Key Points
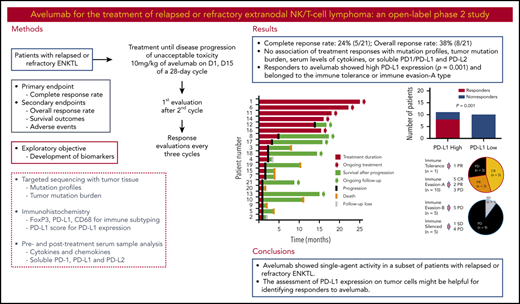
Avelumab showed single-agent activity in a subset of patients with relapsed or refractory extranodal natural killer/T-cell lymphoma.
The assessment of PD-L1 expression and immune subtyping could help to identify responders to avelumab.
Abstract
This study aimed to assess the efficacy and safety of treatment with avelumab, an anti–programmed death ligand 1 (PD-L1) antibody, in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma (ENKTL). In this phase 2 trial, 21 patients with relapsed or refractory ENKTL were treated with 10 mg/kg of avelumab on days 1 and 15 of a 28-day cycle. The primary end point was the complete response (CR) rate based on the best response. Targeted sequencing and immunohistochemistry were performed using pretreatment tumor tissue, and blood samples were drawn before and after treatment for measurement of cytokines and soluble programmed cell death protein 1 (PD1), PD-L1, and PD-L2. The CR rate was 24% (5 of 21), and the overall response rate was 38% (8 of 21). Although nonresponders showed early progression, 5 responders currently continue to receive treatment and have maintained their response. Most treatment-related adverse events were grade 1 or 2; no grade 4 adverse events were observed. Treatment responses did not correlate with mutation profiles, tumor mutation burden, serum levels of cytokines, or soluble PD1/PD-L1 and PD-L2. However, the response to avelumab was significantly associated with the expression of PD-L1 by tumor tissue (P = .001). Therefore, all patients achieving CR showed high PD-L1 expression, and their tumor subtyping based on PD-L1 expression correlated with treatment response. In summary, avelumab showed single-agent activity in a subset of patients with relapsed or refractory ENKTL. The assessment of PD-L1 expression on tumor cells might be helpful for identifying responders to avelumab. This trial was registered at www.clinicaltrials.gov as #NCT03439501.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma (ENKTL) is an aggressive malignancy that arises from NK or cytotoxic T cells associated with Epstein-Barr virus (EBV) infection and is more frequently encountered in East Asia and Central America than in Western countries.1,2 In a recent prospective cohort study from the international T-cell project, ENKTL comprised 11% of 1553 eligible registered cases, and the frequency of ENKTL was also higher in Asian countries than in Europe and the United States.3 ENKTL is resistant to anthracycline because of its expression of p-glycoprotein; thus, non–anthracycline-based chemotherapy incorporating l-asparaginase has been the primary treatment of patients newly diagnosed with ENKTL.4-6 However, even with this treatment, the international multicenter retrospective study establishing the prognostic index for NK cell lymphoma-EBV (PINK-E) showed that the 3-year overall survival (OS) of patients at high risk of PINK-E remains less than 30%.7 The poor prognosis of high-risk patients is related to the limited salvage treatment options available. Thus, once patients relapse or become refractory to the first-line treatment, their treatment outcomes are extremely poor, even after allogeneic stem cell transplantation (SCT).8,9
Recently, immune checkpoint inhibitors have been proposed as an alternative treatment option for relapsed or refractory ENKTL. EBV-infected tumor cells frequently express programmed death ligand 1 (PD-L1), the binding of which to programmed cell death protein 1 (PD1) on T cells transduces inhibitory signals to the T cells, resulting in immune escape of tumor cells.10,11 Therefore, inhibition of the interaction between PD1 and PD-L1 could reverse this suppression of T-cell cytotoxicity. Previous studies using pembrolizumab, an immunoglobulin G4 anti-PD1 antibody, have shown that it has single-agent activity in patients with relapsed or refractory ENKTL.12,13 However, not all patients respond to pembrolizumab, and some patients with ENKTL show rapid disease progression during pembrolizumab treatment.13 Our recent comprehensive molecular analysis of ENKTL suggested 4 tumor immune subtypes based on the immune shift. Thus, the tumor immune microenvironment of ENKTL might change from immune tolerance to immune evasion and silence during disease progression, and the groups with enriched PD-L1 expression and activated immune cells, immune tolerance, and immune evasion-A groups showed a better response to pembrolizumab.14 Given the association of PD-L1 expression and the outcome of pembrolizumab treatment in ENKTL patients, blockade of PD-L1 may be a better therapeutic approach than blockade of PD1, as indicated by the accelerated approval of avelumab, an anti–PD-L1 immunoglobulin G1 monoclonal antibody, for treatment of metastatic Merkel cell carcinoma.15 However, the data regarding the outcome of treatment with PD-L1 inhibitors compared with PD1 inhibitors are sparse, especially for T-cell or NK-cell lymphomas, although several clinical trials of PD-L1–blocking antibodies, including atezolizumab, durvalumab, and avelumab, are underway in B-cell lymphoma patients.16 Therefore, we conducted a phase 2 study to evaluate the efficacy of avelumab in patients with relapsed or refractory ENKTL. Here, we report their outcomes together with the results of comprehensive genetic and immunologic analyses of the responders to identify biomarkers predicting responsiveness to avelumab.
Patients and methods
Study design and patient population
This study was a phase 2, single-arm trial that investigated the efficacy of avelumab in patients with relapsed or refractory ENKTL. It was conducted at 2 institutes: Samsung Medical Center and Seoul National University Hospital within the Consortium for Improving Survival of Lymphoma.17 Patients who fulfilled the following inclusion criteria were enrolled: (1) histologically confirmed ENKTL; (2) relapsed or refractory disease after at least 1 type of chemotherapy regimen; (3) performance status ≤ grade 2 on the Eastern Cooperative Oncology Group (ECOG) scale; (4) age ≥18 years; (5) more than 1 measurable lesion on computed tomography (CT) or positron-emission tomography (PET)/CT scan (patients who had only unmeasurable lesions on CT or PET/CT [eg, bone marrow involvement or malignant effusion] were assessed by an investigator to determine whether these were associated with relapse or disease progression; based on the investigator’s decision, these patients could be enrolled); (6) serum creatinine <2.0 × upper limit of normal (ULN), transaminases <3.0 × ULN, and bilirubin <2.0 × ULN (if the values for liver function tests above these criteria were related to liver involvement by disease, a patient could be enrolled based on the investigator’s decision if his or her values were ≤5.0 × ULN); and (7) absolute neutrophil count ≥1000/mm3, platelets ≥50 000/mm3, and hemoglobin ≥8.0 g/dL. The exclusion criteria were as follows: patients with secondary central nervous system involvement detected on CT or magnetic resonance imaging scan and patients who had previously undergone allogeneic SCT. The use of systemic high-dose steroids before enrollment was not allowed except for (1) intranasal, inhaled, topical steroids, or local steroid injection (eg, intra-articular injection); (2) corticosteroids at ≤10 mg/day of prednisone or equivalent; or (3) steroids as premedication for hypersensitivity reactions (eg, CT scan premedication). The last date of data update, including survival and disease status, was 29 February 2020. Written informed consents were obtained from all patients before study enrollment, and the institutional review boards of the participating institutes approved this phase 2 study, which was registered at www.clinicaltrials.gov as NCT03439501.
Treatments and assessments
The treatment was intravenous infusion of avelumab at 10 mg/kg body weight on days 1 and 15 of a 28-day cycle. Treatment continued until either withdrawal of consent, an unacceptable adverse event (AE), disease progression, or investigator decision. The first response evaluation by PET/CT and CT scan was done after day 15 of the second cycle based on the recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma (the Lugano classification).18 If complete response (CR), partial response (PR), or stable disease (SD) was documented, the treatment was continued, and the response evaluation was repeated every 3 cycles (CT scan was mandatory, but PET/CT scan was optional) after the first until progressive disease (PD) was documented. Safety was monitored throughout the study, and AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Dose modification was not permitted for avelumab because of hematologic toxicity. However, administration of avelumab was withheld in cases of grade 3 hematologic and nonhematologic toxicities until they resolved to grade 0 or 1.
End points
The primary end point was the CR rate, which was based on the best response during the study period. The secondary end points included the overall response rate (ORR), which was defined as the percentage of the total enrolled patients who achieved CR or PR; OS, which was calculated from the date of enrollment to final follow-up or death from any cause; progression-free survival (PFS), which was calculated from the date of enrollment to the date of disease progression or death from any cause; and safety profiles. To identify a biomarker predicting response, we assessed PD-L1 expression by immunohistochemistry and analyzed mutation profiles by targeted-probe sequencing using formalin-fixed paraffin-embedded (FFPE) tumor tissue that was archived at diagnosis or before avelumab treatment. We also measured serum levels of cytokines and soluble proteins using pre-and posttreatment serum samples.
Clinical risk factor analysis
Pretreatment clinical and laboratory parameters at the time of enrollment, including age, sex, Ann Arbor stage, involved nodal and extranodal sites, serum lactate dehydrogenase, and blood EBV DNA titer, were collected and analyzed in conjunction with treatment response. Based on the PINK-E, which is composed of 5 variables (age >60 years, stage III/IV, distant lymph node involvement, nonnasal type, and blood EBV DNA), patients were stratified into low- (none or 1 factor), intermediate- (2 factors), and high-risk groups (≥3 factors).7 Pretreatment disease status was dichotomized into relapsed disease, which was designated as relapsed disease occurring during follow-up, and refractory disease, which was designated as disease refractory to the last treatment before enrollment.
In situ hybridization and immunohistochemistry
All patients were pathologically diagnosed with ENKTL based on a positive result for EBV in situ hybridization, and the pathology was centrally reviewed by 2 pathologists (K.Y.H. and J.H.). Immunohistochemistry was performed for PD-L1, FoxP3, and CD68 as described in supplemental Table 1, available on the Blood Web site. Tumor cells expressing PD-L1 and PD-L1–positive tumor-associated macrophages (TAMs) were evaluated because the expression of PD-L1 in TAMs was reported in ENKTL.19 To represent the extent of PD-L1 expression where tumor cells were present, the percentage of total PD-L1–positive cells including PD-L1–positive tumor cells and TAMs among tumor cells was calculated as a PD-L1 score, and PD-L1 score >10 was designated as high PD-L1 expression based on our previous study for immune subtyping of ENKTL.14 The number of FoxP3-positive cells in one high-power field area was counted by light microscopy, and the patients with more than 500 FoxP3-positive cells/high-power field in a hot spot were classified as the high–T regulatory cell group. The CD68 stain was used to evaluate whether the cytoplasm of TAMS was abundant (granular) or scanty (process type). Based on the results of FoxP3, PD-L1, and CD68 staining, tumor immune microenvironment (TIME) subgroups of ENKTL were determined by the previously described algorithm as immune tolerance (IT), immune evasion-A/B (IE-A/B), and immune silenced (IS) subgroups.14
Targeted-probe sequencing and tumor mutation burden
Genomic DNA was extracted from the archival FFPE tissues, and the library was generated from 10 to 200 ng DNA for the Illumina Paired-End Sequencing Library according to the manufacturer’s instructions. The RNA-based probe was designed with SureDesign (Agilent Technologies) to target-capture 708 genes (supplemental Table 2). The targeted option was used when targeted-capture sequencing data were analyzed. The tumor mutation burden was calculated as nonsilent mutational load per mega base pairs. The detailed information about the targeted-probe sequencing and variant calling is described in supplemental Methods.
Measurement of cytokines and soluble proteins
Pre- and posttreatment serum samples were collected before the first cycle of avelumab treatment and after the completion of the second cycle and stored at –80°C. Cytokines were measured using the Procarta Cytokine Profiling Kit (Human ProcartaPlex, Panomics, San Diego, CA) to detect 34 cytokines and chemokines simultaneously (supplemental Table 3). All measurements were performed in duplicate according to the manufacturer’s instructions. Serum levels of PD1, PD-L1, and PD-L2 were measured as described in the supplemental Methods.
Statistical analyses
Sample size was calculated to reject the null hypothesis with a 20% CR rate but was increased to favor a target rate of 40%, with α significance of 0.05 and power of 80% using Simon’s Minimax 2-stage design.20 Thus, if the CR rate was ≤4/18 during the first stage, the study would be stopped. If not, patient recruitment would continue until the number reached 33, and given the 10% dropout rate, the final target number would be 37. The χ2 test was used to analyze the association of molecular and laboratory parameters with treatment response. The Wilcoxon rank-sum 2-tailed test was used for comparison of gene mutation profiles in responders and nonresponders. The median potential follow-up time with 95% confidence intervals (CIs) was determined using the Kaplan-Meier method.21 Survival was estimated based on Kaplan-Meier curves and compared using the log-rank test. Two-sided statistical tests yielding P < .05 were considered significant. Statistical analyses were performed using the IBM PASW version 24.0 software program (SPSS Inc., Chicago, IL).
Results
Patient characteristics
A total of 21 patients with a median age of 54 years (range, 24-78 years) were enrolled between January 2018 and April 2019. The ECOG performance of all but 1 patient was grade 0 or 1 (n = 20), and 17 patients had stage IV disease at the time of enrollment. The PINK-E–based risk classification before avelumab treatment showed that 11 patients were at high risk; the remaining patients were classified as intermediate (n = 6) and low (n = 4) risk (Table 1). All patients were initially treated with non–anthracycline-based chemotherapy, and 14 patients received at least 2 lines of treatment before participating in the study. Five patients relapsed after autologous SCT, and 13 patients were refractory to their most recent treatments. Detailed information about previous treatments is summarized in supplemental Table 4. The categorization of patients according to TIME immune subtyping showed IT (n = 1), IE-A (n = 10), IE-B (n = 5), and IS (n = 5) (Table 1; supplemental Figure 1A). The distribution of stage and PINK-E risk did not differ significantly between immune subtypes (supplemental Figure 1B).
Response and safety
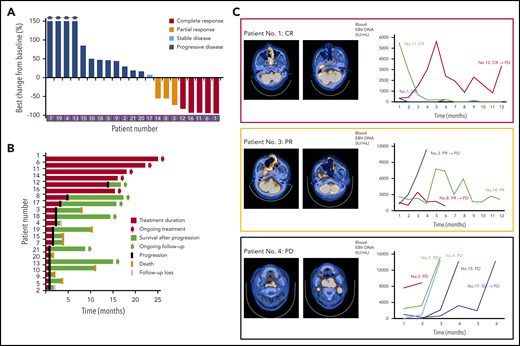
The first response evaluation showed 7 responders (CR [n = 2], PR [n = 5]) and 2 patients with SD. However, the remaining patients (n = 12) did not respond to treatment, resulting in disease progression at the time of first evaluation. During subsequent response evaluations with repeated treatment, 3 patients with PR achieved CR and 1 patient with SD showed PR. As a result, the CR rate was 24% (5 of 21), and the ORR based on the best response was 38% (8 of 21), although this study did not meet the primary end point because of early discontinuation of the trial before enrolling the planned number of patients (Figure 1A). At the time of analysis, 5 patients were continuing treatment (median number of cycle: 18; range: 13-25) and maintaining their response, and 8 patients died because of disease progression (Figure 1B). The EBV DNA titer in whole blood was correlated with response to avelumab in most patients (Figure 1C). Thus, patients responding to avelumab showed decrease of blood EBV DNA, whereas patients having disease progression showed rapid elevation of EBV DNA (Figure 1C). Most treatment-related AEs were grade 1 or 2; no grade 4 AEs were observed (Table 2). Grade 3 AEs, including neutropenia (10%, 2 of 21), thrombocytopenia (5%, 1 of 21), fatigue (5%, 1 of 21), infusion-related reaction (5%, 1 of 21), and sore throat (5%, 1 of 21), were manageable; therefore, there was no discontinuation of treatment because of these AEs. With a median follow-up of 15.7 months (95% CI: 14.5-16.9 months), the median PFS was 2.7 months (95% CI: 1.35-4.05 months) because of frequent early progression, but the median OS was not reached because of subsequent salvage treatments.
Treatment outcome of avelumab in relapsed or refractory ENKTL. (A) Waterfall plot of 21 patients. (B) Swimmer plot according to treatment duration and survival. (C) Representative images of patients showing complete and partial response and progression and the serial changes of EBV DNA titer in whole blood.
Treatment outcome of avelumab in relapsed or refractory ENKTL. (A) Waterfall plot of 21 patients. (B) Swimmer plot according to treatment duration and survival. (C) Representative images of patients showing complete and partial response and progression and the serial changes of EBV DNA titer in whole blood.
Comprehensive characterization of responders and nonresponders
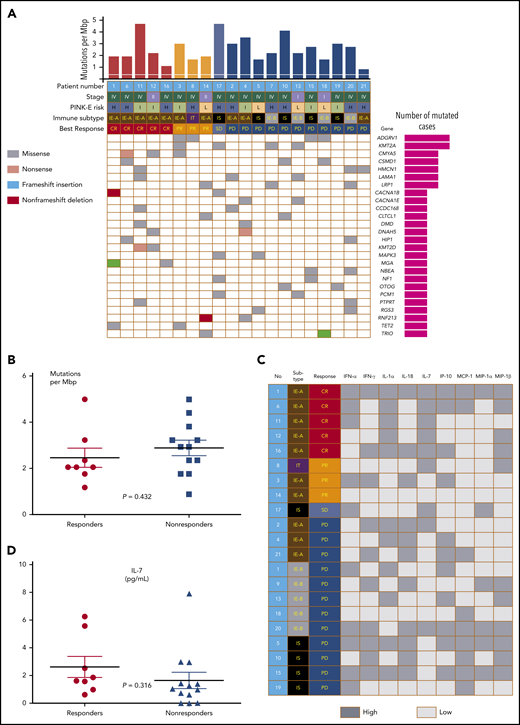
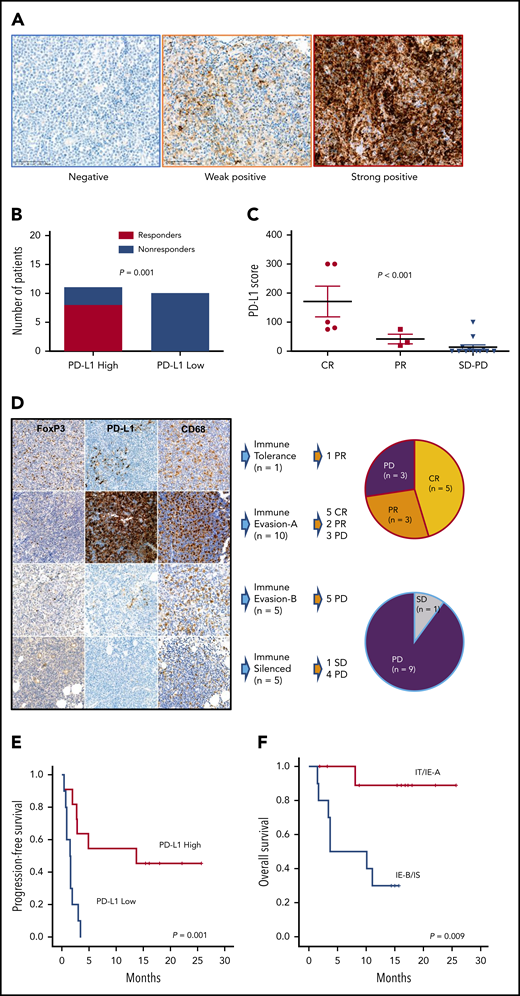
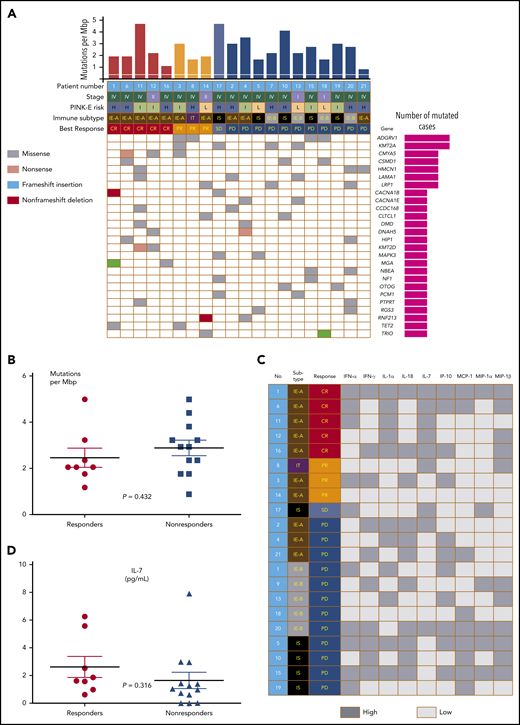
The response to avelumab was not associated with clinical characteristics such as stage I/II vs III/IV, primary refractory disease vs relapsed disease, blood EBV DNA levels, and PINK-E risk (Table 1; supplemental Figure 1C-D). The level of PD-L1 expression by tumor tissue was variable from negative to strongly positive (Figure 2A). When patients were dichotomized as PD-L1 high (n = 11) and low (n = 10) according to their PD-L1 score (>10 vs ≤10), the number of responders to avelumab was significantly higher in patients with high PD-L1 (8 of 11, 73%) than patients with low PD-L1 (0 of 10, 0%) expression (Figure 2B; P = .001), and all patients achieving CR (n = 5) showed higher PD-L1 scores than those with PR and SD/PD (Figure 2C; P < .001). Because high PD-L1–expressing tumor cells were enriched in the IE-A subtype compared with other immune subtypes, the IE-A type (n = 10) showed 5 CR and 2 PR (7 of 10, 70%,); however, none of the patients classified as IE-B (n = 5)/IS (n = 5) achieved CR or PR (P = .022; Figure 2D). Accordingly, the PFS of patients with high PD-L1 was significantly better than that of patients with low PD-L1 (Figure 2E). When patients were dichotomized into IT/IE-A and IE-B/IS groups, the IT/IE-A groups showed significantly better OS than patients in the IE-B/IS groups (Figure 2F; P = .009). Targeted sequencing using pretreatment FFPE tumor tissue from 20 patients, after excluding 1 case (no. 9) for whom the tumor tissue was not available for sequencing, showed high mutation rates in 2 genes (4 of 20; 20%): ADGRV1, also known as GPR98, G-protein coupled receptor 98, and KMT2A, known as MLL1, an important epigenetic regulator (Figure 3A). Although MGA (nos. 1, 16) and KMT2D (nos. 11, 12) were mutated only in patients who achieved CR, this did not reach statistical significance. Similarly, mutations in CMYA5 (nos. 3, 6, 12) and TET2 (nos. 1, 3) were also identified in the responders but did not reach significance. There was no significant difference in tumor mutation burden between responders and nonresponders (Figure 3B; P = .167). Pretreatment serum (s) levels of sPD1, sPD-L1, and sPD-L2 and the comparison of pre- and posttreatment sPD1 did not show an association with treatment response (supplementa Figure 1E-F). Although cytokines were detected in pretreatment serum from more than half of the patients, these cytokine levels were not associated with response to avelumab (Figure 3C). Although the number of patients with high interleukin-7 (IL-7) levels (above the median value) was higher in responders, no significant difference was found between responders and nonresponders (Figure 3D).
PD-L1 expression and immune subtypes of responders to avelumab. (A) Images of PD-L1–negative, focal weak positive, and diffuse strong positive cases by immunohistochemistry. (B) Comparison of responders and nonresponders based on high and low PD-L1 expression (PD-L1 score >10 vs ≤10). (C) Distribution of PD-L1 scores according to responses. (D) Association of response and immune subtypes: representative immunohistochemistry images of each TIME subgroup. FoxP3-positive regulatory T cells were very abundant in IT but then decreased rapidly. PD-L1 expression was highest in IE-A patients. The CD68-positive macrophages of IS showed a unique morphology of the process type. (E) Progression-free survival of high and low PD-L1 patients. (F) Overall survival of 4 immune subtypes.
PD-L1 expression and immune subtypes of responders to avelumab. (A) Images of PD-L1–negative, focal weak positive, and diffuse strong positive cases by immunohistochemistry. (B) Comparison of responders and nonresponders based on high and low PD-L1 expression (PD-L1 score >10 vs ≤10). (C) Distribution of PD-L1 scores according to responses. (D) Association of response and immune subtypes: representative immunohistochemistry images of each TIME subgroup. FoxP3-positive regulatory T cells were very abundant in IT but then decreased rapidly. PD-L1 expression was highest in IE-A patients. The CD68-positive macrophages of IS showed a unique morphology of the process type. (E) Progression-free survival of high and low PD-L1 patients. (F) Overall survival of 4 immune subtypes.
Tumor mutations and serum cytokines of responders and nonresponders. (A) Comparison of mutation profiles according to responses. (B) Tumor mutation burden of responders and nonresponders. (C) Comparison of cytokines and chemokines according to responses. (D) IL-7 levels of responders and nonresponders.
Tumor mutations and serum cytokines of responders and nonresponders. (A) Comparison of mutation profiles according to responses. (B) Tumor mutation burden of responders and nonresponders. (C) Comparison of cytokines and chemokines according to responses. (D) IL-7 levels of responders and nonresponders.
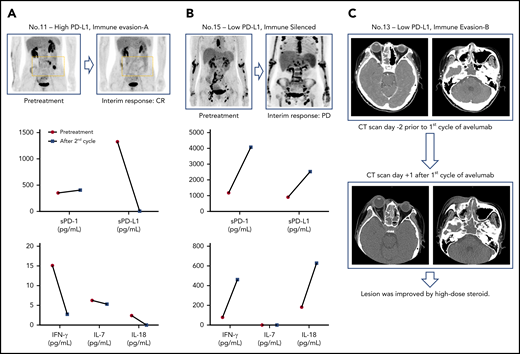
The comparison of pre-and posttreatment interferon-γ (IFN-γ) showed a tendency to a decrease in IFN-γ in responders after avelumab treatment (eg, the patient achieving CR, no. 11; Figure 4A; supplemental Figure 1G), whereas the serum level of sPD-L1, IFN-γ, and IL-18 were markedly increased in a patient showing rapid progression after the second cycle of avelumab (Figure 4B). Similarly, 1 patient showed signs of disease progression immediately after the first dose of avelumab; however, this patient had low expression of PD-L1 in their tumor tissue and nondetectable pretreatment serum sPD-L1 (no. 13; Figure 4C). This patient was excluded from the trial, and the symptoms subsided with high-dose steroid administration. The patient was rescued by subsequent salvage treatments and was being followed up at the time of analysis (Figure 1B).
Posttreatment changes of serum cytokines and a case of flare reaction. (A-B) Serial assessment of sPD1 and sPD-L1 and cytokines in patients showing complete response and disease progression. (C) A case of flare reaction occurring immediately after the first dose of avelumab.
Posttreatment changes of serum cytokines and a case of flare reaction. (A-B) Serial assessment of sPD1 and sPD-L1 and cytokines in patients showing complete response and disease progression. (C) A case of flare reaction occurring immediately after the first dose of avelumab.
Discussion
Our study analyzed the efficacy and safety of treatment with the anti–PD-L1 antibody avelumab and explored biomarkers predicting response to avelumab in patients with relapsed or refractory ENKTL. Although the study was terminated early because of a lower response rate than expected, the blockade of PD-L1 by avelumab could induce CR in a subset of patients, leading to a CR rate of 24% (5 of 21) and an ORR of 38% (8 of 21). The safety and tolerability of avelumab were acceptable, and all AEs could be resolved by appropriate supportive therapy. Although the number of patients achieving CR was lower than expected, the response was remarkably durable, with the longest response duration being greater than 25 months (Figure 1B). These results implied that avelumab could induce prolonged remission if patients respond to this drug. Thus, the selection of patients with ENKTL who have a high probability of responding to avelumab might be important to improve the efficacy of avelumab.
A comprehensive analysis of responders showed that the response to avelumab was not associated with clinical parameters such as stage, PINK-E risk, and refractoriness to previous treatments (Table 1). However, PD-L1 expression in tumor area was significantly higher in responders than in nonresponders, and all patients achieving CR had high PD-L1 expression (Figure 2B-C). Consistent with this finding, immune subtyping based on the enrichment of PD-L1 expression and effector cells showed a close relationship with the response to avelumab. Therefore, better response and survival outcomes were observed in the IT/IE-A compared with the IE-B/IS groups (Figure 2E-F). A mutation analysis showed different frequencies of ADGRV1 and KMT2A than those reported in previous studies of ENKTL, and mutations of KMT2D, MGA, TET2, and CMYA5 were found only in responders.22,23 However, our analysis failed to identify mutation profiles predicting a response to avelumab, and the tumor mutation burden was not related to the response to avelumab (Figure 3A-B). In this study, we also analyzed pre- and posttreatment serum levels of sPD-L1 and sPD1 because a correlation of sPD-L1 level with PD-L1 expression by lymphoma cells was previously reported in patients with ENKTL.19 Furthermore, sPD1 has been reported to promote T-cell responses through blocking the interactions between PD-L1 and PD1.24 However, the pretreatment serum levels of sPD-L1 and sPD1 failed to show a significant association with the response in our study. Because IFN-γ secreted by lymphoma cells can influence PD-L1 expression of ENKTL,22 and γ-chain cytokines such as IL-2, IL-7, IL-15, and IL-21 can induce PD1 and PD-L1 expression,25 we analyzed their association with response to avelumab. We found no difference in the cytokine profiles between responders and nonresponders (Figure 3C-D). Thus, based on our findings, we suggest that the use of the PD-L1 inhibitor avelumab should be considered in the context of PD-L1 expression by tumor tissue and the immune subtype of ENKTL.
One patient who showed rapid disease progression after avelumab treatment had a marked increase of serum sPD-L1 and IFN-γ, which differed from the findings in the patient achieving CR at the interim response evaluation (Figure 4A-B). This rapid progression might be associated with high serum IFN-γ because of the upregulation of IFN-γ receptor 1 (IFNGR1) identified in a previous gene expression profile of ENKTL,26 and the binding of IFN-γ to IFNGR1 was reported to activate the Janus kinase/signal transducer and activator of transcription pathway in ENKTL.27 Furthermore, IFN-γ is a key component of hemophagocytosis, a frequently occurring phenomenon during disease progression of ENKTL.28 Indeed, the patient showed an elevation of IL-18 that was related to hemophagocytosis. The other patient also experienced a flare reaction immediately after the first dose of avelumab (Figure 4C). These events seemed to be similar to a recent observation reporting rapid disease progression in 3 patients with adult T-cell leukemia/lymphoma (ATLL) after they received nivolumab.29 As an underlying mechanism for hyperprogression of ATLL, PD1 blockade could drive expression of additional checkpoints that promote immune suppressive activity and induce expression of ATLL-promoting growth factors, among several suggested mechanisms.30 Considering the pre- and posttreatment cytokine changes in our patients, the blockade of PD-L1 might induce hyperprogression in a subgroup of patients. However, more clinical experience is required to clarify this phenomenon and its underlying mechanism.
According to the final results of this study, the outcomes of avelumab were worse than that of previous studies reporting promising single-agent activity of pembrolizumab in relapsed or refractory ENKTL.12,13 However, it was difficult to directly compare ours with previous results because those studies were retrospective analyses that could be influenced by selection bias. Furthermore, there are some limitations interpreting the efficacy of avelumab based on our results because of early discontinuation of trial before enrolling the planned number of patients. Although the first evaluation after the second cycle might be too early to determine the response to avelumab because of the possibility of pseudo-progression, and this design might influence the failure of our study to meet the primary end point, most patients with early progression showed clinical deterioration and/or rapid elevation of EBV DNA titer in blood (Figure 1C), implying that they were less likely to have pseudo-progression. Thus, our results suggested that the achievement of early response might have significant clinical relevance in ENKTL patients receiving avelumab, and this drug should be cautiously used for selected patients who are more likely to respond according to the expression of PD-L1 expression.
In summary, avelumab showed single-agent activity in a subset of patients with relapsed or refractory ENKTL; therefore, the assessment of PD-L1 expression and immune subtyping might be helpful for identifying patients more likely to respond to this drug. However, more studies focusing on PD-L1 high-expressing ENKTL patients are needed.
For original data from this study, please e-mail the corresponding author at wskimsmc@skku.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by research funding from Merck, Inc., and grants from the following: Singapore Ministry of Health National Medical Research Council (NMRC-OFLCG-18May0028 and NMRC-ORIRG16nov090), Tanoto Foundation Professorship in Medical Oncology and Ling Foundation, the 20-20 Project of Samsung Medical Center, and the National Research Foundation of Korea (NRF-2017R1A2B4005136).
The investigational product (avelumab) was donated by Merck, Inc. Merck KGaA (Darmstadt, Germany), and Pfizer reviewed the manuscript for medical accuracy only before journal submission. The authors are fully responsible for the contents of this manuscript, and the views and opinions described in the publication reflect solely those of authors.
Authorship
Contribution: S.J.K. wrote the manuscript; W.S.K. performed the trial as a principal investigator; S.J.K. and W.S.K. designed the trial and analyzed the data; J.Q.L., Y.L., S.T.L., and C.K.O. performed targeted-probe sequencing and analyzed the data; J.C. and Y.H.K. reviewed the pathology and analyzed the results of immunohistochemistry; S.E.Y. and Y.K. participated in the trial and analyzed the clinical data; J.Y.L., K.J.R., and D.C. participated in the cytokine analysis and laboratory investigation; and M.B.E., F.D., and M.B. analyzed soluble PD-L1/2 and PD1.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Won Seog Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea; e-mail: wskimsmc@skku.edu.