Key Points
VTE risk is high for NDMM patients receiving treatment and only modestly reduced by IMWG-guided thromboprophylaxis.
VTE risk is equivalent for thalidomide and lenalidomide regimens, and in these trials, VTE was not associated with reduced PFS or OS.
Abstract
Newly diagnosed multiple myeloma (NDMM) patients treated with immunomodulatory drugs are at high risk of venous thromboembolism (VTE), but data are lacking from large prospective cohorts. We present thrombosis outcome data from Myeloma IX (n = 1936) and Myeloma XI (n = 4358) phase 3 randomized controlled trials for NDMM that treated transplant-eligible and transplant-ineligible patients before and after publication of thrombosis prevention guidelines. In Myeloma IX, transplant-eligible patients randomly assigned to cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CVAD) induction had higher risk of VTE compared with patients treated with cyclophosphamide, thalidomide, and dexamethasone (CTD) (22.5% [n = 121 of 538] vs 16.1% [n = 89 of 554]; adjusted hazard ratio [aHR],1.46; 95% confidence interval [95% CI], 1.11-1.93). For transplant-ineligible patients, those randomly assigned to attenuated CTD (CTDa) induction had a higher risk of VTE compared with those treated with melphalan and prednisolone (MP) (16.0% [n = 68 of 425] vs 4.1% [n = 17 of 419]; aHR, 4.25; 95% CI, 2.50-7.20). In Myeloma XI, there was no difference in risk of VTE (12.2% [n = 124 of 1014] vs 13.2% [n = 133 of 1008]; aHR, 0.92; 95% CI, 0.72-1.18) or arterial thrombosis (1.2% [n = 12 of 1014] vs 1.5% [n = 15 of 1008]; aHR, 0.80; 95% CI, 0.37-1.70) between transplant-eligible pathways for patients treated with cyclophosphamide, lenalidomide, and dexamethasone (CRD) or CTD. For transplant-ineligible patients, there was no difference in VTEs between attenuated CRD (CRDa) and CTDa (10.4% [n = 95 of 916] vs 10.7% [n = 97 of 910]; aHR, 0.97; 95% CI, 0.73-1.29). However, arterial risk was higher with CRDa than with CTDa (3.1% [n = 28 of 916] vs 1.6% [n = 15 of 910]; aHR, 1.91; 95% CI, 1.02-3.57). Thrombotic events occurred almost entirely within 6 months of treatment initiation. Thrombosis was not associated with inferior progression-free survival (PFS) or overall survival (OS), apart from inferior OS for patients with arterial events (aHR, 1.53; 95% CI, 1.12-2.08) in Myeloma XI. The Myeloma XI trial protocol incorporated International Myeloma Working Group (IMWG) thrombosis prevention recommendations and compared with Myeloma IX, more patients received thromboprophylaxis (80.5% vs 22.3%) with lower rates of VTE for identical regimens (CTD, 13.2% vs 16.1%; CTDa, 10.7% vs 16.0%). However, thrombosis remained frequent in spite of IMWG-guided thromboprophylaxis, suggesting that new approaches are needed.
Introduction
Venous thromboembolism (VTE) has a well-established association with cancer and is one of the leading causes of death in cancer patients.1 In addition to mortality risk, VTE is an important cause of long-term morbidity, impaired quality of life, and adverse psychological impact, and it is a burden on health care resources.2,3 Multiple myeloma (MM) is the second most common blood cancer and is associated with a high risk of VTE.4-7 A recent review of nearly 5000 myeloma patients showed that those with VTE had an increased risk of mortality at 2 and 5 years after diagnosis, independent of other known prognostic factors.8
A large retrospective study of more than 4 million US veterans demonstrated a ninefold increased risk of deep vein thrombosis (DVT) in those with myeloma and a threefold increased risk of DVT in patients with monoclonal gammopathy of uncertain significance (MGUS).9 Another large retrospective population-based study from Sweden demonstrated an increased risk of VTE for patients with MM (adjusted hazard ratio [aHR], 7.5 at 1 year after MM diagnosis, 4.6 at 5 years, and 4.1 at 10 years), and to a lesser extent, patients with MGUS (aHR, 3.4 at 1 year after MM diagnosis, 2.1 at 5 years, and 2.1 at 10 years). Of interest, this group also showed an increased risk of arterial thrombosis for patients with MM (aHR, 1.9 at 1 year after MM diagnosis, 1.5 at 5 years, and 1.5 at 10 years) and with MGUS (aHR, 1.7 at 1 year after diagnosis, 1.3 at 5 years and 1.3 at 10 years).7
The pathogenesis of VTE in MM is complex and only partially understood; patients can develop thrombosis at any stage in the disease trajectory, with the highest risk being in the first year from diagnosis.6,9 The plasma cell cancer, its treatment, and patient-related factors all contribute to the mechanism of thrombosis in MM.5,10 Treatments for MM have improved over the last decade with the introduction of immunomodulatory drugs (IMiDs) such as thalidomide, lenalidomide, and pomalidomide. However, these drugs further increase the risk of VTE, as do corticosteroids, which are included in most treatment regimens.11,12 Newly diagnosed MM (NDMM) patients receiving initial treatment with IMiDs and high-dose corticosteroids are at high risk of thrombosis; there are many reported incidences from heterogeneous studies but a lack of data from large prospective patient cohorts. In addition, it is not known whether the 2 most commonly used IMiDs in induction therapy combinations, thalidomide and lenalidomide, have the same risk of thrombosis because they differ in potency and adverse effect profile. Recently, myeloma-specific VTE risk assessment scores have been developed and validated (IMPEDE VTE and SAVED).13-15 These scores help stratify the risk of VTE and may help identify patients who warrant thromboprophylaxis. The International Myeloma Working Group (IMWG) guidelines help to discriminate the risk of VTE, and this has been demonstrated in both the IMPEDE VTE and SAVED publications.
The optimal thrombosis prevention strategy for patients with MM at high risk of VTE remains controversial. Data from randomized trials suggest that aspirin, low molecular weight heparin (LMWH), and therapeutic warfarin reduce risk to an acceptable bleeding risk, but it is not clear which of these strategies is better.16-25 Emerging data suggest that thromboprophylaxis with apixaban may be an option, but it has not yet been compared with conventional approaches in a randomized trial.10,26-28
In 2008, the IMWG published guidance on the prevention of IMiD-associated thrombosis in myeloma.12 These guidelines recommended that all patients be assessed for risk and offered thromboprophylaxis with LMWH if they have ≥2 thrombosis risk factors or if they are receiving concurrent IMiDs and high-dose corticosteroids, whereas those with ≤1 risk factors should be offered aspirin. More recent guidelines have made consistent recommendations.29,30 However, it is recognized that the guidelines are based on limited evidence, and the expected reduction in risk if they are implemented is unknown.30 It is also unclear how deliverable the recommendations are in the real world, that is, whether once-per-day injections of LMWH are acceptable to patients and whether the logistics of initiating heparin is achievable for health care providers.
The Medical Research Council Myeloma IX and National Cancer Research Institute Myeloma XI trials are the largest randomized trials published to date that used IMiD and corticosteroid regimens for NDMM patients. Myeloma IX recruited patients before the IMWG thrombosis prevention guidance was published and Myeloma XI recruited afterward. Here, we present the thrombotic outcome data from both trials.
Methods
Trial design and treatment
Myeloma IX and XI trials, based in the United Kingdom, are phase 3, multicenter, open-label, parallel group, randomized controlled trials for NDMM patients. Myeloma IX (ISRCTN684564111) recruited patients between May 2003 and November 2007. Myeloma XI (ISRCTN49407852) recruited patients between May 2010 and April 2016. The trials were approved by the National Research Ethics Service (London, United Kingdom), institutional review boards of the participating centers, and the competent regulatory authority (Medicines and Healthcare Products Regulatory Agency, London, United Kingdom), and were undertaken according to the Declaration of Helsinki and the principles of Good Clinical Practice as espoused in the Medicines for Human Use (Clinical Trials) Regulations. All patients provided written informed consent. Inclusion criteria were similar in both trials: adult patients with newly diagnosed and histologically confirmed symptomatic MM.
Both trials had pathways for transplant-eligible and transplant-ineligible patients with pathway choice made by the individual’s physician or by the patient on the basis of the patient’s performance status and comorbidities without age restrictions. Methods and results from both trials have been published previously.31-35 In brief, transplant-eligible patients in Myeloma IX were randomly assigned either to cyclophosphamide, thalidomide, and dexamethasone (CTD) or to cyclophosphamide, vincristine, doxorubicin, and dexamethasone (CVAD) before autologous stem cell transplantation (ASCT). Transplant-ineligible patients were randomly assigned either to attenuated CTD (CTDa) or to melphalan plus prednisolone (MP) induction chemotherapy. After initial therapy, patients were randomly assigned to thalidomide maintenance or observation in both pathways. All patients were also randomly assigned to receive a bisphosphonate, either sodium clodronate or zoledronic acid.
Transplant-eligible patients in Myeloma XI were randomly assigned to CTD or cyclophosphamide, lenalidomide, and dexamethasone (CRD). There was a second random assignment for patients achieving a partial or minimal response between intensification with cyclophosphamide, bortezomib, and dexamethasone (CVD) or no further therapy before ASCT. Patients with stable or progressive disease underwent intensification therapy before ASCT, and patients with a very good partial response or complete response proceeded directly to ASCT. Transplant-ineligible patients were randomly assigned either to CTDa or to attenuated CRD (CRDa). Transplant-ineligible patients also underwent the intensification randomization. Patients in both pathways were randomly assigned to either lenalidomide (with or without vorinostat) or observation. The induction randomization of the transplant-eligible pathway of the Myeloma XI trial was amended in June 2013 to include a random assignment either to the response-adapted approach described above (CTD or CRD with or without CVD) or to the quadruplet carfilzomib, cyclophosphamide, lenalidomide, and dexamethasone (KCRD).
VTE prophylaxis
The Myeloma IX trial protocol did not include specific recommendations for preventing thrombosis, although it was stated that anticoagulation with either warfarin or LMWH should be considered in those at high risk for VTE. In contrast, the Myeloma XI trial protocol incorporated IMWG guidance for preventing thrombosis and specified that all patients should receive thromboprophylaxis for at least the first 3 months of treatment.12 It was recommended that low-risk patients should receive aspirin and high-risk patients should receive LMWH (the definition of high risk for VTE followed IMWG guidance).12
Collection of VTE and arterial events
The objectives of this secondary analysis of the Myeloma IX and XI trials were to estimate the frequency, incidence, and types of thrombosis events occurring on trial according to baseline characteristics, trial pathway, and treatment; to investigate the thromboprophylaxis received before thrombosis events according to treatment and thrombosis risk category before the event; and to estimate the median progression-free survival (PFS) and overall survival (OS) according to thrombosis occurrence.
In Myeloma IX, thrombotic events were collected from the adverse effect case report form (CRF) and follow-up CRFs that included a thromboembolism section. In Myeloma XI, thrombotic events were collected from a specific thromboembolism CRF. Treatment CRFs also included an indication of the occurrence of thromboembolism. For both trials, thrombosis events categorized as “other” site were reviewed by a clinician to determine whether they were venous or arterial events.
Statistical analysis
Analyses were carried out separately for each trial and pathway. In Myeloma IX, only VTEs were analyzed because there were only 11 arterial events recorded. In Myeloma XI, both venous and arterial thrombosis were analyzed. Analysis of patients receiving KCRD in Myeloma XI was performed using only patients contemporaneously randomly assigned to CTD and CRD and included only VTE. Analyses were conducted using the safety population, which included all patients who received at least 1 dose of study treatment. This population classified patients according to the treatment they received rather than to the treatment they were randomly assigned to receive.
Baseline characteristics were compared between those experiencing and those not experiencing a thrombosis event. Continuous baseline variables were evaluated with a 2-sample Student t test, and categorical baseline variables were evaluated with a χ2 test; the nonparametric equivalent was used where appropriate. The Fine and Gray competing risks regression model compared the hazard of thrombosis events by treatment group accounting for the minimization factors, excluding recruiting center (Myeloma IX: hemoglobin, corrected serum calcium, serum creatinine, and platelets; Myeloma XI: β2 microglobulin, hemoglobin, corrected serum calcium, serum creatinine, and platelets), with unrelated death defined as death without a preceding thrombosis event specified as a competing risk.
Person-years on trial were calculated as the sum of all patients receiving at least 1 dose of study treatment and time in years from random assignment to death or last date known to be alive. The incidence was calculated with the number of events as the numerator and the number of person-years on trial as the denominator. Confidence intervals (CIs) for incidence were calculated using approximations to the Poisson distribution. Cumulative incidence function curves of thrombosis events split by treatment group were estimated by nonparametric maximum likelihood estimation and compared by Gray’s test, accounting for unrelated deaths as a competing risk.
Site of thrombosis, risk of thrombosis, and thromboprophylaxis were summarized in those who had an event. Thromboprophylaxis was also assessed in patients who had not had an event in Myeloma IX; these data were not available for Myeloma XI. PFS and OS were compared between those who did and did not experience a thrombosis event using the Kaplan-Meier method and Cox regression models, and hazard ratios (HRs) were estimated, accounting for the minimization factors, excluding recruiting center. Statistical analyses were performed using SAS (version 9.4). All reported P values were 2-sided and were considered significant at the 5% significance level.
Results
The median follow-up after random assignment for this analysis was 71 months (interquartile range [IQR], 60-83 months) in Myeloma IX and 60 months (IQR, 48-77 months) in Myeloma XI. In both trials, the majority of the events occurred during induction (96.2% of events in Myeloma IX and 83.8% in Myeloma XI). The median time to first VTE in Myeloma IX was 2.2 months (IQR, 1.31-3.44 months), and in Myeloma XI, it was 2.9 months (IQR, 1.59-4.73 months).
In the Myeloma IX trial, VTE (368 events) occurred in 15.2% (295 of 1936) of all patients receiving treatment, 19.2% (210 of 1092) of transplant-eligible patients, and 10.1% (85 of 844) of transplant-ineligible patients. In the Myeloma XI trial, at least 1 thrombotic event (746 total events) occurred in 13.7% (599 of 4358) of all patients receiving treatment, VTE occurred in 12.2% (532 of 4358), and arterial thrombosis occurred in 1.8% (79 of 4358). Of note, some patients suffered both VTEs and arterial events. Of transplant-eligible patients, thrombotic events occurred in 14.7% (371 of 2532), VTE occurred in 13.4% (340 of 2532), and arterial thrombosis occurred in 1.4% (36 of 2532). Of transplant-ineligible patients, thrombotic events occurred in 12.5% (228 of 1826), VTE occurred in 10.5% (192 of 1826), and arterial thrombosis occurred in 2.4% (43 of 1826). A small number of peritransplant-associated thrombotic events occurred in both trials. In the 100 days after the administration of melphalan (autograft conditioning), there were 3 thrombotic events in Myeloma IX and 17 in Myeloma XI.
Baseline characteristics
The baseline characteristics for the safety population of patients within each trial and pathway are similar (supplemental Table 1, available on the Blood Web site). In both trials, transplant-eligible patients were younger than transplant-ineligible patients. In Myeloma IX, sex, age, and paraprotein type were significantly different between patients who did and did not experience a VTE; no other characteristics differed (Table 1). Compared with patients who did not develop thrombosis, the patients who did develop thrombosis were younger, more likely to be female, and more likely to have an immunoglobulin G paraprotein. When the transplant-eligible and ineligible pathways were analyzed separately, only paraprotein type differed in the transplant-eligible pathway and only sex differed in the transplant-ineligible pathway (supplemental Table 2).
In Myeloma XI, β2 microglobulin and hemoglobin were significantly different between patients who did and did not experience a thrombosis event (Table 1). Compared with patients without thrombosis, the patients with thrombosis had higher hemoglobin and lower β2 microglobulin levels. When the transplant-eligible and ineligible pathways were analyzed separately, sex, age, World Health Organization performance status, β2 microglobulin, calcium, hemoglobin, and light chain type were significantly different according to thrombosis incidence within the transplant-eligible pathway (supplemental Table 3). No baseline characteristics differed according to thrombosis incidence within the transplant-ineligible pathway.
Thrombosis events according to treatment group
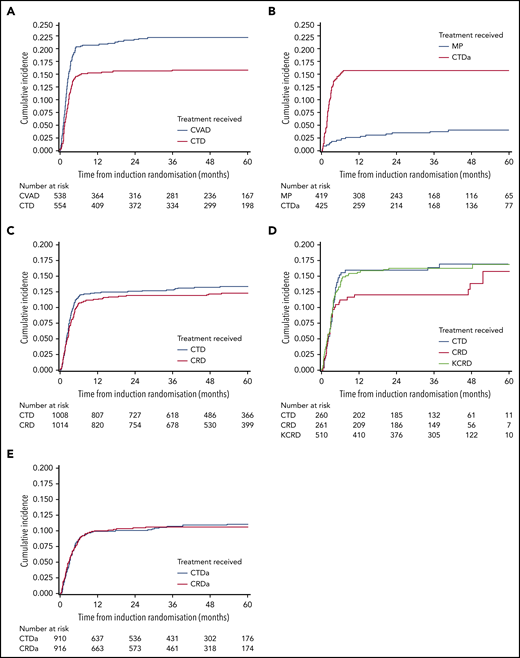
In the Myeloma IX transplant-eligible pathway, there was a higher risk of VTE in patients receiving CVAD than in those receiving CTD (22.5% [n = 121 of 538] vs 16.1% [n = 89 of 554]; aHR, 1.46; 95% CI, 1.11-1.93). For patients in the transplant-ineligible pathway, there was a higher risk of VTE in patients receiving CTDa than in those receiving MP (16.0% [n = 68 of 425] vs 4.1% [n = 17 of 419]; aHR, 4.25; 95% CI, 2.50-7.20). Within the maintenance phase, there were few thrombotic events and no difference in the number of patients with VTE between the thalidomide maintenance and the observation-only groups (1.5% [n = 6 of 391] vs 1.7% [n = 7 of 402]; P = .82).
In the Myeloma XI transplant-eligible pathway, there was no difference in risk of VTE between those treated with CRD and those treated with CTD (12.2% [n = 124 of 1014] vs 13.2% [n = 133 of 1008]; aHR, 0.92; 95% CI, 0.72-1.18). In the KCRD treatment group, 16.3% (n = 83 of 510) of patients experienced a VTE, which was not significantly different from concurrently randomly assigned patients receiving CRD (aHR, 0.79; 95% CI, 0.53-1.18) or CTD (aHR, 1.02; 95% CI, 0.7-1.47). For patients in the transplant-ineligible pathway, there was no difference in risk of VTE between those receiving CRDa and those receiving CTDa (10.4% [n = 95 of 916] vs 10.7% [n = 97 of 910]; aHR, 0.97; 95% CI, 0.73-1.29).
In the Myeloma XI transplant-eligible pathway, there was no difference in risk of arterial thrombosis between those receiving CRD and those receiving CTD (1.2% [n = 12 of 1014] vs 1.5% [n = 15 of 1008]; aHR, 0.80; 95% CI, 0.37-1.70). For patients in the transplant-ineligible pathway, there was a higher risk of arterial thrombosis in patients receiving CRDa than in those receiving CTDa (3.1% [n = 28 of 916] vs 1.6% [n = 15 of 910]; aHR, 1.91; 95% CI, 1.02-3.57).
Within the maintenance phase, significantly more patients in the lenalidomide maintenance group than the observation group had a VTE, although the absolute incidence was very low (4.1% [n = 44 of 1082] vs 0.6% [n = 5 of 889]; P < .0001). Arterial events were also more frequent in those receiving lenalidomide maintenance than in those under observation (1.3% [n = 14 of 1082] vs 0.3% [n = 3 of 889]; P = .022).
Incidence rate of thrombosis and comparison of equivalent treatment regimens in Myeloma IX and Myeloma XI trials
The VTE incidence rate for patients receiving CTD was slightly higher in Myeloma IX than in Myeloma XI (5.4 events per 100 person-years [PY]; 95% CI, 4.5-6.5 vs 4.3 events per 100 PY; 95% CI, 3.7-5.0). The VTE incidence rate for patients receiving CTDa was higher in Myeloma IX than in Myeloma XI (7.6 events per 100 PY; 95% CI, 6.2-9.5 vs 4.2 events per 100 PY; 95% CI, 3.4-5.0).
Cumulative incidence of thrombosis
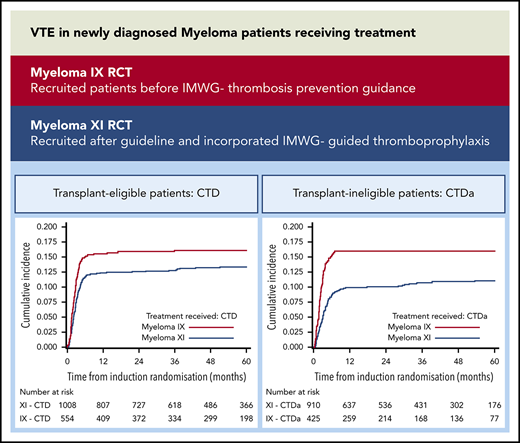
Across both trials and all treatments, the cumulative incidence of VTE increased most rapidly during the first 6 months after random assignment, after which it plateaued (Figure 1). All plots in Figure 1 have been cut at 60 months because all curves remain unchanged after this point. In Myeloma IX, the 6-month VTE cumulative incidence was higher in the CVAD group than in the CTD group (20.7% [95% CI, 17.3%-24.1%] vs 15.0% [95% CI, 12.0%-18.0%]; Gray’s test P = .006). In addition, the 6-month VTE cumulative incidence was higher in the CTDa group than in the MP group (15.6% [95% CI, 12.1%-19.0%] vs 2.2% [95% CI, 0.76%-3.55%]; Gray’s test P < .0001).
Cumulative incidence of VTE in transplant-eligible and -noneligible patients treated in Myeloma IX and XI clinical trials. VTE cumulative incidence function curves for (A) Myeloma IX trial transplant-eligible and (B) transplant-ineligible pathways and (C) Myeloma XI trial transplant-eligible, (D) transplant-eligible including KCRD, and (E) transplant-ineligible pathways.
Cumulative incidence of VTE in transplant-eligible and -noneligible patients treated in Myeloma IX and XI clinical trials. VTE cumulative incidence function curves for (A) Myeloma IX trial transplant-eligible and (B) transplant-ineligible pathways and (C) Myeloma XI trial transplant-eligible, (D) transplant-eligible including KCRD, and (E) transplant-ineligible pathways.
In Myeloma XI, the 6-month VTE cumulative incidence was comparable between treatment groups (10.7% [95% CI, 8.77%-12.6%] for CRD and 11.7% [95% CI, 9.69%-13.7%] for CTD; Gray’s test P = .54). In addition, there was no difference between the cumulative incidence function curves for KCRD, CRD, and CTD (Gray’s test P = .46). This was also the case within the transplant-ineligible pathway (8.7% [95% CI, 6.83%-10.5%] for CRDa and 8.7% [95% CI, 6.83%-10.5%] for CTDa; Gray’s test P = .82).
For arterial events, the 6-month cumulative incidence was similar between groups in the transplant-eligible pathway (0.7% [95% CI, 0.18%-1.21%] for CRD and 0.9% [95% CI, 0.31%-1.48%] for CTD; Gray’s test P = .56), but in the transplant-ineligible pathway, the 6-month cumulative incidence of arterial events was greater in the CRDa group than in the CTDa group [2.2% [95% CI, 1.25%-3.16%] vs 0.9% [95% CI, 0.28%-1.52%]; Gray’s test P = .05) (supplemental Figure 2).
Thrombosis site
Within both trials and pathways, the most common sites of thrombosis were DVT and pulmonary embolism (Table 2). However, for patients randomly assigned to CVAD, line-associated VTE was the most common thrombosis site (37.1%; n = 59 of 159 events), and line-associated VTE was almost exclusively restricted to patients treated with CVAD (96.7% of all line-associated VTEs in Myeloma IX). There were no other clear differences in the patterns of VTE presentations according to regimens.
Thromboprophylaxis before thrombosis
In Myeloma IX, before the VTE event, 22.3% of patients received thromboprophylaxis (Table 3). When thromboprophylaxis was given, treatment-dose warfarin was given most frequently, and patterns of thromboprophylaxis were similar between treatment groups. Of the patients who did not develop VTE, 19.7% received thromboprophylaxis, with therapeutic warfarin given most frequently. In Myeloma XI, before the VTE event, 80.5% of patients received thromboprophylaxis (Table 3). When thromboprophylaxis was given, LMWH was given most frequently. Patterns of thromboprophylaxis were similar between treatment groups.
VTE risk assessment before thrombosis
In Myeloma IX, before thrombosis, 21.0% of patients had been assessed as having a high risk for VTE, and 79.0% were assessed as having a low risk, but the patterns of thromboprophylaxis were similar between these groups (Table 4). In Myeloma XI, before VTE, 54.7% had been assessed as having a high risk of VTE and 45.3% had been assessed as having a low risk. Thromboprophylaxis was not given to 13.7% of high-risk patients or to 20.7% of low-risk patients. When thromboprophylaxis was given, slightly more high-risk patients received thromboprophylaxis, and of these, more received LMWH and fewer received aspirin than was the case for low-risk patients (Table 4).
PFS and OS
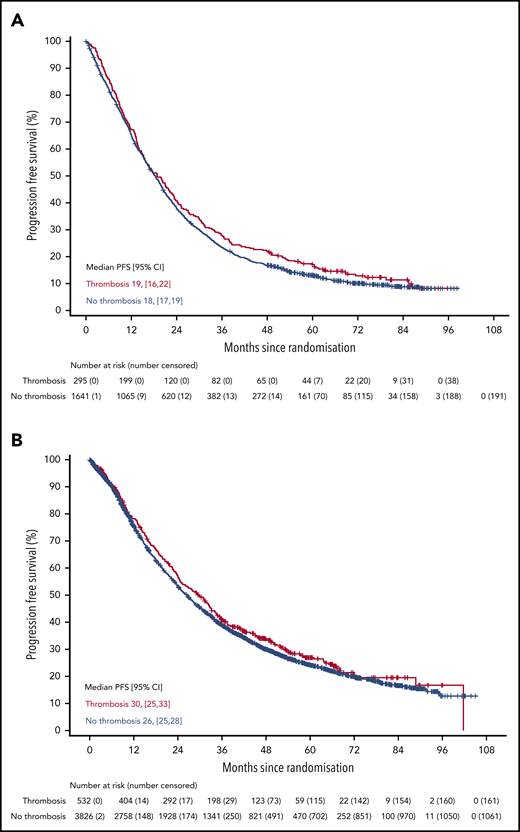
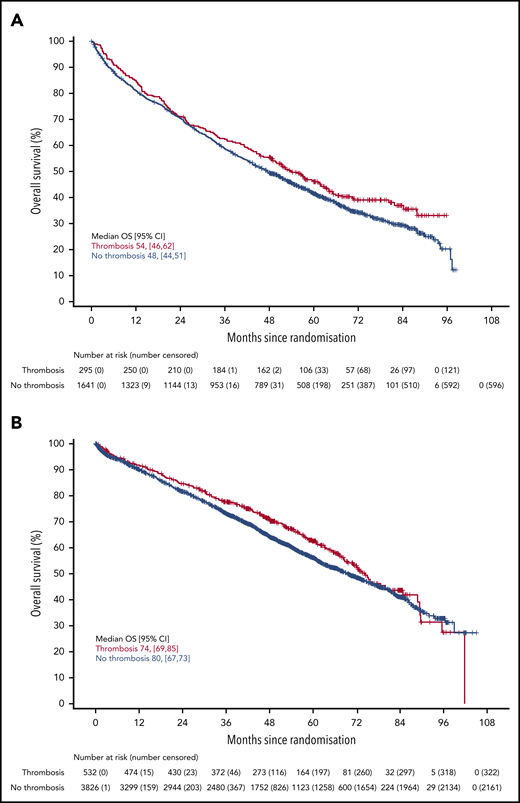
There was no difference in PFS in either trial for patients who developed VTE compared with those who did not (Figure 2) (Myeloma IX aHR, 0.92; 95% CI, 0.80-1.05; Myeloma XI aHR, 0.92; 95% CI, 0.83-1.03). There was also no difference in PFS in Myeloma XI for patients who developed arterial thrombosis compared with those who did not (aHR, 1.12; 95% CI, 0.86-1.47) (supplemental Figure 3). There was no difference in OS in either trial for patients who developed VTE compared with those who did not (Figure 3) (Myeloma IX aHR, 0.87; 95% CI, 0.74-1.02; Myeloma XI aHR, 0.90; 95% CI, 0.78-1.04). In Myeloma IX, aHR for OS of patients with VTE remains virtually unchanged if results are adjusted for bisphosphonate allocation, zoledronate, or clodronate (aHR, 0.88; 95% CI, 0.75-1.03). In Myeloma XI, there was no random assignment for bisphosphonate, and patients received bisphosphonate as standard of care. In Myeloma XI, there was an increased mortality risk for patients who developed arterial thrombosis (aHR, 1.53; 95% CI, 1.12-2.08) (supplemental Figure 4).
PFS in patients with or without VTE occurrence in Myeloma IX and XI clinical trials. PFS by VTE occurrence in (A) Myeloma IX and (B) Myeloma XI trials.
PFS in patients with or without VTE occurrence in Myeloma IX and XI clinical trials. PFS by VTE occurrence in (A) Myeloma IX and (B) Myeloma XI trials.
OS in patients with or without VTE occurrence in Myeloma IX and XI clinical trials. OS by VTE occurrence in the (A) Myeloma IX and (B) Myeloma XI trials.
OS in patients with or without VTE occurrence in Myeloma IX and XI clinical trials. OS by VTE occurrence in the (A) Myeloma IX and (B) Myeloma XI trials.
Discussion
Previous evidence from large retrospective cohorts has demonstrated that patients with myeloma are at increased risk of venous and arterial thrombosis, particularly in the first year after diagnosis.7,9 NDMM patients who received initial treatment with IMiDs and corticosteroids are at particularly high risk of thrombosis.36 However, the range of reported incidences is very broad and timing of risk is unclear, reflecting that the data arose from heterogeneous relatively small studies. There is a need for data from large prospective cohorts to better define this risk. Myeloma IX and XI are the largest randomized trials for first-line treatment of NDMM patients using regimens that include IMiDs with corticosteroids and therefore add significant new data to the literature. In addition, Myeloma IX recruited patients before the IMWG VTE prevention guidance, and XI recruited patients after the IMWG VTE prevention guidance,12 which allowed only indirect evaluation of the impact of these recommendations by comparing identical regimens used in both trials.
Both trials confirm and highlight the significant risk of thrombosis for NDMM patients, with nearly all events occurring within 6 months of treatment initiation, regardless of treatment regimen. Data from Myeloma IX allow comparison between induction regimens that use IMiDs and corticosteroids and alternative regimens. For transplant-eligible patients, it is perhaps surprising that those treated without IMiDs in regimens with CVAD had an even higher rate of thrombosis than those treated with CTD. The high rate of thrombosis for CVAD therapy for NDMM patients may in part be related to the high-dose dexamethasone and anthracycline chemotherapy (both drugs are known to contribute to VTE risk) but perhaps more importantly, the high rate may be related to the requirement for a long-term (3-6 months) central line for administration unlike the alternative oral only regimens. Of interest, in Myeloma IX, line-related VTEs were almost exclusively restricted to patients treated with CVAD and represented 37.1% of VTE events in the CVAD group. For transplant-ineligible patients, as expected, thrombosis risk was far higher for the CTDa regimen that contained IMiDs than for MP, although even patients treated with MP were at higher risk of VTE than the expected background population (<1% per year).37
Thalidomide and lenalidomide are the most commonly used IMiDs for treating myeloma. Although the drugs are structurally similar, lenalidomide is more potent and has a different adverse effect profile, but it was not previously known whether the 2 drugs had equivalent risk of thrombosis. A recent retrospective cohort (n = 2397) suggested that the risk of venous and arterial events was the same for both drugs when used to treat NDMM patients; only a few of these patients (<20%) received thromboprophylaxis.38 Data from Myeloma XI allow a direct comparison between lenalidomide and thalidomide treatment regimens for NDMM patients in a large prospective randomized NDMM patient cohort. In both transplant-eligible and ineligible patients, there was no difference in risk of VTE between regimens that contained thalidomide and lenalidomide combined and no difference in arterial event rate with CRD vs CTD. Patients receiving CRDa had a higher rate of arterial thrombosis than those treated with CTDa, but this needs to be interpreted with caution because of the low incidence of arterial events, which could also be affected by underreporting.
In the Myeloma IX trial, thalidomide maintenance did not increase the risk of thrombosis; in contrast, in the Myeloma XI trial, lenalidomide maintenance did increase the risk of venous and arterial thrombosis. However, thalidomide maintenance was delivered only for a median of 7 months in Myeloma IX, because many patients stopped treatment before progression as a result of toxicity not related to VTE.39 In contrast, within the lenalidomide maintenance phase of Myeloma XI, patients had a median of 18 cycles. Although the risk of thrombosis in the patients receiving lenalidomide was increased compared with that in the observation group, the absolute risk was low and far less than when lenalidomide was used for induction as part of CRD or CRDa regimens, probably because of the higher disease burden and additional corticosteroids in induction.
Previous data on large retrospective cohorts demonstrated that arterial and venous thrombosis were associated with inferior survival in myeloma.40 In contrast to this, in both the Myeloma IX and XI trials, VTE events were not associated with an inferior OS. It is possible that this reflects differences between clinical trial and real-world patient cohorts. Although both Myeloma clinical trials included a proportion of elderly patients with poor performance status within the transplant-ineligible pathways, this may not reflect the full spectrum of frailty and comorbidity in patients who were not part of any clinical trial. It is also important to recognize that there may be other important adverse impacts of VTE such as chronic morbidity, impaired quality of life, and psychological impact, but these have not been assessed in this study. In Myeloma XI, arterial events were associated with reduced OS, consistent with previous evidence. Thrombotic events (arterial or venous) did not adversely impact PFS, which suggests no meaningful reductions, delays, or omissions of myeloma-directed treatment resulted from the thrombotic events.
Myeloma IX recruited patients before the IMWG thrombosis prevention guideline,12 and accordingly, there were no specific recommendations for preventing thrombosis. Only a minority of patients received thromboprophylaxis, predominantly with warfarin. Myeloma XI recruited patients after the IMWG guidance was published, and the trial protocol contained recommendations for preventing thrombosis. The majority of patients received thromboprophylaxis before a thrombotic event, predominantly with LMWH and aspirin rather than warfarin. When identical treatment regimens were compared between trials (CTD and CTDa), the risk of VTE was lower in Myeloma XI compared with Myeloma IX. However, in spite of implementation of IMWG guidance and widespread thromboprophylaxis, VTE incidence remained high with only a modest reduction between trials.
In both trials, patterns of thromboprophylaxis before VTE events did not significantly differ between treatment groups. In Myeloma XI, patients identified as being at high risk for VTE before their event were more likely to be receiving thromboprophylaxis prescribed earlier, and the differences in thromboprophylaxis patterns between high-risk and low-risk patients were surprisingly small. This suggests that additional factors are being considered when making decisions regarding thromboprophylaxis, which may include patient and clinician choice, logistical difficulties with LMWH daily injections, and bleeding risk.
Overall, these findings suggest that patients with NDMM remain at an unacceptably high risk of VTE in spite of implementation of IMWG-guided thromboprophylaxis. Therefore, new approaches are needed, particularly in the initial 6 months of treatment.
For original data, please contact Charlotte Bradbury via e-mail at c.bradbury@bristol.ac.uk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients who took part in both studies as well as all the principal investigators and other study staff at more than 120 recruiting centers throughout the United Kingdom. Both trials were coordinated by the Clinical Trials Research Unit (CTRU) at the University of Leeds (Leeds, United Kingdom). The authors are grateful to all current and past members of the CTRU staff for their hard work and commitment to these studies.
This study was supported by the Medical Research Council (G0100132) for the Myeloma IX trial and by Cancer Research UK (C1298/A10410) for the National Cancer Research Institute Myeloma XI trial. Unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, Ortho Biotech, Amgen, and Merck Sharp & Dohme, and funding from Myeloma UK supported trial coordination and laboratory studies.
Authorship
Contribution: J.A.C., G.H.J., and G.J.M. were chief investigators for the Medical Research Council Myeloma IX study; G.H.J., F.E.D., and G.J.M. were chief investigators for the National Cancer Research Institute Myeloma XI study; A.H. and A.P. coordinated the data collection and regulatory and governance requirements; Z.C., D.A.C., W.M.G., and C.A.B. developed and carried out the statistical analysis plan; M.F.K., M.T.D., R.G.O., and G.J.M. coordinated the central laboratory investigations; G.H.J., F.E.D., C.P., J.R.J., M.W.J., G.C., M.F.K., R.G.O., and G.J.M. helped recruit patients; M.F.K., M.T.D., R.G.O., and G.J.M. coordinated the central laboratory investigations; C.A.B., Z.C., C.P., G.C., and G.H.J. developed the first drafts of the manuscript; and all authors contributed to reviewing and amending the manuscript for important intellectual content and approved the final version for submission.
Conflict-of-interest disclosure: C.A.B. received consultancy fees, honoraria, and speakers’ bureau fees from BMS, Pfizer, Novartis, Janssen, and Ablynx and funds to attend conferences from Bayer, Novartis, and Amgen. Z.C. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. G.C. received consultancy fees, honoraria, research funding, and speakers’ bureau fees from Takeda, Celgene, Janssen, and Amgen; consultancy fees and honoraria from Glycomimetics and Bristol-Myers Squibb; and consultancy fees, honoraria, and speakers’ bureau fees from Sanofi. C.P. received grants from Celgene. during the study and personal fees and nonfinancial support from Amgen, Takeda Oncology, Janssen, and Celgene outside the submitted work. D.A.C. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. A.H. received grants and nonfinancial support from Celgene, Merck Sharpe & Dohme, Amgen, and Takeda. M.W.J. received consultancy fees, honoraria, travel support, and research funding from Janssen; consultancy fees, honoraria, and travel support from Takeda and Amgen; consultancy fees, honoraria, and research funding from Celgene; and consultancy fees and honoraria from Novartis. J.R.J. received honoraria and research funding from Celgene. M.T.D. has equity ownership in and is on the board of directors and advisory committee for Abingdon Health. R.G.O. received honoraria and travel support from Takeda; consultancy fees and travel support from Janssen; and consultancy fees, honoraria, and research funding from Celgene Corporation. M.F.K. received consultancy fees and travel support from Bristol-Myers Squibb and Takeda; consultancy fees from Chugai; consultancy fees and honoraria from Janssen and Amgen; and consultancy fees, honoraria, and research funding from Celgene. W.M.G. received consultancy fees and research funding from Celgene; research funding from Amgen and Merck Sharp and Dohme; and honoraria from Janssen. F.E.D. received consultancy fees and honoraria from Amgen, AbbVie, Takeda, Janssen, and Celgene. G.J.M. received research funding from Janssen; consultancy fees and honoraria from Bristol-Myers Squibb and Takeda; and consultancy fees, honoraria, and research funding from Celgene. G.H.J. received consultancy fees, honoraria and speakers’ bureau fees from Roche, Amgen, Janssen, and Merck Sharp and Dohme; and consultancy fees, honoraria, travel support, research funding, and speakers’ bureau fees from Celgene and Takeda. The remaining authors declare no competing financial interests.
A complete list of the members of the United Kingdom National Cancer Research Institute Haemato-oncology Clinical Studies Group appears in “Appendix.”
Correspondence: Charlotte A. Bradbury, Bristol Haematology and Oncology Centre, Horfield Rd, Bristol BS2 8ED, United Kingdom; e-mail: c.bradbury@bristol.ac.uk.
Appendix
The members of the United Kingdom National Cancer Research Institute Haemato-oncology Clinical Studies Group are: Peter Hillman, Lesley Anderson, Stephen O’Brien, Jim Cavet, Oliver Ottman, Alan Chant, Alasdair Rankin, Gordon Cook, Clare Rowntree, Charles Craddock, Anna Schuh, Lavinia Davey, Shamyla Siddique, Walter Gregory, Simon Stanworth, Sally Killick, Simon Watt, Amy Kirkwood, Kwee Yong, Dragana Milojkovic, Thomas Fox, Adam Mead, Gillian Horne, Gillian Murphy, and Kikkeri Naresh.