Key Point
Dual targeting of CD38 and CD47 elevates in vitro antibody-dependent phagocytosis in T-ALL.
Abstract
Acute lymphoblastic leukemia (ALL) is the most common malignant disease affecting children. Although therapeutic strategies have improved, T-cell acute lymphoblastic leukemia (T-ALL) relapse is associated with chemoresistance and a poor prognosis. One strategy to overcome this obstacle is the application of monoclonal antibodies. Here, we show that leukemic cells from patients with T-ALL express surface CD38 and CD47, both attractive targets for antibody therapy. We therefore investigated the commercially available CD38 antibody daratumumab (Dara) in combination with a proprietary modified CD47 antibody (Hu5F9-IgG2σ) in vitro and in vivo. Compared with single treatments, this combination significantly increased in vitro antibody-dependent cellular phagocytosis in T-ALL cell lines as well as in random de novo and relapsed/refractory T-ALL patient-derived xenograft (PDX) samples. Similarly, enhanced antibody-dependent cellular phagocytosis was observed when combining Dara with pharmacologic inhibition of CD47 interactions using a glutaminyl cyclase inhibitor. Phase 2–like preclinical in vivo trials using T-ALL PDX samples in experimental minimal residual disease–like (MRD-like) and overt leukemia models revealed a high antileukemic efficacy of CD47 blockade alone. However, T-ALL xenograft mice subjected to chemotherapy first (postchemotherapy MRD) and subsequently cotreated with Dara and Hu5F9-IgG2σ displayed significantly reduced bone marrow infiltration compared with single treatments. In relapsed and highly refractory T-ALL PDX combined treatment with Dara and Hu5F9-IgG2σ was required to substantially prolong survival compared with single treatments. These findings suggest that combining CD47 blockade with Dara is a promising therapy for T-ALL, especially for relapsed/refractory disease harboring a dismal prognosis in patients.
Introduction
Antibody-based immunotherapies have become an important component in the treatment of hematologic malignancies.1 However, immunotherapy for acute lymphoblastic leukemia (ALL) arising from immature T-cell lymphoblasts (ie, T-ALL) is practically nonexistent. Beyond allogeneic stem cell transplantation, the overall survival of patients with relapsed T-ALL is devastating, emphasizing the need for novel therapeutic options.2 CD38 is an attractive therapeutic target in many hematologic malignancies, including T-ALL.3 CD38, a cell membrane–expressed cyclic adenosine 5′-diphosphate ribose hydrolase, is a major regulator of nicotinamide adenine dinucleotide (NAD)+ levels, required for the generation of cyclic adenosine 5′-diphosphate ribose.4 CD38 expression remains stable during chemotherapy and thus represents a suitable target in refractory disease.5 The CD38-targeting antibody daratumumab (Dara) alone or in different combinations has been approved for the treatment of relapsed/refractory (r/r) multiple myeloma (MM) as well as for patients newly diagnosed with MM.6-8 Dara exerts a broad spectrum of killing mechanisms, including antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity, as well as antibody-dependent cellular phagocytosis (ADCP) of tumor cells.9 Only recently have preclinical data suggested a broad efficacy of Dara in T-ALL,10,11 and case reports have suggested clinical benefits in r/r disease.12,13 Using a T-ALL patient-derived xenograft (PDX) model, we were able to show that Dara can cause MRD negativity and that it outperforms chemotherapy alone.11 Further approaches using early T-cell precursor ALL and non–early T-cell precursor ALL xenograft mice also showed a positive response to Dara in vivo.10
In vitro studies described elevated macrophage-mediated phagocytosis upon treatment with Dara.9 A major immune checkpoint associated with phagocytosis is the CD47 molecule, which acts as a “don’t eat me signal” preventing phagocytosis of cells by interacting with the signal regulatory protein α (SIRPα) on macrophages.14,15 CD47 is a ubiquitously expressed molecule upregulated in a wide variety of neoplasms.16 Elevated CD47 expression has been associated with an inferior outcome for high-risk ALL patients,16,17 although the prognostic value of CD47 expression specifically for T-ALL has not yet been described. In addition, elevated CD47 expression was associated with poor survival in 3 adult cohorts of acute myeloid leukemia (AML) patients.18 Interestingly, CD47 expression in T-ALL is higher than in any B-cell precursor ALL subgroup17 or in AML.19 Different strategies to target CD47 alone and to combine CD47 inhibition with other antibodies or with chemotherapeutic drugs have been proposed.20,21 Combination of the CD47 antibody Hu5F9-G4 (magrolimab) with the CD20 antibody rituximab showed durable responses in patients with rituximab-refractory diffuse large B-cell lymphoma.22 Moreover, dual treatment of magrolimab with azacitidine showed favorable results in patients with AML.15,23 Recent studies described increased ADCP after genetic knockout of the enzyme glutaminyl cyclase (QPCTL).24 QPCTL catalyzes the formation of an N-terminal pyroglutamate residue from glutamine or glutamic acid residues on CD47, which is important for proper binding to SIRPα.25 These results suggest another approach to target CD47 by using QPCTL inhibitors similar to SEN177, which increased ADCP in combination with rituximab or cetuximab in various tumor cell lines.26
The current study found that the combination of Dara with an engineered CD47 antibody lacking binding to Fc-γ receptors and complement component 1q27 increased in vitro ADCP in T-ALL cell lines as well as in random de novo and r/r T-ALL PDX samples. Our in vivo experiments further showed significant long-term survival of T-ALL xenograft mice after therapy with Dara and CD47 blockade. These preclinical data indicate a novel and highly promising therapeutic option for T-ALL, particularly in relapsed disease.
Methods
Cell lines
P12, MOLT-13, and HSB-2 cells were purchased from DSMZ. Patients were treated according to the clinical ALL-BFM 2000/2009 protocols. Informed consent was obtained in accordance with institutional regulations.
Antibodies
The CD47 antibody variant Hu5F9-IgG2σ was generated from variable light and heavy chain sequences of Hu5F9-G4, which were synthesized de novo according to published sequences.28 Variable light and heavy sequences were ligated into derivatives of the expression vector pSectag2/Hygro C (Invitrogen) encoding either the constant domain of human antibody κ light chain or the immunoglobulin G2σ (IgG2σ) heavy chain,27 respectively.
Xenograft
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Charles River Laboratories) were maintained as approved by the governmental animal care and use committees. In all in vivo approaches, 1 × 105 random de novo or r/r T-ALL PDX cells were injected intravenously into the tail vein of mice. Antibodies were applied by intraperitoneal injection of 1 mg/kg (Dara) or 2 mg/kg (Hu5F9-IgG2σ) body weight on days 1, 3, 5, 7, 10, and 15 and then every third week thereafter as previously described.29 For all in vivo approaches, a “one animal per model per treatment” was applied as described by others.30,31
In vitro antibody-dependent cellular phagocytosis
In vitro phagocytosis was measured by using the IncuCyte System. Macrophages from healthy human donors were generated as previously described.29 Then, 2 × 104 macrophages were plated on a 96-well plate and allowed to adhere at room temperature for 30 minutes; next, 1 × 106 target cells were stained with 0.5 µg/mL pHrodo (Thermo Fisher Scientific) for 1 hour at room temperature. Antibodies were applied to a final concentration of 10 µg/mL for Dara and cetuximab and 20 µg/mL for Hu5F9-IgG2σ. Engulfed cells are displayed as red object counts per image. A positive response to combination treatment was defined as increased phagocytosis detected with macrophages of at least 2 of all analyzed donors.
Additional information can be obtained in the supplemental Methods (available on the Blood Web site).
Results
CD47 and CD38 expression levels in T-ALL patient samples and antibody generation
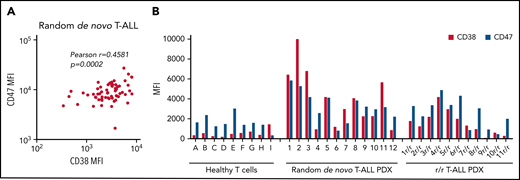
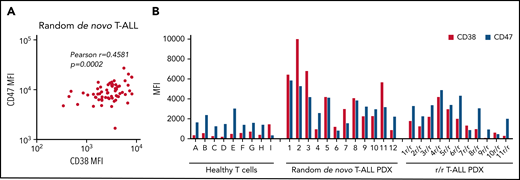
To examine CD47 and CD38 as potential targets for antibody therapy in T-ALL, CD47 and CD38 surface expression was determined in diagnostic bone marrow/blood samples of a randomly selected cohort of T-ALL patient samples (n = 63) (Figure 1A). CD47 and CD38 are ubiquitously expressed membrane molecules, and to exclude detection in nonmalignant cells, expression measurements were restricted to CD7+ cells. Both antigens were expressed in all analyzed samples. Moreover, a positive correlation between CD47 and CD38 expression was observed. In addition, surface expression was evaluated in 12 random de novo and 11 r/r T-ALL PDX primograft samples generated by intrafemoral injection of T-ALL patient samples at initial diagnosis as previously described.11 All samples displayed surface expression of both antigens, which was generally higher than in healthy T cells (Figure 1B).
CD47 and CD38 expression levels in T-ALL patient samples. (A) Pearson correlation between CD38 and CD47 mean fluorescence intensity (MFI) values in initial T-ALL patient samples as analyzed by flow cytometry. (B) Surface expression of CD38 and CD47 in T-cells from healthy donors (ie, donors A-I), random de novo T-ALL (ie, 1-12), and r/r T-ALL (1r/r-11r/r) PDX samples.
CD47 and CD38 expression levels in T-ALL patient samples. (A) Pearson correlation between CD38 and CD47 mean fluorescence intensity (MFI) values in initial T-ALL patient samples as analyzed by flow cytometry. (B) Surface expression of CD38 and CD47 in T-cells from healthy donors (ie, donors A-I), random de novo T-ALL (ie, 1-12), and r/r T-ALL (1r/r-11r/r) PDX samples.
To investigate a potential therapeutic role of CD47 targeting in T-ALL, we generated an antibody, which retains its blocking properties by carrying the variable regions of magrolimab (Hu5F9) and a modified Fc domain to prevent Fc-γ receptor and complement component 1q binding (labeled Hu5F9-IgG2σ), as previously described.27,28,32 Purity of protein was determined by detection of the heavy and light chains of Hu5F9-IgG2σ compared with Dara (supplemental Figure 1A). In vitro binding properties of Dara and Hu5F9-IgG2σ were analyzed in the T-ALL cell line P12, displaying binding in a concentration-dependent manner (supplemental Figure 1B).
Combination of Dara and Hu5F9-IgG2σ enhances in vitro ADCP
We next determined the in vitro antileukemia effects of Dara and Hu5F9-IgG2σ in T-ALL. ADCP was therefore examined in three T-ALL cell lines (P12, MOLT-13, and HSB-2) with diverging surface expression of CD38 and CD47 (Figure 2A). In vitro phagocytosis using human macrophages isolated from healthy donors was measured by determining the proportion of engulfed tumor cells as the increase in red object counts over time by using live cell imaging. Cells were left untreated, treated with Dara or Hu5F9-IgG2σ, or treated with the combination of both. In all cell lines, blocking of CD47 alone was insufficient to trigger phagocytosis (Figure 2B-C). Tumor cells subjected to Dara alone displayed slightly elevated levels of phagocytosis compared with CD47 blockade or control. However, a strong increase in phagocytosis was detected when cells were treated with the combination of Dara and Hu5F9-IgG2σ in all cell lines. The extent of phagocytosis correlated with surface expression of CD38 and CD47 on tumor cells, with P12 and MOLT-13 showing the highest and HSB-2 the lowest increase in red object counts. Taken together, blocking of CD47 alone is insufficient to boost in vitro phagocytosis. Moreover, CD47 blockade combined with Dara elevated in vitro ADCP in T-ALL cell lines. In addition, cells with high surface expression of both antigens are more vulnerable to this type of combination therapy.
Combination of Dara and Hu5F9-IgG2σ increases in vitro phagocytosis in T-ALL cell lines. (A) CD47 and CD38 surface expression in P12, MOLT-13, and HSB-2 examined by using flow cytometry. (B) Antibody-dependent cellular phagocytosis analyzed in P12, MOLT-13, and HSB-2 treated with Dara, a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Graph illustrates 5 independent experiments with different human macrophages (n = 5, standard error of the mean). (C) Representative pictures of phagocytosed P12 cells (engulfed cells in red) subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D-E) ADCP in CD47-depleted P12 (D) and MOLT-13 (E) cells subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]) of both antibodies, or untreated (control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). Statistical analysis: nonparametric Mann-Whitney U test (two-tailed). *P < .05, **P < .01. MFI, mean fluorescence intensity; ns, not significant; ROI, red object counts per image.
Combination of Dara and Hu5F9-IgG2σ increases in vitro phagocytosis in T-ALL cell lines. (A) CD47 and CD38 surface expression in P12, MOLT-13, and HSB-2 examined by using flow cytometry. (B) Antibody-dependent cellular phagocytosis analyzed in P12, MOLT-13, and HSB-2 treated with Dara, a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Graph illustrates 5 independent experiments with different human macrophages (n = 5, standard error of the mean). (C) Representative pictures of phagocytosed P12 cells (engulfed cells in red) subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D-E) ADCP in CD47-depleted P12 (D) and MOLT-13 (E) cells subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]) of both antibodies, or untreated (control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). Statistical analysis: nonparametric Mann-Whitney U test (two-tailed). *P < .05, **P < .01. MFI, mean fluorescence intensity; ns, not significant; ROI, red object counts per image.
To confirm target antigen specificity of Hu5F9-IgG2σ, CD47 was knocked out in P12 and MOLT-13 cells by using CRISPR/Cas9, and in vitro phagocytosis was evaluated. Importantly, CD38 surface expression was not affected by depletion of CD47 (supplemental Figure 2A-B). P12 and MOLT-13 control cells (Cas9 only) displayed elevated phagocytosis when Dara was combined with Hu5F9-IgG2σ compared with single treatments (Figure 2D-E). In both CD47 knockout cell lines, Dara treatment significantly boosted phagocytosis, which was not further enhanced by addition of Hu5F9-IgG2σ. Hence, Hu5F9-IgG2σ–mediated effects are specifically caused by CD47 blockade. Moreover, combination with Dara seems to be essential for induction of phagocytosis, as sustained depletion of CD47 alone only weakly elevates phagocytosis.
Pharmacologic inhibition of CD47 in combination with Dara elevates in vitro phagocytosis
To explore an alternative strategy to target CD47 besides the application of antibodies, we examined if pharmacologic disruption of the CD47/SIRPα axis using the QPCTL inhibitor SEN177 in combination with Dara also resulted in increased phagocytosis. Treatment of P12 and MOLT-13 with SEN177 reduced binding of CC2C6, a CD47 antibody selectively binding to CD47 with an N-terminal pyroglutamyl residue.26 In contrast, binding of B6H12, an antibody binding the CD47 Ig-domain,33 was not affected (supplemental Figure 2C-D). Similarly, to block CD47 via an antibody, treatment with SEN177 alone did not boost phagocytosis significantly in any of the cell lines (Figure 3A-B). Dara alone was able to slightly increase phagocytosis levels; however, the highest rise was observed when Dara was combined with SEN177. Similar results were also obtained in four T-ALL PDX samples when subjected to Dara with SEN177 (Figure 3C). In summary, pharmacologic inhibition of CD47 in combination with Dara also led to increased ADCP.
Pharmacologic inhibition of CD47 with Dara elevates ADCP in T-ALL cells. (A-B) ADCP in P12 (A) and MOLT-13 (B) subjected to Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or dimethyl sulfoxide (DMSO) (solvent control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). (C) ADCP in 2 random de novo (patients #2 and #10) and 2 r/r (patients #6r/r and #10r/r) T-ALL PDX samples treated with Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or DMSO (solvent control). One representative experiment of 4 experiments with different donor macrophages is depicted. Nonparametric Mann-Whitney U test (two-tailed). *P < .05. ROI, red object counts per image.
Pharmacologic inhibition of CD47 with Dara elevates ADCP in T-ALL cells. (A-B) ADCP in P12 (A) and MOLT-13 (B) subjected to Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or dimethyl sulfoxide (DMSO) (solvent control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). (C) ADCP in 2 random de novo (patients #2 and #10) and 2 r/r (patients #6r/r and #10r/r) T-ALL PDX samples treated with Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or DMSO (solvent control). One representative experiment of 4 experiments with different donor macrophages is depicted. Nonparametric Mann-Whitney U test (two-tailed). *P < .05. ROI, red object counts per image.
Combination of Dara and Hu5F9-IgG2σ enhances ADCP in random de novo T-ALL PDX samples
Cell lines do not reliably depict the clinical heterogeneity of patients with T-ALL. Therefore, in vitro ADCP with Dara, Hu5F9-IgG2σ, or the combination of both was re-examined in 12 random de novo T-ALL PDX cells described earlier (Figure 1B; Table 1). To directly compare phagocytosis levels between samples, simultaneous analysis of all T-ALL PDX samples with healthy human macrophages was conducted with 4 independent donors. Blocking CD47 alone was insufficient to boost ADCP significantly in the majority of samples; however, a slight increase was detected in 3 (25%) of 12 T-ALL PDX samples (patients #8, #9, and #10) (Figure 4A). Treatment with Dara led to an increase in phagocytosis in 9 (75%) of 12 T-ALL PDX samples (patients #1-#3, #5, and #7-#11) compared with CD47 blockade alone and control (Figure 4A; supplemental Figure 3). Interestingly, the same 9 (75%) of 12 samples exhibited substantial further elevation of phagocytosis after combination of Dara with Hu5F9-IgG2σ compared with single treatments (Figure 4A-B). Of note, T-ALL PDX samples from patients #4, #6, and #12 displayed only low surface expression of CD38 and CD47 (Figure 1B), which might explain the comparatively low rise of phagocytosis and the lack of benefit when combining both antibodies. Overall, we observed a clear increase of ADCP in T-ALL cell lines as well as in a panel of various random de novo T-ALL PDX samples with different human macrophage donors after combination of Dara with Hu5F9-IgG2σ.
Combination of Dara and Hu5F9-IgG2σ in random de novo T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in 12 random de novo T-ALL PDX samples treated with daratumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars show standard error of the mean of technical triplicates. One representative experiment of 4 experiments with different donor macrophages is shown. The remaining experiments with other donor macrophages are depicted in supplemental Figure 3. (B) Summary of all ADCP in T-ALL PDX samples with human macrophages from 4 healthy donors. (C) In vivo phase 2–like preclinical study in an MRD-like setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D) In vivo phase 2–like preclinical study in an overt leukemia setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). The set of mice from patient #1 was excluded due to non-engraftment in this experiment. (E) Determination of blood and postmortem bone marrow (BM) blasts analyzed by using flow cytometry in mice injected with one random de novo T-ALL PDX sample and subjected to chemotherapy only (chemo only), the combination of Dara + chemo, Hu5F9-IgG2σ + chemo, both antibodies with chemo, or no treatment (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. Nonparametric Mann-Whitney U test (two-tailed). **P < .01, ***P < .001. ns, not significant; ROI, red object counts per image.
Combination of Dara and Hu5F9-IgG2σ in random de novo T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in 12 random de novo T-ALL PDX samples treated with daratumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars show standard error of the mean of technical triplicates. One representative experiment of 4 experiments with different donor macrophages is shown. The remaining experiments with other donor macrophages are depicted in supplemental Figure 3. (B) Summary of all ADCP in T-ALL PDX samples with human macrophages from 4 healthy donors. (C) In vivo phase 2–like preclinical study in an MRD-like setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D) In vivo phase 2–like preclinical study in an overt leukemia setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). The set of mice from patient #1 was excluded due to non-engraftment in this experiment. (E) Determination of blood and postmortem bone marrow (BM) blasts analyzed by using flow cytometry in mice injected with one random de novo T-ALL PDX sample and subjected to chemotherapy only (chemo only), the combination of Dara + chemo, Hu5F9-IgG2σ + chemo, both antibodies with chemo, or no treatment (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. Nonparametric Mann-Whitney U test (two-tailed). **P < .01, ***P < .001. ns, not significant; ROI, red object counts per image.
CD47 blockade is sufficient to prolong survival of random de novo T-ALL xenografts
To explore the efficacy of the CD38/CD47 cotargeting strategy in a variety of T-ALL samples, we conducted a phase 2–like preclinical trial using 6 random de novo T-ALL PDX samples. For this, PDX cells (patients #1-#6) (Figure 1B; Table 1) were injected intravenously into NSG mice and antibody therapy started 1 day after cell injection (MRD-like model).29 Mice were left untreated, subjected to Dara or Hu5F9-IgG2σ alone, or treated with a combination of both. All control animals died of leukemia, with a median survival of 58 days (Figure 4C), evidenced by the occurrence of leukemic blasts in the peripheral blood and postmortem bone marrow infiltration (supplemental Figure 4A). Animals treated with Dara displayed prolonged survival in 4 (67%) of 6 cases. All mice treated with Hu5F9-IgG2σ exhibited long-term survival, with none of the animals developing leukemia. The combination with Dara had no further survival benefit in this setting. Surviving mice in all groups were euthanized on day 153, and, to assess the depth of remission, MRD was determined in DNA isolated from the murine bone marrow if MRD markers were available (MRD markers for patients #1 and #3 had not been determined). All surviving animals with cells from patients #2, #4, #5, and #6 were MRD negative, irrespective of treatment conditions, showing that CD47 blockade alone is highly efficient, with or without the addition of Dara in this setting (supplemental Figure 4B).
We also examined the efficacy of Dara and Hu5F9-IgG2σ combination therapy in vivo in an overt leukemia situation without prior chemotherapy, starting therapy when 1% blasts were detected in the peripheral blood using the same 6 T-ALL PDX samples (overt leukemia setting). Control mice displayed leukemic engraftment, with a median survival of 58 days confirmed by analysis of peripheral blasts in the blood over time and postmortem bone marrow infiltration (Figure 4D; supplemental Figure 5). Three of five mice treated with Dara only (60%) responded to Dara monotherapy. Of note, the same three PDX samples responding to Dara in the MRD-like setting also displayed prolonged survival in the overt leukemia situation. In overt leukemia, 1 mouse treated with CD47 blockade alone (patient #6) also developed signs of leukemia and had to be euthanized, resulting in 4 (80%) of 5 mice showing prolonged survival. Mice injected with this particular PDX sample were resistant to single-agent treatment with Dara or Hu5F9-IgG2σ. Combined treatment in overt leukemia, however, led to long-term survival in all animals in vivo, including the patient not responding to the single agents.
To mimic a further frequent clinical situation, we next examined a sequential treatment of chemotherapy and antibody therapy in vivo (termed “postchemo MRD”). Cells from one T-ALL PDX (patient #5) was injected into replicate mice, and the mice were then subjected to chemotherapy when >10% blasts were detected in the peripheral blood (supplemental Figure 6A). Two weeks after completion of chemotherapy, the presence of residual leukemic cells was confirmed, and treatment with Dara, Hu5F9-IgG2σ alone, the combination of both, or no further treatment (chemo only) was initiated. Animals treated with chemo only or with Dara/chemo were euthanized due to leukemic development at 84 days (supplemental Figure 6B). Both groups showed high percentages of blasts in blood and bone marrow (Figure 4E). Animals in the remaining groups were euthanized on day 103 for further analysis. Animals treated with Hu5F9-IgG2σ/chemo displayed significantly higher median percentages of blasts in blood and bone marrow upon postmortem examination compared with the combi/chemo group (12% vs 1% and 80% vs 10%, respectively). Combined therapy with Dara/Hu5F9-IgG2σ therefore also exhibited a clear benefit in this postchemo MRD setting also.
Overall, in experimental MRD-like and overt leukemia situations using random de novo T-ALL PDX samples, CD47 blockade was sufficient to significantly prolong survival of T-ALL xenografts and, due to the favorable performance of CD47 blockade alone, combined treatment with Dara had no additional benefit. However, mice subjected to chemotherapy first (postchemo MRD) clearly benefited from cotreatment compared with a single application.
Combination of Dara and CD47 blockade is highly efficient in r/r T-ALL xenografts
Therapeutic options are limited for patients with r/r T-ALL, and novel immunotherapeutic approaches are urgently needed. Hence, we recapitulated our in vitro and in vivo evaluations of Dara and Hu5F9-IgG2σ combination therapy using PDX samples from patients refractory to standard chemotherapy (Table 2). First, ADCP was examined in the eleven r/r T-ALL PDX samples described earlier (Figure 1B; Table 2). Phagocytosis was simultaneously determined in all eleven r/r T-ALL PDX samples with healthy human macrophages from 3 independent donors. CD47 blockade alone was insufficient to significantly elevate phagocytosis levels (Figure 5A). Five (45%; patients #1r/r, #5r/r-#7r/r, and #10r/r) of 11 samples exhibited elevated phagocytosis when subjected to Dara only (Figure 5A; supplemental Figure 7). However, in vitro phagocytosis was markedly elevated in 8 of 11 r/r samples (72%; patients #1r/r, #3r/r-#7r/r, #10r/r, and #11r/r) when Dara was combined with Hu5F9-IgG2σ (Figure 5A-B).
Combination of Dara and Hu5F9-IgG2σ in r/r T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in eleven r/r T-ALL PDX samples treated with datatumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars indicate standard error of the mean of technical triplicates. One representative experiment of 3 experiments with different donor macrophages is depicted. The remaining experiments with the other donor macrophages are depicted in supplemental Figure 7. (B) Summary of all ADCP in r/r T-ALL PDX samples with human macrophages from 3 different healthy donors. (C) In vivo phase 2–like preclinical study in overt leukemia with eleven r/r T-ALL PDX samples injected into NSG mice subjected to Dara, Hu5F9-IgG2σ alone, or the combination (Dara + Hu5F9-IgG2σ [combi]), or left untreated (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. **P < .01, ***P < .001, ****P < .0001. ns, not significant; ROI, red object counts per image.
Combination of Dara and Hu5F9-IgG2σ in r/r T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in eleven r/r T-ALL PDX samples treated with datatumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars indicate standard error of the mean of technical triplicates. One representative experiment of 3 experiments with different donor macrophages is depicted. The remaining experiments with the other donor macrophages are depicted in supplemental Figure 7. (B) Summary of all ADCP in r/r T-ALL PDX samples with human macrophages from 3 different healthy donors. (C) In vivo phase 2–like preclinical study in overt leukemia with eleven r/r T-ALL PDX samples injected into NSG mice subjected to Dara, Hu5F9-IgG2σ alone, or the combination (Dara + Hu5F9-IgG2σ [combi]), or left untreated (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. **P < .01, ***P < .001, ****P < .0001. ns, not significant; ROI, red object counts per image.
Next, we aimed to examine the efficacy of Dara/Hu5F9-IgG2σ in vivo. NSG mice were injected with PDX cells of eleven r/r T-ALL cases in a preclinical phase 2–like experimental setting, and therapy was started when 1% blasts were observed in the peripheral blood (overt leukemia) according to the same scheme as noted earlier. All control animals were euthanized due to development of leukemia, with a median survival of 45 days (Figure 5C). Leukemic engraftment was detected in the peripheral blood over time (supplemental Figure 8). Surprisingly, mice subjected to Dara monotherapy (median survival, 62 days) displayed no significant survival benefit compared with control, and all animals died of the disease, highlighting that Dara response may be divergent in de novo vs r/r T-ALL. Interestingly, animals treated with Hu5F9-IgG2σ monotherapy showed a prolongation of median survival to 101 days; however, 8 (72%) of 11 mice developed leukemia at later stages and had to be euthanized, and only 3 (27%) of 11 displayed long-term survival. Most notably, mice subjected to a combined therapy of Dara and CD47 blockade showed long-term survival in 9 (82%) of 11 cases. The experiment was terminated on day 130, and surface expression of CD38 and CD47 via flow cytometry was determined in bone marrow samples of all animals with leukemic engraftment. The majority of mice displayed no significant change of antigen surface expression, excluding immune escape by downregulation of target antigens as the underlying mechanism for disease development (supplemental Figure 9A-B).
In summary, ADCP was elevated in r/r T-ALL PDX samples after combination of Dara with Hu5F9-IgG2σ. In an overt leukemia setting with r/r T-ALL PDX samples, simultaneous treatment resulted in long-term survival in 82% of the cases.
Discussion
The application of antibodies revolutionized the therapy of hematologic malignancies, especially in relapsed disease when chemotherapeutic options are exhausted.1 Although T cell–related antigens were among the first to be identified by monoclonal antibodies,34 clinically approved immunotherapeutic interventions for T-ALL remain a medical challenge. In line with previous reports, we show expression of CD38 and CD47 in patients with T-ALL, making both attractive targets for antibody therapy.10,11,17 Accordingly, we describe elevated in vitro ADCP in T-ALL cell lines, as well as in de novo and r/r T-ALL PDX samples after dual treatment with Dara and an optimized CD47-blocking antibody. Most importantly, mice injected with r/r T-ALL PDX samples displayed long-term survival after combination of Dara with Hu5F9-IgG2σ compared with single-agent treatments in a large preclinical phase 2–like trial of overt leukemia in mice.
Antibodies may exert antitumoral activity by directly inducing cell death of malignant cells as described for the CD47-targeting antibody CC2C6 clone in T-ALL and chronic lymphocytic leukemia cells.35 However, in our study, neither Hu5F9-IgG2σ nor Dara alone, nor the combination of both antibodies, was able to directly induce cell death in T-ALL cells (data not shown), which is in line with published data showing no direct apoptosis-promoting effects of Dara on cancer cells.36 Dara executes its cytotoxic effects by a complex set of effector functions, including effector cell recruitment and complement activation, initiating antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity.3 Both are regarded as further key effector functions for the elimination of malignant cells.37 In addition, macrophage-mediated ADCP as a mechanism of tumor cell killing has also become a focus of interest in cancer immunotherapy.38 Elevated ADCP induced by Dara has been described in chronic lymphocytic leukemia and MM cells.39,40 In our study, Dara-mediated in vitro phagocytosis was detected in T-ALL cell lines as well as in random de novo and r/r T-ALL PDX samples but to a rather low extent. The detailed mechanisms of how Dara exerts its cytotoxic effects in patients remain elusive. However, it has been described that enzymatic activity of CD38 can be modulated by Dara.3 CD38 catalyzes the hydrolyzation of NAD+, important for generation of extracellular adenosine,3,41 a key metabolite enabling tumor cells to escape immune surveillance, impairing macrophage-mediated phagocytosis.41,42 Hence, perturbation of adenosine generation due to Dara-mediated modulation of NAD+ might render cells more susceptible to ADCP. Of interest, MM cells expressing low levels of CD38 are less vulnerable for Dara-mediated ADCP.43 In line with this observation, in our studies, red object counts as a surrogate parameter for engulfed cells were higher in T-ALL cell lines and PDX samples expressing higher levels of CD38.
Increasing evidence shows that tumor cell phagocytosis by macrophages is regulated by a plethora of positive and negative signals, provided by a number of heterogeneous molecules on tumor cells and receptors on phagocytes.44 In our study, application of the CD47 antibody Hu5F9-IgG2σ alone was insufficient to drive leukemia cell phagocytosis by most donors, which is in line with published data showing that CD47 blockade by itself, mediated by effector function silent antibody blockade, does not affect phagocytosis.26,45 This suggests that an additional stimulus besides CD47 blockade is needed to trigger ADCP. Indeed, in our study, the highest rise of ADCP was observed when the CD47-blocking antibody was coadministered with Dara, which will provide a positive phagocytic signal by binding to activating Fcγ receptors on macrophages. A recent study described that macrophage-mediated phagocytosis can be predicted by balancing the ratio of activating to inhibiting antibody.46 Hence, Hu5F9-IgG2σ–mediated blockade of CD47 might lower the threshold for macrophage activation, and the addition of a Fc-functional antibody might boost phagocytosis.
Overexpression of CD47 has been shown to rescue tumor growth of MOLM-13 AML cells, which express exceptionally low levels of the surface marker, suggesting that CD47 is essential for tumorigenesis in vivo.23 In accordance with this, we observed no leukemic engraftment of P12 or MOLT-13 cells lacking CD47 expression (data not shown). Moreover, in models of random de novo T-ALL, CD47 blockade was able to maintain mice MRD negativity, underpinning the necessity of CD47 for leukemic development, particularly in the in vivo situation. Furthermore, mice with full-blown T-ALL treated with chemotherapy and antibodies sequentially displayed reduced bone marrow infiltration when cotreated with Dara and Hu5F9-IgG2σ compared with single application of either antibody. This was further supported by an overt leukemia setting with r/r T-ALL, in which mice subjected to CD47 blockade displayed prolonged survival but died of the disease at later stages. Here, only dual treatment with Dara showed long-term survival of animals.
The therapeutic activity of certain CD47 antibodies seems to be Fc dependent,47 and several clinical studies were discontinued due to nonselective depletion of CD47-expressing normal hematopoietic cells such as erythrocytes or thrombocytes, thereby causing anemia or thrombocytopenia.48,49 In our study, we used a CD47-antibody harboring a silent Fc domain incapable of inducing Fc-mediated cytotoxicity. Furthermore, our in vitro data showed corresponding antileukemic effects when modulating the CD47/SIRPα axis by preventing pyroglutamate formation. Other in vitro studies showed elevated phagocytosis upon QPCTL inhibitors with antibodies.26 In line with this, we also detected elevated phagocytosis in T-ALL cell lines after use of the combination of Dara with the QPCTL inhibitor SEN177. No clinical study examining QPCTL inhibitors for tumor patients is yet available. A current phase 2 study is evaluating the safety, tolerability, and efficacy of PQ912 (#NCT04498650). Hence, QPCTL inhibitors alone or in combination with antibodies might open an additional therapeutic window for hematologic malignancies, particularly in T-ALL expressing high CD47 levels.
Current clinical trials are examining daratumumab (#NCT03384654) and isatuximab (#NCT03860844) in combination with chemotherapy for r/r B-ALL and T-ALL. In addition, several trials examining diverse CD47 antibodies are ongoing21; however, a trial combining Dara with CD47 blockade does not yet exist. It will become important to determine which patients might benefit from CD38/CD47–targeted immunotherapy and potentially identify corresponding biomarkers. Higher expression levels of CD38 have been associated with superior clinical response to daratumumab in patients with MM,50 and similar results were observed preclinically for CD47 in MM samples.51 Hence, high surface expression of both antigens might serve as a predictor of therapy response.
Taken together, dual targeting of CD38 and CD47 alone or in combination with chemotherapy might represent a novel therapeutic approach for patients with T-ALL, especially in relapsed disease warranting evaluation in clinical studies, and may be applicable to both pediatric and adult patients.
Acknowledgments
The authors thank Katrin Neumann, Katrin Timm-Richert, Martina Kähler, Gabriele Riesen, Birthe Fedders, Irene Pauls, Miriam Bultmann, and Kathrin Richter for excellent technical assistance. The manuscript contains data in partial fulfillment of the requirements for a thesis by K.M. at the Medical Faculty of the Christian-Albrechts University Kiel.
This work was funded by the Stiftung Deutsche Krebshilfe (70113524 and 70113533, to C.K. and D.M.S.)
Authorship
Contribution: F.V., K.M., and D.W. designed and performed experiments and analyzed data; M.-P.A., C.L.G., T.R., L.L., and L.B. performed experiments and analyzed data; B.B. and J.-P.B. contributed ALL PDX material; A.K.B. contributed genetic data; G.C., M.S., A.E.K., C.E., and M.B. provided patient information; T.R., C.K., and M.P. designed and cloned antibodies; D.M.S., T.V., and F.V. supervised the research direction; and D.M.S., T.V., and F.V. wrote the manuscript. All authors discussed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denis M. Schewe, Department of Pediatrics, Otto-von-Guericke University, Leipziger Str. 44, 39120 Magdeburg, Germany; e-mail: denis.schewe@med.ovgu.de.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
K.M. and F.V. contributed equally to this study.


![Combination of Dara and Hu5F9-IgG2σ increases in vitro phagocytosis in T-ALL cell lines. (A) CD47 and CD38 surface expression in P12, MOLT-13, and HSB-2 examined by using flow cytometry. (B) Antibody-dependent cellular phagocytosis analyzed in P12, MOLT-13, and HSB-2 treated with Dara, a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Graph illustrates 5 independent experiments with different human macrophages (n = 5, standard error of the mean). (C) Representative pictures of phagocytosed P12 cells (engulfed cells in red) subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D-E) ADCP in CD47-depleted P12 (D) and MOLT-13 (E) cells subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]) of both antibodies, or untreated (control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). Statistical analysis: nonparametric Mann-Whitney U test (two-tailed). *P < .05, **P < .01. MFI, mean fluorescence intensity; ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f2.png?Expires=1768584028&Signature=znRVznUKUeqeey1ocuo0PJS7vy7BwAypFhJPjYD0vWl9U9P2LJFITpmIohHwUj9NGZvH2MoGdqqjS3uA3tG6130aWl4Pl~DFp6hjzgjsj17vbWcR9Sx69o-3E5WP2cs7F7o9BARvO2FY-ujThfv1GOsqgfyg9EwHJSJ-ZM~AkD0y7DFwgeK40SSSm3D8YQFhpi-QIuGDLt1GUecZHV2vv2e7Xx8gctSy8OetsDhZGZ8sb8IIjXXUERaag0W4y684fzA99Kkpscp6XMKhkAj8Y0VZcRD6suGb1FiR1wmTK6c8K1yYG9~OCLm~VHk-WCoeRPpP0mSHT2dTNTXMcAZ-EQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Pharmacologic inhibition of CD47 with Dara elevates ADCP in T-ALL cells. (A-B) ADCP in P12 (A) and MOLT-13 (B) subjected to Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or dimethyl sulfoxide (DMSO) (solvent control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). (C) ADCP in 2 random de novo (patients #2 and #10) and 2 r/r (patients #6r/r and #10r/r) T-ALL PDX samples treated with Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or DMSO (solvent control). One representative experiment of 4 experiments with different donor macrophages is depicted. Nonparametric Mann-Whitney U test (two-tailed). *P < .05. ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f3.png?Expires=1768584028&Signature=vf217EGdanKkd9hf93Ozq~XPp4fsrX0i2pA2Ihvbk~BVS5uYnISOtzyYi3cuNB4cZ3bXMqB95G~rVKcFtAbsYqRnCh1GJNbNiFN10zTHLgdRW5uH1Rs7C-88mRRRxPm0oo8J6HdiJyT8Fma86cnfRjnWlpi3Ktsh6bXLkc7N25403aisIB6Vb2giP~BF4hOh6Jgs1dh2kuqwzhveuITT2UyfHyNwyLygnf96f4mQWckTAamZj6pC3NNLmK70EoQnJjnpnYCIrFoLvWXsRdD4Tdl7oskfZJnZg0HHq7gjv8ZDsgwEVddcXVo6JQOiSXLO-Xa0yWoUbbLEPTNYiEt2gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Combination of Dara and Hu5F9-IgG2σ in random de novo T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in 12 random de novo T-ALL PDX samples treated with daratumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars show standard error of the mean of technical triplicates. One representative experiment of 4 experiments with different donor macrophages is shown. The remaining experiments with other donor macrophages are depicted in supplemental Figure 3. (B) Summary of all ADCP in T-ALL PDX samples with human macrophages from 4 healthy donors. (C) In vivo phase 2–like preclinical study in an MRD-like setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D) In vivo phase 2–like preclinical study in an overt leukemia setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). The set of mice from patient #1 was excluded due to non-engraftment in this experiment. (E) Determination of blood and postmortem bone marrow (BM) blasts analyzed by using flow cytometry in mice injected with one random de novo T-ALL PDX sample and subjected to chemotherapy only (chemo only), the combination of Dara + chemo, Hu5F9-IgG2σ + chemo, both antibodies with chemo, or no treatment (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. Nonparametric Mann-Whitney U test (two-tailed). **P < .01, ***P < .001. ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f4.png?Expires=1768584028&Signature=TrHJpOQS8gvkbkxtJMAPkRUg-RTK08rhEja6gML8xc5T5L0JJ2CZXU2a5fgSw8m2XSkAbxY3E6RcSoWeoJGycW0BRncflXJFIQcqYjcRDCQwkvatdkl8erFfu8N2Q6KlcR-WNhsN7ynYSTDZk7D6joJgZqU~YrmEbX2K7jgzPehGv-elYmgZCxzJ-b3LxuhIK1osM2YOClb7UuFACL1yrBeZgOuDHN6RlHjbwwnA7GYvtwBtfzaldFEyG8aQeu62~ou4CE7tYJo~ZIw4n0TuudMcpeWYxgbMx~RQ1TOhXgtNPZMlCHayDbsHROkpNZb2mCDGOohVltcOhiVIZiHYOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Combination of Dara and Hu5F9-IgG2σ in r/r T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in eleven r/r T-ALL PDX samples treated with datatumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars indicate standard error of the mean of technical triplicates. One representative experiment of 3 experiments with different donor macrophages is depicted. The remaining experiments with the other donor macrophages are depicted in supplemental Figure 7. (B) Summary of all ADCP in r/r T-ALL PDX samples with human macrophages from 3 different healthy donors. (C) In vivo phase 2–like preclinical study in overt leukemia with eleven r/r T-ALL PDX samples injected into NSG mice subjected to Dara, Hu5F9-IgG2σ alone, or the combination (Dara + Hu5F9-IgG2σ [combi]), or left untreated (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. **P < .01, ***P < .001, ****P < .0001. ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f5.png?Expires=1768584028&Signature=YipTmeROCcA9hbY81Km1flXdekWA9SjDoxc7Pi0O7F7xp3fOSrLIeuiln-~gAGdM3OUZ1i41-KS8BNoGLKk9c7IUz1V9v9XU5RQkOpz2iDSiBXo65YkGYgszolwwrgFcl8MlGIM~UijiUh2crzeSs4PIEsOoiX05ulLFjaC89wnfR3XgAV2csK7RvT6PUJ-iOfRA5iapsaM8TnnXgVMzKV-V1tO7yWH9TIKE-1xw7IIwiX6gcqpgQPLdUelJiwKcM36sH2BnLNGEmGYn9cYrDywfbGJmaZA2QJOkg6XrdFbMftwCr3MYjc7-kU85wA1HwKQNHXgABtldorQ29CDcEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Combination of Dara and Hu5F9-IgG2σ increases in vitro phagocytosis in T-ALL cell lines. (A) CD47 and CD38 surface expression in P12, MOLT-13, and HSB-2 examined by using flow cytometry. (B) Antibody-dependent cellular phagocytosis analyzed in P12, MOLT-13, and HSB-2 treated with Dara, a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Graph illustrates 5 independent experiments with different human macrophages (n = 5, standard error of the mean). (C) Representative pictures of phagocytosed P12 cells (engulfed cells in red) subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D-E) ADCP in CD47-depleted P12 (D) and MOLT-13 (E) cells subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]) of both antibodies, or untreated (control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). Statistical analysis: nonparametric Mann-Whitney U test (two-tailed). *P < .05, **P < .01. MFI, mean fluorescence intensity; ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f2.png?Expires=1768584029&Signature=yVYfK8SafdtIVkAm5CchFwSoFj23CRwDMFufI~xLX~c0dXuHaWjW2yQHFeLc62sf-ez0rVWgH9w7dD2ECMZUcpBBvPfWPmgUCIz1eYsJ9nxMsNLnVyGw~rlLdhLO-RdJDYXzbIkGn-ejwmAJFFLlu3N~MP3-BpdN5zlUOpazDGpv~5KeF0lW7Q4RHt-Xl-7orIAXbaYDiLCW00tphahFJElXhOUoHNqZsUrpEU5jWrRPdwNBC0cGE1rYrT3g0TNQ10ZC0gU1oJrwb~lkcSRBArQlGrthJJS-~lMWUUpILWaKehZQ2dwMkf8n-0gg77gtrd062l~Uhx9iTKu1DPmVWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Pharmacologic inhibition of CD47 with Dara elevates ADCP in T-ALL cells. (A-B) ADCP in P12 (A) and MOLT-13 (B) subjected to Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or dimethyl sulfoxide (DMSO) (solvent control). Graph depicts 4 independent experiments with different human macrophages (n = 4, standard error of the mean). (C) ADCP in 2 random de novo (patients #2 and #10) and 2 r/r (patients #6r/r and #10r/r) T-ALL PDX samples treated with Dara or SEN177 (10 µM; 72 hours) alone, the combination of both (Dara + SEN177 [combi]), or DMSO (solvent control). One representative experiment of 4 experiments with different donor macrophages is depicted. Nonparametric Mann-Whitney U test (two-tailed). *P < .05. ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f3.png?Expires=1768584029&Signature=USXRBvaWKakedbVSc1zkndKeova67yApVOkmLqI83Pdc-CKTYOjBHj97QujEx6wVKyV9XaBWuijo7D9SY9bUWyK8Pzml1QRe8PEE6SrepFLzni4xW9867M~X8HRszVoHiKg4sAaeUIRskoSwx4iRzYqhxxwvIilUA8HDKt9rKAcBEMVDeJ2W2XDtHuSYwWiSVeA-uRUbp2Oxr~34IXYvyvOvdXSDg9qB1ULSbSZQnPLwQxe6Jb6lfdlZuS9-HVn6J7k7TYTFyKi0-H4dZZMul8sc93erX5yS31tKOXgSbcdyQXogEWhs3437cvG9E5cWI4rqy2ktmZAKkblgP50lhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Combination of Dara and Hu5F9-IgG2σ in random de novo T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in 12 random de novo T-ALL PDX samples treated with daratumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars show standard error of the mean of technical triplicates. One representative experiment of 4 experiments with different donor macrophages is shown. The remaining experiments with other donor macrophages are depicted in supplemental Figure 3. (B) Summary of all ADCP in T-ALL PDX samples with human macrophages from 4 healthy donors. (C) In vivo phase 2–like preclinical study in an MRD-like setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). (D) In vivo phase 2–like preclinical study in an overt leukemia setting with 6 random de novo T-ALL PDX samples injected into NSG mice and subjected to Dara, Hu5F9-IgG2σ, the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control). The set of mice from patient #1 was excluded due to non-engraftment in this experiment. (E) Determination of blood and postmortem bone marrow (BM) blasts analyzed by using flow cytometry in mice injected with one random de novo T-ALL PDX sample and subjected to chemotherapy only (chemo only), the combination of Dara + chemo, Hu5F9-IgG2σ + chemo, both antibodies with chemo, or no treatment (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. Nonparametric Mann-Whitney U test (two-tailed). **P < .01, ***P < .001. ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f4.png?Expires=1768584029&Signature=pS26EXYGR4X7-xU74ddfO8bUI8xA1XlO-QUWvt1hgl8UqGhddw1gIC7y6YDB4mKUQWyUuH6GkAwajwwAMAHU4HuQ1rtkwbYfVwkdaYabFN~~EWbwpOX45pa4o67zpCj~53iRHLmgHnhJwfFxeY3Kddx4Ue3njLbql9zJFXScpzz3rj2NmnUdFCgAdAbd8wR76G4MWis3ZQ324VU8ThnHzTZ21y2OWUebPMEQTXRpA-4wsG9--I0KfP2-SS5RADVW5ySMtOesy2R2WrlM6hi0XvBlnFxfWErz~5NCv6S~pbNaHvVSITpjDkyjFQ9lOfThllNk1I3A0Jpbl8XtgeK6pg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Combination of Dara and Hu5F9-IgG2σ in r/r T-ALL PDX samples. (A) Antibody-dependent cellular phagocytosis in eleven r/r T-ALL PDX samples treated with datatumumab (Dara), a CD47-blocking antibody (Hu5F9-IgG2σ), the combination (Dara + Hu5F9-IgG2σ [combi]), or no treatment (control) using live cell imaging. Error bars indicate standard error of the mean of technical triplicates. One representative experiment of 3 experiments with different donor macrophages is depicted. The remaining experiments with the other donor macrophages are depicted in supplemental Figure 7. (B) Summary of all ADCP in r/r T-ALL PDX samples with human macrophages from 3 different healthy donors. (C) In vivo phase 2–like preclinical study in overt leukemia with eleven r/r T-ALL PDX samples injected into NSG mice subjected to Dara, Hu5F9-IgG2σ alone, or the combination (Dara + Hu5F9-IgG2σ [combi]), or left untreated (control). Survival was analyzed by using the Kaplan-Meier method and log-rank statistics. **P < .01, ***P < .001, ****P < .0001. ns, not significant; ROI, red object counts per image.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/1/10.1182_blood.2021014485/2/m_bloodbld2021014485f5.png?Expires=1768584029&Signature=Fx5B6zqYvoUIV8QVyBOLVNB-uPcQGwM-VWquIWm964kzvWBxHDJuYrnhGYbfQwDqUpVTkS4hGvo3L~tXzNG00bO~1xgyS0nqVCwlcYvgZ1~fnoxpgV1pFqgxOmvBIfw1PPhNczPljL4baXiF8wYZeHiIAL3md1PcSQok87ZOBnsWhLJtJe91TMb7QD-PoAle1JHCeBRP5vlfznx053kc-QBLnNR9YkoSVZLj2RYVkfoLZRhyeGqQFCCjm3w2WCZqLS17xTZawR-D~w2YcfktF86HGO~kIto6x53CLjIbQVQ0RpHPPseXC21j4cYGVKQW4syeyePbHdpvROsQKsJ1tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)