TO THE EDITOR:
A wide array of cutaneous manifestations can be observed in myelodysplastic syndromes (MDS), ranging from infections, MDS-associated vasculitis, Sweet syndrome (SS) (sterile mature neutrophils dermal infiltrate), and leukemia cutis (blast cell skin infiltration, corresponding to AML progression in the skin, concomitant with or heralding MDS bone marrow [BM] progression).1
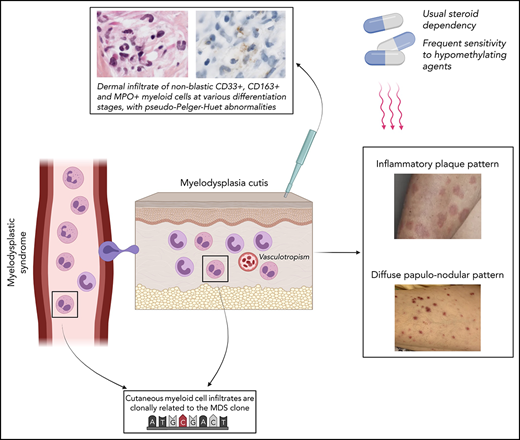
Histiocytoid-SS is a variant of SS composed of immature myeloid cells at various differentiation stages that is strongly associated with MDS.2,3 Our group introduced the term “myelodysplasia cutis” (MDS-cutis) to describe specific myelodysplastic cells infiltration within the skin of MDS patients previously diagnosed as histiocytoid-SS.4,5 The relevance of this entity has been a matter of debate because the clonal origin of skin-infiltrating myeloid cells was not clearly demonstrated.6-8 Recently, however, we found in a patient with MDS-cutis the same clonal origin of myeloid cells present in the BM and infiltrating the skin, supporting MDS-cutis as a true entity.5
Here, we report a series of documented MDS-cutis cases, related to the MDS clone, in 7 MDS patients, further characterizing this entity.
We reviewed MDS patients with nonblastic, specific skin infiltrate followed at Hôpital Saint-Louis, Paris, between January 2007 and April 2021. Patients fulfilling the following criteria: (1) diagnosis of MDS according to the World Health Organization 2016 criteria,9 (2) cutaneous pathology consistent with “histiocytoid” immature myeloid cells, and (3) identical mutation of at least 1 gene recurrently mutated in MDS in the BM and skin were considered to have MDS-cutis. This definition ruled out leukemia cutis and neutrophilic MDS-associated–SS.
Targeted next-generation sequencing (NGS) of 80 genes recurrently mutated in MDS (and UBA1 gene by Sanger sequencing) were performed on DNA extracted from BM and from formalin-fixed paraffin-embedded tissue sections of skin-lesion samples. Patients were classified according to the International Prognostic Scoring System (IPSS), considering IPSS low or intermediate-1 as lower risk and IPSS intermediate-2 and high as higher risk10
Characteristics of the 7 patients (6 males, 1 female) are summarized in supplemental Table 1. Median age was 65 years (range, 40-79) at MDS diagnosis and 68 years (range, 42-83) at MDS-cutis diagnosis. Median interval from MDS diagnosis to MDS-cutis occurrence was 11 months (range, 1-203).
At diagnosis of MDS-cutis, 6 patients had higher-risk and 1 lower-risk MDS. MDS features worsened at the time of MDS-cutis diagnosis in 2 patients (acquisition of [del]12p in patient number 4 and of trisomy 8 in patient number 7) and 6 months after MDS-cutis diagnosis in patient number 2 (progression from lower risk to higher risk).
Clinically, 4 patients presented with an erythematous, edematous (inflammatory) plaque pattern of the limbs and/or face (SS-like) (Figure 1A-B) and 3 patients presented with a diffuse papulo-nodular pattern (leukemia cutis–like) (Figure 1C-D). Pathology showed in all patients a dermal infiltrate of immature nonblastic myeloid cells at various differentiation stages, some of them presenting pseudo–Pelger-Huet abnormality (Figure 1E). A perivascular pattern was seen in all cases, without vascular wall necrosis or leukocytoclasia, and a peri-adnexal pattern in 4 patients.
Clinical and pathological pictures of myelodysplasia cutis. (A) Arm annular edematous, erythematous (inflammatory) plaques (SS-like) in patient number 1. (B) Forehead edematous, erythematous (inflammatory) papules (SS-like) in patient number 7. (C) Dark red diffuse papulo-nodules (leukemia cutis–like) in patient number 2. (D) Pink red diffuses papulo-nodules (leukemia cutis–like) in patient number 4. (E) Inflammatory infiltrate consisting of myeloid cells with indented nuclei corresponding to metamyelocytes or band neutrophils, with some of them carrying pseudo–Pelger-Huet abnormality (hematoxylin-eosin-saffron, original magnification ×1000). (F) Myeloid cells expressed myeloperoxidase (immunostaining, ×1000).
Clinical and pathological pictures of myelodysplasia cutis. (A) Arm annular edematous, erythematous (inflammatory) plaques (SS-like) in patient number 1. (B) Forehead edematous, erythematous (inflammatory) papules (SS-like) in patient number 7. (C) Dark red diffuse papulo-nodules (leukemia cutis–like) in patient number 2. (D) Pink red diffuses papulo-nodules (leukemia cutis–like) in patient number 4. (E) Inflammatory infiltrate consisting of myeloid cells with indented nuclei corresponding to metamyelocytes or band neutrophils, with some of them carrying pseudo–Pelger-Huet abnormality (hematoxylin-eosin-saffron, original magnification ×1000). (F) Myeloid cells expressed myeloperoxidase (immunostaining, ×1000).
Immature myeloid cells expressed CD33, CD163, CD14, CD68-PGM1, and myeloperoxidase (MPO) (Figure 1F) but did not express CD34, CD56, or CD117. Dermal edema and a population of CD3+ T cells were associated with the myeloid infiltrate in all cases.
NGS analysis (supplemental Table 1) identified 1 to 5 genes mutated both in the BM (with a median variant allele frequency [VAF] of 30%) and the skin paired sample (with a median VAF of 11%). Some mutations were detected in 1 tissue and not the other, supporting clonal evolution in 1 of the tissues.
One patient had UBA1 mutation in the blood and the skin, whereas the 6 other patients tested were UBA1 wild-type in both tissues. The patient with UBA1 mutation had a 12 months history of vasculitis, arm and leg venous thrombosis, relapsing fever, and cytoplasmic vacuoles in myeloid and erythroid precursors in BM aspirate. The histologic profile of his leukemia cutis–like lesions did not differ from that of the other patients.
Six of the 7 patients received corticosteroids, and 4 (number 1 to 4) became steroid dependent. Azacytidine (75 mg/m2 per day in 5-day cycles) was introduced in those last 4 patients, yielding complete resolution (CR) of MDS-cutis in all cases, with hematological improvement in 3 of them. Patient number 5 received low-dose cytarabine, also with CR of MDS-cutis. Three patients (number 2, number 3, and number 5) died of MDS progression within 9 months, 2 had more prolonged survival (>25 and >32 months, respectively), and the remaining 2 had short follow-up (>4 and >12 months).
This study provides, to our knowledge, for the first time, molecular analysis of MDS-cutis in a consecutive case series. We showed that the cutaneous immature myeloid cell infiltrate was clonally related to the BM–MDS clone, as the same mutations were found in both tissues. Most mutations detected in skin lesion samples were at relatively high allelic burden (median VAF: 11%), and mature neutrophils were not observed in the skin samples, suggesting that they were due to skin infiltration by clonal MDS cells rather than to blood contamination.
Clinically, we found 2 different dermatological pictures: (1) erythematous, edematous (inflammatory) papules and plaques of the head and limbs (SS-like) and (2) erythematous diffuse papules and nodules (leukemia cutis–like). Those polymorphic clinical pictures contrasted with a uniform pathological picture of perivascular and periadnexal infiltrate of MPO+ immature nonblastic myeloid cells with pseudo–Pelger-Huet abnormality, at different stages of differentiation, associated with a lymphocytic infiltrate and extensive dermal edema. Interestingly, 3 patients had a previous diagnostic of MDS-associated vasculitis, and no patient fulfilled SS diagnostic criteria.11
Although the small patient number precludes any conclusion, MDS-cutis may frequently be associated with advanced MDS. Indeed, 6 patients had higher-risk MDS, 3 progressed at diagnosis or within 6 months of MDS-cutis diagnosis, and survival was 9 months or less in 3 of 7 patients. In contrast to classical SS,12 MDS-cutis appeared to be frequently steroid dependent but potentially responded to azacytidine.
Our findings suggest that MDS-cutis should be considered as differential diagnosis of leukemia cutis, classical SS, and MDS-associated vasculitis. MDS-cutis may potentially be underdiagnosed due to the lack of skin molecular investigation, particularly in patients labeled as histiocytoid-SS.2
The present description of MDS-cutis also potentially allows new insights into the specific skin involvement in MDS pathophysiology. In AML-associated–SS, we and others found, by cytogenetic and NGS, that neutrophils infiltrating the skin could be part of the leukemic clone, corresponding to differentiation of AML blasts and suggesting that leukemia cutis and SS, in AML, could be part of a same “disease spectrum.”13-15 Likewise, MDS patients may develop skin involvement by immature myeloid dysplastic cell as MDS-cutis (this report) or infiltration by more mature clonal cells leading to mature SS (as also previously described by our team).15 This suggests, in MDS, a specific cutaneous disease spectrum ranging from MDS-cutis to MDS-associated classical SS (and leukemia cutis in case of AML progression).
In conclusion, MDS-cutis is a rare specific entity in MDS patients, characterized by a stereotypical pathological picture (immature myeloid cells with pseudo–Pelger-Huet, MPO+ perivascular infiltrate) but a more heterogeneous clinical picture (SS-like or leukemia cutis–like). This entity should be distinguished from leukemia cutis and from classical SS observed in MDS. MDS patients presenting with a histological picture of histiocytoid-SS (the term histiocytoid, proposed by Requena in 2005,16 being misleading as those cells correspond to immature myeloid cells) may be better considered as MDS-cutis. MDS-cutis may respond to hypomethylating agents, although this sensitivity will have to be confirmed in larger prospective series.
Informed consent was acquired for all patients as per the Declaration of Helsinki.
Authorship
Contribution: J.D., M. Battistella, P.F., and J.-D.B. designed research; J.D., R.K., E.C., P.F., and M. Battistella provided and analyzed biological data; M. Battistella and M.-D.V.-P. reviewed histopathological data; J.D., L.-P.Z., A.d.M., C.C., C.R.-W., M. Bagot, L.A., M.S., J.-D.B., M. Battistella, and P.F. managed patients and provided clinical data; J.D., J.-D.B., P.F., and M. Battistella wrote the manuscript; all authors reviewed the manuscript.
J.D., P.F., and M. Battistella had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jérémie Delaleu, Dermatology Department, APHP, Saint Louis Hospital, 1, avenue Claude Vellefaux, 75010 Paris, France; e-mail: delaleu.jeremie@gmail.com.
Raw NGS data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-9554.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
REFERENCES
Author notes
P.F. and M. Battistella contributed equally as last authors.