children and 60% in young adults with relapsed T-ALL.6,7
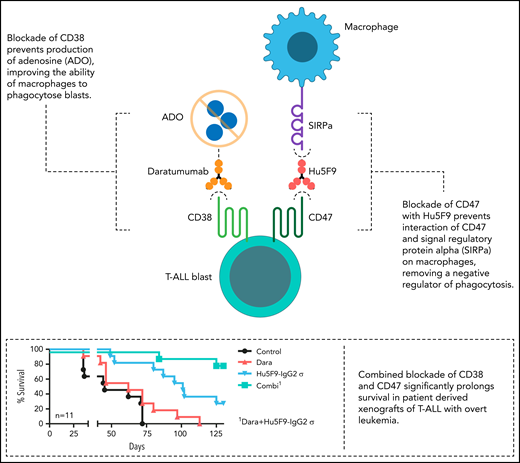
Muller et al identify CD47 as an additional target that is highly expressed on pediatric T-ALL blasts, with CD47 and CD38 expression correlated (r = 0.46). CD47 acts as an inhibitor of phagocytosis, with blasts using CD47 expression to send a “don’t eat me” signal to host macrophages via signal regulatory protein α (see figure). Blocking CD47 removes this negative signal, thereby allowing macrophages to phagocytose T-ALL blasts. Why targeting CD38 is effective is not fully understood; however, CD38 has a key role in the generation of extracellular adenosine, which also negatively regulates phagocytosis by macrophages (see figure). Thus, cotargeting CD38 and CD47 may have a synergistic effect that improves antibody-dependent cellular phagocytosis. Indeed, Muller et al demonstrate the efficacy of this strategy in multiple preclinical models and by targeting CD47 with both pharmacologic inhibition and antibody-based approaches.
Blockade of CD38 prevents production of adenosine (ADO), improving the ability of macrophages to phagocytose blasts (top left). Blockade of CD47 with Hu5F9 prevents interaction of CD47 and signal regulatory protein α (SIRPa) on macrophages, removing a negative regulator of phagocytosis (middle right). Combined blockade of CD38 and CD47 significantly prolongs survival in patient-derived xenografts of T-ALL with overt leukemia (bottom right). Professional illustration by Somersault18:24.
Blockade of CD38 prevents production of adenosine (ADO), improving the ability of macrophages to phagocytose blasts (top left). Blockade of CD47 with Hu5F9 prevents interaction of CD47 and signal regulatory protein α (SIRPa) on macrophages, removing a negative regulator of phagocytosis (middle right). Combined blockade of CD38 and CD47 significantly prolongs survival in patient-derived xenografts of T-ALL with overt leukemia (bottom right). Professional illustration by Somersault18:24.
Using patient-derived xenograft models of T-ALL, Muller et al show that targeting CD47 alone is an efficacious strategy in mice in a minimal residual disease–like state and with overt leukemia. Having data in both settings is important, because some immunotherapies may not be effective in the setting of bulk disease but may be highly effective at clearing minimal residual disease. Most importantly, in a relapsed model of leukemia, they demonstrate that targeting both CD47 and CD38 significantly prolongs survival, while targeting either alone is insufficient (see figure). This is of particular importance, because relapsed disease most closely recapitulates the clinical scenario where CD47 and CD38 dual targeting could be considered.
The optimal strategy for targeting CD47, whether in combination with daratumumab or alone, remains to be determined. Muller et al show the efficacy of both pharmacologic and antibody-based therapies in in vitro and in vivo models. As the authors acknowledge, some previous clinical studies of anti-CD47 antibodies were discontinued because of destruction of normal hematopoietic cells.8 However, in the setting of relapsed or refractory T-ALL, this may be an acceptable risk, because these patients typically require hematopoietic stem cell transplantation for cure of their disease.
Several promising immunotherapeutic approaches are currently in preclinical and clinical development for T-ALL. Chimeric antigen receptor (CAR) T cells have shown particular promise in B-cell ALL, and the first results from clinical trials applying CAR T cells in T-ALL were recently published, using CD7 as a target.9 In addition, several trials testing autologous and allogenic CAR T cells targeting CD2, CD5, CD7, and CD38 are in clinical development.10 Anti-CD47 monoclonal antibodies are particularly attractive, because they could theoretically synergize with CAR T-cell therapies. Critically, preclinical data testing CAR T cells with anti-CD47 monoclonal antibodies are needed, and trials combining anti-CD47 monoclonal antibodies plus CAR T cells would need to be carefully designed, because activating macrophages could worsen cytokine release syndrome. The work by Muller et al highlights the critical importance of preclinical studies testing combinatorial immunotherapy approaches. Finally, although the current report is focused on T-ALL, it is important to highlight that these results could also affect other T-cell malignancies, including T-cell lymphoblastic lymphoma and Sezary syndrome.
With a rigorous series of experiments, Muller et al demonstrate the potential of dual targeting of both CD47 and CD38 as an efficacious strategy in relapsed or refractory T-ALL. This work represents an important foundation for future clinical studies and the promise of a new therapeutic avenue for a population of patients with few options.
Conflict-of-interest disclosure: D.T.T. has patents pending on chimeric antigen receptor T cells for acute lymphoblastic leukemia; receives research funding from BEAM Therapeutics and NeoImmune Tech; and serves on advisory boards for Sobi, Janssen, and BEAM Therapeutics. C.D. declares no competing financial interests.