Abstract
Hematopoietic transplantation is the preferred treatment for many patients with hematologic malignancies. Some patients may develop invasive fungal diseases (IFDs) during initial chemotherapy, which need to be considered when assessing patients for transplantation and treatment posttransplantation. Given the associated high risk of relapse and mortality in the post–hematopoietic stem cell transplantation (HSCT) period, IFDs, especially invasive mold diseases, were historically considered a contraindication for HSCT. Over the last 3 decades, advances in antifungal drugs and early diagnosis have improved IFD outcomes, and HSCT in patients with a recent IFD has become increasingly common. However, an organized approach for performing transplantation in patients with a prior IFD is scarce, and decisions are highly individualized. Patient-, malignancy-, transplantation procedure–, antifungal treatment–, and fungus-specific issues affect the risk of IFD relapse. Effective surveillance to detect IFD relapse post-HSCT and careful drug selection for antifungal prophylaxis are of paramount importance. Antifungal drugs have their own toxicities and interact with immunosuppressive drugs such as calcineurin inhibitors. Immune adjunct cytokine or cellular therapy and surgery can be considered in selected cases. In this review, we critically evaluate these factors and provide guidance for the complex decision making involved in the peri-HSCT management of these patients.
Introduction
Invasive fungal diseases (IFDs), invasive candidiasis (IC) in the 1990s, and subsequently invasive infections caused by Aspergillus or other molds have been feared complications of treatment for malignant hematologic disease.1-3 In the era of azole prophylaxis, IC is less common, and invasive pulmonary aspergillosis (IPA) is currently the most common IFD in patients with hematologic malignancies. In the last 2 decades, IPA has been typically diagnosed earlier based on prompt use of high-resolution computed tomography (CT), early bronchoscopy and bronchoalveolar lavage (BAL), and serum fungal biomarkers such as galactomannan.4,5 Historically, a prior IFD, especially IPA, was considered a contraindication for hematopoietic stem-cell transplantation (HSCT) because of studies showing high rates of IFD relapse and fungal-related mortality post-HSCT.6-8 However, earlier diagnosis and the introduction of potent broad-spectrum antifungals such as the triazoles have significantly improved the prognosis of patients with IFDs. In this context, HSCT in patients with a prior IFD has become routine practice.9-12
Because autologous HSCT is associated with lesser immunosuppression (short neutropenia duration, no risk for graft-versus-host disease [GVHD]), we focus on patients undergoing allogeneic HSCT (allo-HSCT) with a history of IFD, with an emphasis on IPA as a complication of prior cytotoxic chemotherapy.
Patient
A 65-year-old patient with acute myeloid leukemia is evaluated for HSCT. He has underlying obesity, type 2 diabetes mellitus, and chronic renal insufficiency. Two months ago, after consolidation chemotherapy, he developed IPA diagnosed by positive serum galactomannan and chest CT showing 3 nodules (1 dominant, 2 smaller). He received 4 weeks of voriconazole (VRC) with improvement of all lung nodules by ∼50% to 60% by follow-up chest CT. He currently has no associated signs or symptoms of IPA. Repeated serum Aspergillus galactomannan is negative. He continues to receive VRC, with a serum level of 2.5 mg/dL. Performance status is 1, serum creatinine is 1.8 mg/dL, and white blood cell count is 2.5 × 103/μL (absolute neutrophil count, 1650 per μL), with mild elevation of transaminases. He has a suitable matched unrelated donor and is in complete remission (CR) but is at high risk for relapse based on cytogenetics. He is cytomegalovirus (CMV) immunoglobulin G positive.
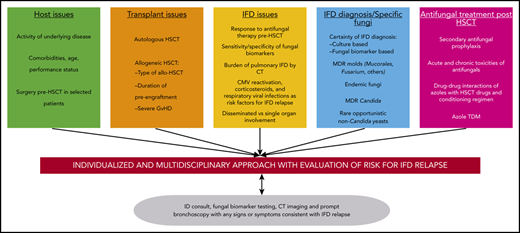
What are the issues surrounding the decision to proceed with transplantation in this scenario? There are few modern cohorts of patients with prior IFDs undergoing HSCT (Table 1). In this review, we aim to critically evaluate the many host-, procedure- (SCT), fungal-, and antifungal treatment–related issues (Table 2) and provide guidance on the complex decision making involved in the peritransplantation management of these patients.
Representative studies regarding HSCT in patients with prior IFD
| Study type and IFD before HSCT . | n . | Post-HSCT IFD relapse . | Percentage and type of secondary antifungal prophylaxis . | Main results . | Reference and year of publication* . |
|---|---|---|---|---|---|
| Prior IA: 10 proven and 38 probable; retrospective | 48 | 33% at 1 y | 85%†: 69% AMB based, 33% itraconazole | No RFs for IA relapse was identified | 8; 1998 |
| 88% mortality among relapsed IA | |||||
| Prior IA: 32 proven, 5 probable, 8 possible; retrospective | 42 | 29% vs 10% (if prior IA) at 1 y | NS | RFs for IA relapse: <30 d of antifungal therapy, BM or cord blood as source of stem cells, engraftment failure, radiologic persistence | 69; 2004 |
| Patients with prior IA had lower OS (56% vs 77%) and higher transplantation-related mortality (38% vs 21%) | |||||
| Prior IA: 49 proven, 80 probable; retrospective | 129 | 22% at 2 y | 95%†: 72% azoles,‡ 45% AMB based, 20% echinocandins | RFs for IA relapse: >20 d of neutropenia, advanced status of underlying hematologic malignancy, <6 wk from IA to HSCT, myeloablative conditioning, CMV disease, BM or cord blood as source of stem cells | 27; 2006 |
| Low risk: 0-1 RF, 6% incidence; intermediate risk: 2-3 RFs, 27% incidence; high risk: >3 RFs, 72% incidence | |||||
| Prior IFD: 18 IA, 8 invasive candidiasis, 23 undetermined; retrospective | 49 | 18% at 2 y | 88% azoles,‡ 6% echinocandins, 2% AMB based, 4% fluconazole | RFs for IFD relapse: <12 wk from IFD to HSCT, residual disease before HSCT, CMV reactivation, glucocorticoids for GVHD | 66; 2009 |
| Prior IFD: 31 IA, 5 Candida spp., 6 others (including 4 IMD); prospective, open label | 42 | 7% at 6 mo (including 1 Mucorales and 1 Scedosporium) | 100% voriconazole | Low overall mortality (24% at 12 mo) | 121; 2010 |
| Prior cryptococcosis: 6 pulmonary, 1 meningeal; retrospective | 7 | No relapses | 100%: 71% fluconazole, 29% voriconazole | No evidence of relapse and no mortality | 98; 2010 |
| Prior IFD: 4 proven, 40 probable, 46 possible; retrospective | 90 | 25% at 1 y | 100%: 53% azoles,‡ 16% echinocandins, 31% fluconazole | RFs for IFD relapse: neutropenia >18 d (HR, 7.3), grade 3-4 acute GVHD (HR, 7.6), <70 d from IFD to HSCT (HR, 4), use of fluconazole as prophylaxis (HR, 11.5) | 16; 2013 |
| Prior IMD: 20 IA, 5 Mucorales, 3 Curvularia, 2 Fusarium, 1 Basidiomycetes; retrospective | 29 | 14% at 1 y | 100%†: 93% azoles,‡ 66% echinocandins, 5% AMB based | High overall mortality (48% at 12 mo) | 15; 2013 |
| No IMD relapse post-HSCT | |||||
| Prior IPA: 21 proven, 115 probable; prospective | 136 | 27% at 1 y; 23% vs 42% in stable vs active IPA | 100%: 72% azoles,† 24% echinocandins, 4% AMB based | RFs for IPA relapse: active IPA at HSCT (HR, 2.4), immunosuppressive treatment of GVHD (HR, 2.2) | 67; 2014 |
| Active IPA was associated with higher rate of breakthrough IFDs and lower OS | |||||
| Prior IFD: 51 IA, 5 Candida spp., 3 Mucorales, 2 other IMD, 32 undetermined; prospective | 93 | 9% vs 16% at 1 y (overall vs prior IFD) | 99%, agent NS | Overall RFs for IFD: unrelated donor, cord blood, active leukemia when HSCT, prior IFD, GvHD | 122; 2014 |
| Prior IFD: 199 Candida spp., 281 IA, 50 others (including 9 Mucorales and 19 other IMD), and 295 suspected; retrospective | 825 | 24% vs 17% at 1 y (prior IFD vs controls) | NS | RFs for IFD relapse: prior IFD, older age, receipt of alemtuzumab, advanced malignancy, ATG exposure, cord blood, mismatched donor | 17; 2017 |
| Prior IFD was associated with higher overall mortality (RR, 1.33) and shorter PFS (RR, 1.24) | |||||
| Prior chronic disseminated candidiasis; retrospective | 15 | No relapses after median follow-up of 27 mo | NS | Prior chronic disseminated candidiasis did not increase time to HSCT, nor did it affect OS | 101; 2018 |
| Prior fusariosis (35 proven, 5 probable) and further immune suppression (5 HSCT); retrospective | 40 | 12.5% at end of follow-up | 80%†: 60% voriconazole, 25% AMB based, 5% posaconazole | Overall fusariosis relapse: 25% no prophylaxis vs 9% prophylaxis (P = .26) | 88; 2019 |
| Relapse in disseminated fusariosis: 100% no prophylaxis vs 12% prophylaxis (P = .03) | |||||
| All relapsed patients had persistent neutropenia and died | |||||
| Prior IPA: 5 proven/probable and 8 possible; retrospective | 13 | 46% at 2 y | Low-dose liposomal AMB or micafungin (NS percentages) | Mortality: 77% (prior IA) vs 40% (no prior IA) | 12; 2019 |
| Study type and IFD before HSCT . | n . | Post-HSCT IFD relapse . | Percentage and type of secondary antifungal prophylaxis . | Main results . | Reference and year of publication* . |
|---|---|---|---|---|---|
| Prior IA: 10 proven and 38 probable; retrospective | 48 | 33% at 1 y | 85%†: 69% AMB based, 33% itraconazole | No RFs for IA relapse was identified | 8; 1998 |
| 88% mortality among relapsed IA | |||||
| Prior IA: 32 proven, 5 probable, 8 possible; retrospective | 42 | 29% vs 10% (if prior IA) at 1 y | NS | RFs for IA relapse: <30 d of antifungal therapy, BM or cord blood as source of stem cells, engraftment failure, radiologic persistence | 69; 2004 |
| Patients with prior IA had lower OS (56% vs 77%) and higher transplantation-related mortality (38% vs 21%) | |||||
| Prior IA: 49 proven, 80 probable; retrospective | 129 | 22% at 2 y | 95%†: 72% azoles,‡ 45% AMB based, 20% echinocandins | RFs for IA relapse: >20 d of neutropenia, advanced status of underlying hematologic malignancy, <6 wk from IA to HSCT, myeloablative conditioning, CMV disease, BM or cord blood as source of stem cells | 27; 2006 |
| Low risk: 0-1 RF, 6% incidence; intermediate risk: 2-3 RFs, 27% incidence; high risk: >3 RFs, 72% incidence | |||||
| Prior IFD: 18 IA, 8 invasive candidiasis, 23 undetermined; retrospective | 49 | 18% at 2 y | 88% azoles,‡ 6% echinocandins, 2% AMB based, 4% fluconazole | RFs for IFD relapse: <12 wk from IFD to HSCT, residual disease before HSCT, CMV reactivation, glucocorticoids for GVHD | 66; 2009 |
| Prior IFD: 31 IA, 5 Candida spp., 6 others (including 4 IMD); prospective, open label | 42 | 7% at 6 mo (including 1 Mucorales and 1 Scedosporium) | 100% voriconazole | Low overall mortality (24% at 12 mo) | 121; 2010 |
| Prior cryptococcosis: 6 pulmonary, 1 meningeal; retrospective | 7 | No relapses | 100%: 71% fluconazole, 29% voriconazole | No evidence of relapse and no mortality | 98; 2010 |
| Prior IFD: 4 proven, 40 probable, 46 possible; retrospective | 90 | 25% at 1 y | 100%: 53% azoles,‡ 16% echinocandins, 31% fluconazole | RFs for IFD relapse: neutropenia >18 d (HR, 7.3), grade 3-4 acute GVHD (HR, 7.6), <70 d from IFD to HSCT (HR, 4), use of fluconazole as prophylaxis (HR, 11.5) | 16; 2013 |
| Prior IMD: 20 IA, 5 Mucorales, 3 Curvularia, 2 Fusarium, 1 Basidiomycetes; retrospective | 29 | 14% at 1 y | 100%†: 93% azoles,‡ 66% echinocandins, 5% AMB based | High overall mortality (48% at 12 mo) | 15; 2013 |
| No IMD relapse post-HSCT | |||||
| Prior IPA: 21 proven, 115 probable; prospective | 136 | 27% at 1 y; 23% vs 42% in stable vs active IPA | 100%: 72% azoles,† 24% echinocandins, 4% AMB based | RFs for IPA relapse: active IPA at HSCT (HR, 2.4), immunosuppressive treatment of GVHD (HR, 2.2) | 67; 2014 |
| Active IPA was associated with higher rate of breakthrough IFDs and lower OS | |||||
| Prior IFD: 51 IA, 5 Candida spp., 3 Mucorales, 2 other IMD, 32 undetermined; prospective | 93 | 9% vs 16% at 1 y (overall vs prior IFD) | 99%, agent NS | Overall RFs for IFD: unrelated donor, cord blood, active leukemia when HSCT, prior IFD, GvHD | 122; 2014 |
| Prior IFD: 199 Candida spp., 281 IA, 50 others (including 9 Mucorales and 19 other IMD), and 295 suspected; retrospective | 825 | 24% vs 17% at 1 y (prior IFD vs controls) | NS | RFs for IFD relapse: prior IFD, older age, receipt of alemtuzumab, advanced malignancy, ATG exposure, cord blood, mismatched donor | 17; 2017 |
| Prior IFD was associated with higher overall mortality (RR, 1.33) and shorter PFS (RR, 1.24) | |||||
| Prior chronic disseminated candidiasis; retrospective | 15 | No relapses after median follow-up of 27 mo | NS | Prior chronic disseminated candidiasis did not increase time to HSCT, nor did it affect OS | 101; 2018 |
| Prior fusariosis (35 proven, 5 probable) and further immune suppression (5 HSCT); retrospective | 40 | 12.5% at end of follow-up | 80%†: 60% voriconazole, 25% AMB based, 5% posaconazole | Overall fusariosis relapse: 25% no prophylaxis vs 9% prophylaxis (P = .26) | 88; 2019 |
| Relapse in disseminated fusariosis: 100% no prophylaxis vs 12% prophylaxis (P = .03) | |||||
| All relapsed patients had persistent neutropenia and died | |||||
| Prior IPA: 5 proven/probable and 8 possible; retrospective | 13 | 46% at 2 y | Low-dose liposomal AMB or micafungin (NS percentages) | Mortality: 77% (prior IA) vs 40% (no prior IA) | 12; 2019 |
ATG, antithymocyte globulin; BM, bone marrow; HR, hazard ratio; IA, invasive aspergillosis; IMD, invasive mold disease; NS, not specified; OS, overall survival; PFS, progression-free survival; RF, risk factor; RR, relative risk.
Arranged chronologically.
Some patients received >1 antifungal.
In this table, mold-active azoles (itraconazole, voriconazole, and posaconazole) are together referred to as azoles. If an azole without mold activity was used (eg, fluconazole), it is specified.
Considerations for performing transplantation in patients with prior IFD
| Type of issue . |
|---|
| Host |
| Importance of CR of hematologic malignancy before HSCT |
| Comorbidities, age,* performance status |
| Surgery pre-HSCT for residual necrotic fungal lesions |
| Transplantation |
| Autologous HSCT |
| Allo-HSCT |
| RIC |
| Type of allo-HSCT: source of stem cells and donor relatedness† |
| Duration of preengraftment |
| Severe (grade >2) GVHD (acute/chronic) requiring systemic immunosuppression |
| IFD/diagnosis |
| Documenting response to antifungal therapy pre-HSCT |
| Certainty of IFD diagnosis |
| Diagnosis of IFD relapse post-HSCT |
| Coinfections with bacteria as confounders in lung infection |
| Sensitivity/specificity of biomarkers, CT |
| CMV reactivation as predictor, GC use as risk factor |
| Respiratory viral infection (eg, influenza, RSV) as risk of relapsing fungal pneumonia |
| Disseminated vs single-organ involvement by IFD |
| Issues for specific fungi |
| MDR molds (Mucorales, Fusarium, Scedosporium, others) |
| Endemic fungi |
| MDR Candida (eg, Candida glabrata) |
| Rare opportunistic non-Candida yeasts (eg, Rhodotorula) |
| Antifungal treatment for IFD post-HSCT |
| Antifungals as secondary prophylaxis |
| Toxicity of antifungals in patients with liver GVHD, sinusoidal obstruction syndrome |
| Drug-drug interactions of azoles with |
| HSCT drugs |
| Conditioning regimen |
| Azole TDM |
| Type of issue . |
|---|
| Host |
| Importance of CR of hematologic malignancy before HSCT |
| Comorbidities, age,* performance status |
| Surgery pre-HSCT for residual necrotic fungal lesions |
| Transplantation |
| Autologous HSCT |
| Allo-HSCT |
| RIC |
| Type of allo-HSCT: source of stem cells and donor relatedness† |
| Duration of preengraftment |
| Severe (grade >2) GVHD (acute/chronic) requiring systemic immunosuppression |
| IFD/diagnosis |
| Documenting response to antifungal therapy pre-HSCT |
| Certainty of IFD diagnosis |
| Diagnosis of IFD relapse post-HSCT |
| Coinfections with bacteria as confounders in lung infection |
| Sensitivity/specificity of biomarkers, CT |
| CMV reactivation as predictor, GC use as risk factor |
| Respiratory viral infection (eg, influenza, RSV) as risk of relapsing fungal pneumonia |
| Disseminated vs single-organ involvement by IFD |
| Issues for specific fungi |
| MDR molds (Mucorales, Fusarium, Scedosporium, others) |
| Endemic fungi |
| MDR Candida (eg, Candida glabrata) |
| Rare opportunistic non-Candida yeasts (eg, Rhodotorula) |
| Antifungal treatment for IFD post-HSCT |
| Antifungals as secondary prophylaxis |
| Toxicity of antifungals in patients with liver GVHD, sinusoidal obstruction syndrome |
| Drug-drug interactions of azoles with |
| HSCT drugs |
| Conditioning regimen |
| Azole TDM |
GC, glucocorticoid; MDR, multidrug resistant; RIC, reduced-intensity conditioning; RSV, respiratory syncytial virus; TDM, therapeutic drug monitoring.
Sorror et al.23
Source of stem cells: peripheral blood, bone marrow, or cord blood. HLA relatedness: matched related, matched unrelated, mismatched related, or mismatched unrelated.
Host issues
Importance of hematologic disease status
The status of underlying hematologic malignancy is a major prognostic determinant of IFD relapse risk post-HSCT. Several studies have shown that patients with active or refractory hematologic malignancy had higher overall and fungal-related mortality,13,14 as well as higher risk of new or relapsed IFD.15-17 Although lack of CR of hematologic cancer is associated with a greater risk of IFD relapse and mortality, this by itself might not constitute an absolute contraindication to HSCT, and decisions should be made on a case-by-case basis.
Comorbidities and performance status
Age is no longer an absolute contraindication to HSCT.18 However, older age and poor performance status have been associated with increased mortality in patients with a prior IFD undergoing HSCT.17 Diabetes mellitus and even uncontrolled hyperglycemia impair innate immunity and have been associated with increased risk of IFDs, particularly mucormycosis,19 although no specific studies have evaluated the effect of hyperglycemia in the risk of relapse of a prior IFD. Iron overload has been related to increased risk of fungal disease20,21 ; however, the use of iron chelators in the peritransplantation period is controversial, because there is concern regarding renal toxicity and cost.22
Prognostic scores have been developed lately to estimate risk of HSCT mortality according to age, comorbidities, and performance status.23 Some authors have demonstrated that these scores may also predict a higher likelihood of relapsed or new IFD post-HSCT.24,25 However, no specific clinical prediction models are available on the risk of IFD relapse/progression in patients with such a history. Further development, validation, and refinement of such scores could be additional tools when addressing risks for particular patients with a prior IFD and when considering secondary antifungal prophylactic strategies.26
Transplantation issues
Reduced-intenstiy conditioning
Reduced-intensity conditioning (RIC) is commonly used in older patients and patients with significant comorbidities, because it is associated with less conditioning-related toxicity and less prolonged cytopenias.27 Because neutropenia is a major outcome determinant of IFDs, even in the era of modern antifungals,28 RIC is frequently preferred when high-risk patients undergo transplantation. In fact, studies evaluating the feasibility of performing HSCT in patients with prior invasive aspergillosis (IA) have shown that RIC was associated with less than half the risk of early IA progression (<30 days) after HSCT.27,29 Also, nonmyeloablative conditioning regimens are associated with less-severe mucositis, a well-known risk factor for IC.30 However, because RIC has less effect for residual leukemia,31 the potential benefit in transplantation-related mortality (as it relates to IFD-related mortality) should be carefully weighed against the risk of leukemia relapse–related mortality.
Type of allo-HSCT
Multiple studies have documented the importance of the graft source and donor relatedness for transplantation-related toxicity and risk of IFD relapse.32,33 In patients with a prior IFD, mobilized peripheral blood stem cells are usually favored over bone marrow because of faster engraftment.34 Conversely, bone marrow and umbilical cord blood stem cells are characterized by delayed engraftment and impaired immune reconstitution and have been therefore associated with increased risk for both IFDs and relapse of a prior IFD.17,27,35
HLA disparity is associated with increased risk of a prior IFD relapse.16 Specifically, in haploidentical transplantation, immune reconstitution is both slower and incomplete and carries a high risk of severe (grade >2) GVHD, requiring potent immunosuppressive therapy.36 In this setting, many studies have shown haploidentical transplantation to be associated with a higher risk of IFDs and/or relapse of a prior IFD.37,38 Similarly, delayed immune reconstitution is seen after T-cell–depleted or CD34-selected transplantation.39,40
Duration of pancytopenia
Because profound neutropenia is a main risk factor for IFDs, antifungal treatment failure,28 and increased mortality in hematologic patients, longer duration of preengraftment post-HSCT will likely be associated with increased risk of IFD relapse. For example, a study evaluating 90 patients with a prior IFD undergoing HSCT showed that duration of neutropenia >18 days was independently associated with a sevenfold increased likelihood of IFD relapse.16 Lymphopenia and monocytopenia are also related to increased risk of IFDs and worse prognosis,33,41 although evidence on outcomes of an IFD pre-HSCT is missing.
GVHD
Acute and/or chronic GVHD is a feared complication of allo-HSCT.42,43 By itself, GVHD is a very immunosuppressive condition.44,45 Moreover, its treatment requires additional immunosuppressive therapy, typically with high-dose corticosteroids. Corticosteroids exhibit pleiotropic immunosuppressive effects, including decrease effector functions of phagocytic cells,46 and have been independently associated with increased risk of various IFDs.47,48 Systemic corticosteroid treatment for GVHD commonly induces diabetes, further increasing the risk for IFDs. In this context, GVHD (grade >2) has emerged as the single most important scenario associated with development of a new or relapsing IFD.27,47,49,50 Liu et al16 reported on 90 patients undergoing allo-HSCT after an IFD and found grade 3 to 4 acute GVHD to be associated with a 7.6-fold greater hazard ratio for the IFD recurrence risk. Therefore, when immunosuppression to treat severe acute and/or chronic GVHD is to be initiated or escalated, physicians should continue mold-active antifungal prophylaxis and careful monitoring for IFD relapse.
CART
Experience in IFD relapse in patients with leukemia receiving chimeric antigen receptor T-cell therapy (CART) is very limited. The independent contribution of CART to IFD relapse risk remains to be determined, because patients treated with CART who develop infectious complications have high-grade cytokine release syndrome and significant prior immunosuppression, including but not limited to prolonged neutropenia, from prior chemotherapy treatment as well as cumulative corticosteroid exposure.51 Because it remains difficult to predict a priori who will develop prolonged cytopenia or need significant corticosteroid treatment for cytokine release syndrome after CART, we recommend mold-active prophylaxis in such patients with a history of IFD.51
Viral infections as risk factors for IFD relapse
CMV infection
Reactivation of herpesviruses or respiratory viruses may induce local and systemic immunosuppression, increasing the risk for secondary bacterial and fungal infections, including IFD relapse. CMV in particular, as CMV organ disease27,33,52,53 or even CMV reactivation,54,55 has been associated with increased risk of both new and relapsed IFDs post-HSCT.56 Apart from the deleterious effect of CMV viremia on innate immunity, these patients often have other risk factors for a new-onset or relapsed IFD, such as the use of glucocorticoids, prolonged lymphopenia, and/or GVHD.56 Ganciclovir treatment of CMV is frequently complicated by neutropenia and also directly impairs recovery of CMV-specific CD8+ T cells.57 Finally, polymorphisms in the innate immune system could predispose patients to increased susceptibility to both CMV and IA.58 Although no data exist for optimal management of high-risk HSCT patients with a prior IFD who develop new-onset CMV disease or CMV reactivation post-HSCT, intensification of antifungal prophylaxis and monitoring should be strongly considered. Letermovir prophylaxis is effective and safe for reducing CMV infection and improving all-cause mortality in CMV-seropositive patients undergoing HSCT and decreasing IFD rates post-HSCT.59,60 Its use could theoretically result in a lower risk of IFD relapse, although such data are currently lacking.
Respiratory viruses
Respiratory viruses such as respiratory syncytial virus or influenza have been associated with increased risk of fungal pneumonia.61-63 These viruses impair the mucociliary activity and alter bronchial and alveolar mucosa, favoring fungal invasion, and additionally hinder local innate immunity and systemic host defenses.61
No specific studies have evaluated the effect of an intercurrent respiratory viral infection on the risk of relapse of a prior fungal pneumonia post-HSCT. A low threshold for viral testing with polymerase chain reaction during the respiratory virus season, coupled with early treatment with oseltamivir or ribavirin, timely influenza vaccination of the patient, family members, and health care professionals, and improvement of isolation measures, could diminish the risk of potential respiratory viral infections and, indirectly, IFD relapse post-HSCT.61
Fungal issues
Documenting response of IFDs before HSCT
Although the criteria for quantifying responses to antifungals have not been carefully validated, there is little doubt that complete or partial response of a previous IFD to treatment is a very important variable as it relates to the risk of IFD relapse post-HSCT.64 However, with an improved antifungal armamentarium and earlier detection of IFDs, the relative magnitude of such an effect is somewhat less. In older studies, patients with a prior IFD who had not completely responded to antifungal treatment at the time of HSCT were more likely to progress/relapse, and this was associated with increased mortality.13,27,65-67 In contrast, recent studies using more-potent antifungals and supportive care have shown that even a relatively active IFD may not be an absolute contraindication to HSCT.14,68 In addition, the optimal duration of antifungal treatment for an IFD pre-HSCT is a matter of debate, particularly in cytopenic patients with leukemia or aplastic anemia,16,65,67,69 in whom prompt HSCT might be helpful to restore immunity and prevent progression of the IFD.
It seems plausible to try to treat an IFD for at least 4 weeks, with documentation of improvement in measurable parameters (associated clinical signs and symptoms, follow-up CT, fungal biomarkers),15 and probably as long as possible before HSCT, weighing the risk of underlying hematologic disease progression if HSCT is delayed too long. In IA, follow-up serum galactomannan levels and clinical and radiologic signs of disease can be used to evaluate treatment response and monitor for a possible relapse.70 Some studies suggest using immunologic biomarkers. For example, Mucorales-specific T cells may appear at the onset of invasive mucormycosis and dissipate after clinical resolution.71 Although T-cell response is typically diminished after HSCT,40 assessment of fungus-specific T-cell or NK titers could be used as a complementary surrogate for relapse monitoring in patients with prior IMD46,72 ; however, this approach remains investigational.
Because it is difficult to distinguish radiologically active fungal lesions from postinflammatory scarring, positron emission tomography (PET)/CT has emerged as a promising tool to assess antifungal treatment efficacy and evaluate potential for IFD relapse.73-75 Several unresolved questions remain: persistent fluorodeoxyglucose uptake may not mean absence of response, because sterile inflammation could result in false-positive results76 ; PET/CT is not optimal to evaluate brain and kidney lesions because of high background uptake; and PET/CT can lead to increased false-positive results, which would probably drive new and unnecessary diagnostic tests and delay HSCT. More studies are needed to address the usefulness of PET/CT in peritransplantation evaluation of IFDs.
Certainty of IFD diagnosis and type of fungal infection
The difficulty of firmly diagnosing IFDs is well known.77 Some studies have shown patients with a proven IFD to have a higher risk of relapse of fungal infection after HSCT than patients with a probable infection and have shown both to have a higher relapse risk and higher fungal-related mortality than patients with possible IFDs.8,10,12,69 This probably reflects that possible IFDs may include episodes of no IFD or at least infections with low fungal burden below the threshold of detection by cultures and/or fungal biomarkers.
Disseminated IFDs, typically by molds, commonly reflect higher fungal burden and worse prognosis than localized infections.78,79 Additionally, surgical source control is commonly not feasible in disseminated IFDs. Although no specific evidence on relapse rates after HSCT in patients with a prior disseminated IFD exists, intensive and prolonged antifungal treatment should be conducted before HSCT.
Diagnosis of IFD relapse post-HSCT
Fungal pneumonia, most commonly IPA, is the most common type of severe IFD in both leukemia and HSCT patients.80 However, diagnosis of relapsed fungal pneumonia post-HSCT can be difficult. Although IFD relapse is more frequent in previous sites of infection,8,69 it might be hard to differentiate worsening of infiltrates resulting from fungal relapse from that produced by intercurrent superinfections, reported in some studies to be as high as 20% to 50%.81,82 In addition, noninfectious mimickers of IFD relapse or posttransplantation inflammatory lung syndromes (eg, bronchiolitis obliterans, diffuse alveolar hemorrhage, lung engraftment syndrome, drug toxicity) are common. Early use of bronchoscopy with BAL for microbiologic, cytologic, and biomarker analyses is important in HSCT patients who develop new lung infiltrates, because the differential diagnosis is broad, and the yield of bronchoscopy is time dependent.83 Although practices vary among institutions, percutaneous CT-guided fine-needle aspiration should also be considered to establish a diagnosis in patients with peripheral lung lesions.83
Studies of the performance of fungal biomarker surveillance as a harbinger of IFD relapse post-HSCT in patients with an IFD history are lacking. There are no standardized strategies to monitor for IFD relapse post-HSCT. However, serial detection of serum Aspergillus galactomannan antigen is helpful only in IA, and its performance is lower in nonneutropenic immunosuppressed patients (eg, those receiving systemic corticosteroids for GVHD) and/or those receiving mold-active prophylaxis. Serum β-d-glucan, although detected in patients with various IFDs, is absent in patients with mucormycosis or cryptococcosis and is prone to false-positive results.84 Eventually, early preemptive antifungal treatment is important, in view of the high mortality associated with IFD relapse. Because these patients typically are receiving mold-active agents as secondary prophylaxis, a nuanced and individualized approach to the workup that includes adjunct fungal biomarkers and treatment for either a relapse or a new breakthrough IFD is needed.85
Peritransplantation strategies to prevent IFD relapse
Surgery
Traditional indications for surgery in patients with an IFD have been source control, especially for recalcitrant molds, prevention of massive hemoptysis when infection threatens the integrity of the pulmonary vessels, and reduction of the fungal burden to diminish the risk of IFD relapse before new intense chemotherapy and/or HSCT. In this sense, some studies have shown residual lesions to be associated with higher relapse rates.13,49
However, most studies indicating benefit of elective surgery for IFDs, mainly IPA pre-HSCT, preceded the introduction of new antifungals and improvement in early diagnosis of IFDs. Indeed, recent studies have not shown a clear benefit of surgery with regard to either IFD relapse rates or mortality.27,86 Older studies may potentially reflect bias in patient selection of higher fungal burden as a result of late, culture-based diagnosis and treatment with less potent antifungals.
We believe surgical management pre-HSCT should be limited to the following selected candidates with a history of nondisseminated IMD: patients whose underlying hematologic disease is in CR; those with good performance status and no comorbidities; those with a sequestered centrally located lung lesion, especially if there is risk for or intermittent hemoptysis; those with persisting isolated sinus or soft tissue lesion, despite adequate antifungal therapy; and those whose leukemia is not at high risk for early relapse during postsurgical recovery. Minimizing the postsurgical recovery period with the least drastic surgery that can resect residual IFD (eg, wedge resection is favored over partial pneumonectomy) is critical. Careful multidisciplinary coordination between hematologists, thoracic surgeons, and mycology experts is paramount.
Specific issues for recalcitrant fungi
Non-Aspergillus molds
Although the experience with prior IMD pre-HSCT is focused on infections by Aspergillus spp., a history of IMD caused by less common opportunistic molds, such as Mucorales, Fusarium, or Scedosporium, is increasingly encountered in HSCT candidates. These fungi have high potential for tissue invasion and early dissemination, resistance to multiple antifungals, and higher mortality than IA.87,88 Multiple, frequently interrelated factors influencing the decision to perform transplantation in patients with a history of IMD resulting from a non-Aspergillus mold should be taken into account: pharmacology of antifungals, net state of immunosuppression, certainty in diagnosis, surgical debulking of residual infectious foci, and possibly immunotherapy (eg, prophylactic white blood cell transfusions) as adjunct secondary prophylaxis.89 Secondary prophylactic strategies should be highly individualized in these patients, and involvement of a mycology expert is recommended. Breakthrough infections resulting from non-Aspergillus molds such as Mucorales can still develop despite active antifungal therapy and are poor prognostic markers.90 The risk of severe, even fatal, IMD relapse vs benefit of HSCT should be carefully assessed in HSCT candidates with a history of such infections when deciding whether to move forward with transplantation.
Endemic fungi
Endemic fungal infections have been rarely reported in the peritransplantation setting, although the risk exists for severe infection, including dissemination after immunosuppression.91 Coccidioidomycosis before HSCT can manifest in different clinical presentations, ranging from disseminated disease involving multiple organs to subclinical radiologic abnormalities (eg, lung nodule) or asymptomatic seropositivity. Evaluating the metabolic activity of lesions using PET/CT may provide useful information regarding the risk of relapse.92 Experience with solid-organ transplant recipients suggests that risk factors for coccidioidomycosis reactivation are African American race and disseminated coccidioidomycosis before transplantation.93 Clinical and radiologic resolution and decrease in serologic Coccidioides titers before transplantation, followed by long-term (perhaps indefinite) secondary azole-based prophylaxis, seem advisable. Blastomycosis is an even less frequent IFD pre-HSCT. Limited information, again from solid-organ transplantation, suggests that after cure, the risk of relapse is very low.94 Histoplasmosis is also rarely reported in this setting, and the factors that predict relapse of histoplasmosis post-HSCT are undefined. Extrapolating from histoplasmosis relapse in patients with HIV/AIDS, severe immunosuppression (poor HIV control, low CD4 count), central nervous system involvement, and high antigenuria levels seem to be risk factors.95 Monitoring of histoplasma urinary antigen could help in early detection of relapse.96
Cryptococcal infection
Cryptococcosis is very uncommon in the setting of acute leukemia, with few patients reported to have undergone subsequent HSCT.97,98 However, it seems relatively safe to proceed with transplantation once the disease is adequately treated, serum cryptococcal antigen test is negative or at least significantly diminished, and secondary antifungal prophylaxis is conducted.98 The optimal secondary prophylaxis is not well defined, but (weight-dosed) fluconazole, VRC, and the other new triazoles seem suitable options. Serum antigen testing to monitor for relapse could be used, although its value in HIV-negative patients has not been clearly demonstrated.
Chronic disseminated candidiasis and multidrug-resistant Candida
Although candidemia is the most common manifestation of IC in neutropenic hematologic patients, acute disseminated infections, and in some patients chronic disseminated candidiasis, can be encountered pre-HSCT.99 IC incidence has declined since the broad introduction of antifungal prophylaxis, and the disease, across its clinical spectrum, is not a contraindication to HSCT.100 Specifically, the risk of relapse after optimally treated chronic disseminated candidiasis is considered very low, and HSCT under secondary prophylaxis should not be delayed.100,101
Widespread prophylactic use of azoles in hematologic patients has led to a shift toward the non-albicans species of Candida.102 Importantly, most strains of C glabrata display multidrug resistance, posing a challenge to treatment and secondary prophylaxis.103 There is virtually no information regarding the management of the rare patient with a history of an IFD resulting from uncommon non-Candida opportunistic yeasts (eg, Rhodotorula)104 or very rare Candida species (eg, C guillermondii, C lusitaniae)105 who is to undergo HSCT.
Issues with antifungal treatment post-HSCT
Secondary antifungal prophylaxis
Over the last 3 decades, the development of new antifungals such as new triazoles and echinocandins has resulted in less toxicity compared with prior treatment with amphotericin B–based formulations. Although no randomized or prospective studies have been performed, different antifungals have been used as secondary prophylaxis with good results,15,16,61,98,99 although publication bias is likely to exist. In cases of prior probable IA, secondary prophylaxis with a mold-active azole is indicated. There are no validated criteria that predict when it is safe to stop antifungal prophylaxis post-HSCT, and decisions in these patients are highly individualized and depend on the etiology, certainty of diagnosis, and extent of the IFD in conjunction with the net state of immunosuppression and activity of underlying disease post-HSCT.75
Toxicity and drug-drug interactions
Favorable VRC pharmacokinetics (eg, VRC serum levels >1 mg/dL) have been associated with good outcomes in IA.106 However, acute and chronic toxicities and drug-drug interactions continue to be major challenges in HSCT patients. This is of particular importance in the posttransplantation context, with patients commonly presenting with hepatic damage as a result of liver GVHD or sinusoidal obstruction syndrome. The effect of triazoles on human CYP450 enzymes causes many toxicities, and important drug interactions may occur with several chemotherapeutic and immunosuppressive agents. Especially relevant is the interaction between calcineurin and mammalian target of rapamycin inhibitors, which when coadministered with triazoles lead to higher levels of tacrolimus, cyclosporine, sirolimus, and related drugs. Clinical experience has shown that coadministration with posaconazole and VRC could be safe and feasible with dose reduction of the immunosuppressants and close drug-level monitoring.107,108 Preliminary pharmacokinetic data showed a somewhat more favorable drug-drug interaction profile for isavuconazole.109 Similarly, coadministration of triazoles with some chemotherapeutic regimens such as busulfan, although usually discouraged, can be considered with careful monitoring of busulfan blood levels and dose adjustments. Assistance of an experienced transplantation pharmacist and development of institution-specific protocols are paramount. Although still a matter of debate, if a patient is to continue with VRC in the peritransplantation period, serum therapeutic drug monitoring might be useful,110 considering the patient’s comorbidities and the introduction of the HSCT regimen.
Hepatotoxicity occurs with all azoles and ranges from mild elevations in transaminases to severe hepatitis, cholestasis, and fulminant hepatic failure, and it is hard to differentiate from other causes of liver dysfunction.111 Gastrointestinal symptoms such as nausea, vomiting, or diarrhea, as well as malabsorption issues in patients with mucositis, GVHD, and chemotherapy secondary effects, add more limitations to the use of these agents. When oral azole uptake and absorption are problematic, bridging to an echinocandin, IV triazole, or amphotericin B–based formulation, based on the particular scenario, may improve antifungal exposure and seems safe.112 Finally, prolonged use of VRC has been associated with various toxicities such as skin phototoxicity/skin cancer and bone fluorosis,113 and its use on a long-term basis should be carefully weighed.
Several questions remained unsolved in the complex topic of azole toxicity post-HSCT. For example, at what degree of hepatic damage could the different antifungals still be used? What is the role of therapeutic azole drug monitoring in minimizing toxicity? Do substitutions within the same azole family have any impact on toxicity? Also, in the future, pharmacogenetic testing may lead to more exact dose titrating and more accurate prediction of patients at risk of antifungal-related liver injury.111
Immune cellular therapy/cytokines as secondary antifungal prophylaxis
Despite the lack of robust evidence for white cell transfusion (WCT) as an adjunct antifungal strategy in the primary treatment of IFDs, there have been some reports of WCT as secondary prophylaxis during the preengraftment HSCT period in patients with prior IFDs.82,107,108 These studies were retrospective, underpowered, and subject to selection biases. Careful selection of those patients who might benefit from this strategy as secondary prophylaxis, but also as an early adjuvant therapy when an IFD relapses, is needed. For example, patients with disseminated disease with a multidrug-resistant mold, such as Fusarium, might be candidates for secondary WCT during the preengraftment period.114 However, WCT can result in alloimmunization and pulmonary toxicity, underlining again the importance of accurate recipient selection.
Despite preclinical evidence of enhancement of effector immune cell activity against various fungi,115,116 there is no clear evidence that interferon γ with or without granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor in the peritransplantation period benefits patients with a history of an IFD. In addition, the theoretic concern of stimulation of residual leukemia clones by growth factors exists.117 Finally, prophylactic infusion of fungus-specific T cells or NK cells, although theoretically appealing, remains investigational.118
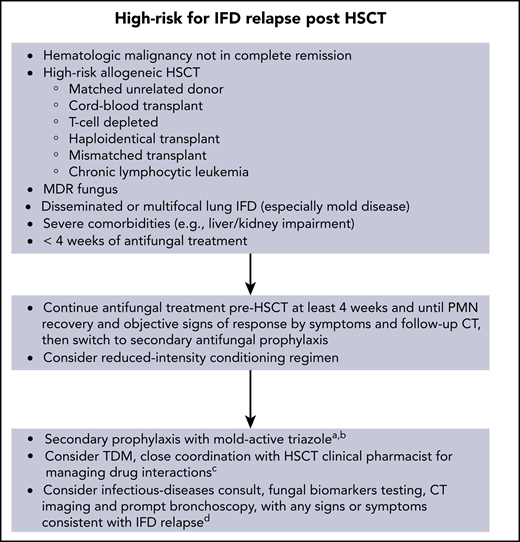
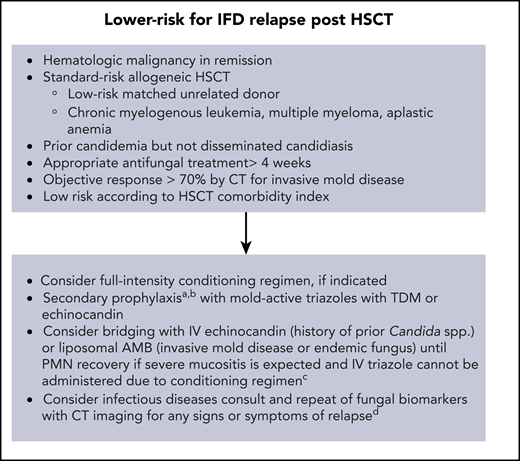
Figures 1 and 2 show our recommendations for peritransplantation management of HSCT in patients who have a history of an IFD.
Recommendations for peritransplantation management of high-risk patients for IFD relapse.aThe duration of secondary antifungal prophylaxis is individualized; consider stopping after up to 1 year post-HSCT (carefully evaluating for acute and chronic toxicities from antifungals) if patient is in CR, has polymorphonuclear leukocyte (PMN) count >1000 cells per mm3, and no signs or symptoms of active IFD. bResume mold-active prophylaxis if GVHD develops (acute or chronic) requiring systemic immunosuppressive therapy. cAny triazole antifungal administered with conditioning regimen (eg, busulphan, cyclophosphamide) or calcineurin inhibitors. dRole of surveillance with fungal biomarkers in asymptomatic patients receiving mold-active prophylaxis is unproven. MDR, multidrug resistant; TDM, therapeutic drug monitoring.
Recommendations for peritransplantation management of high-risk patients for IFD relapse.aThe duration of secondary antifungal prophylaxis is individualized; consider stopping after up to 1 year post-HSCT (carefully evaluating for acute and chronic toxicities from antifungals) if patient is in CR, has polymorphonuclear leukocyte (PMN) count >1000 cells per mm3, and no signs or symptoms of active IFD. bResume mold-active prophylaxis if GVHD develops (acute or chronic) requiring systemic immunosuppressive therapy. cAny triazole antifungal administered with conditioning regimen (eg, busulphan, cyclophosphamide) or calcineurin inhibitors. dRole of surveillance with fungal biomarkers in asymptomatic patients receiving mold-active prophylaxis is unproven. MDR, multidrug resistant; TDM, therapeutic drug monitoring.
Recommendations for peritransplantation management of low-risk patients for IFD relapse.aThe duration of secondary antifungal prophylaxis is individualized; consider stopping after up to 6 months post-HSCT (carefully evaluating for acute and chronic toxicities from antifungals) if patient is in CR, has polymorphonuclear leukocyte (PMN) count >1000 cells per mm3, and no signs or symptoms of active IFD. bResume mold-active prophylaxis if GVHD develops (acute or chronic) requiring systemic immunosuppressive therapy. cTriazole antifungal should be administered with conditioning regimen (eg, busulphan, cyclophosphamide) or calcineurin inhibitors and sirolimus. dRole of surveillance with fungal biomarkers in asymptomatic patients receiving mold-active prophylaxis is unproven. AMB, amphotericin B; TDM, therapeutic drug monitoring.
Recommendations for peritransplantation management of low-risk patients for IFD relapse.aThe duration of secondary antifungal prophylaxis is individualized; consider stopping after up to 6 months post-HSCT (carefully evaluating for acute and chronic toxicities from antifungals) if patient is in CR, has polymorphonuclear leukocyte (PMN) count >1000 cells per mm3, and no signs or symptoms of active IFD. bResume mold-active prophylaxis if GVHD develops (acute or chronic) requiring systemic immunosuppressive therapy. cTriazole antifungal should be administered with conditioning regimen (eg, busulphan, cyclophosphamide) or calcineurin inhibitors and sirolimus. dRole of surveillance with fungal biomarkers in asymptomatic patients receiving mold-active prophylaxis is unproven. AMB, amphotericin B; TDM, therapeutic drug monitoring.
Future research questions
The paucity of evidence in the literature (mainly based on small case series and subject to publication, time-reporting, and selection biases) and the lack of prospectively studied prognostic factors limit the quality of recommendations and leave a wide range of unanswered questions (Table 3). For example, a thoughtful evaluation of attributable vs contributable mortality secondary to IFDs is an important and difficult issue in these patients with complex conditions. Additionally, many different novel therapeutics for hematologic malignancies (eg, ibrutinib, idelalisib, bortezomib, bendamustine, venetoclax) have been recently incorporated. How exactly and for how long these new leukemia drugs may increase the risk of relapse of a prior IFD or increase toxicities when used in conjunction with azoles are not fully understood and pose challenges for the future.119 Also, several investigational antifungals are being tested (eg, olorofim, fosmanogepix, ibrexafungerp, rezafungin),120 which offer some advantages in this field, such as a long half-life, an extended spectrum, and the possibility for combination with conventional antifungals. Finally, advances in immunogenetics may allow identification of genetic polymorphisms associated with increased risk of IFD relapse, allowing personalized risk stratification and potentially facilitating the use of targeted immunotherapy.
Some future research questions regarding HSCT in patients with prior IFD
| Future research questions . |
|---|
| Criteria for assessing response to antifungals, role of PET/CT pre-HSCT |
| Role of isolated positive biomarkers (eg, Aspergillus GM, PCR) or serology (eg, Coccidioiodes) pre-HSCT while there is objective clinical evidence of response |
| Diagnosis of IFD relapse in HSCT patient with new fever/infiltrates |
| Immune adjunct therapy as secondary prophylaxis (eg, WBC or fungal-specific T-cell transfusions with or without cytokines) |
| Bridging antifungal strategies for patients with severe mucositis during preengraftment |
| Assessing contributable vs attributable mortality in HSCT recipient who dies post-HSCT |
| Role of new medications for AML relapse post-HSCT (eg, venetoclax) and risk of IFD relapse post-HSCT |
| Role of new medications for GVHD prevention/treatment (eg, ibrutinib) and risk of IFD relapse post-HSCT, interactions with azoles |
| Developing interventional bundles for secondary prevention of IFD relapse post-HSCT |
| Antifungal stewardship issues in HSCT recipients with history of IFD pretransplantation |
| Immunogenetics (donor/recipient) and IFD relapse |
| Clinical score cards, comorbidity indexes, and IFD relapse |
| Effective and cost-beneficial surveillance for IFD relapse in asymptomatic HSCT patients (eg, periodic CT, biomarkers) who are receiving appropriate antifungal prophylaxis |
| Role of new investigational drugs for treatment of IFD peritransplantation (to avoid toxicity, drug-drug interactions) |
| Role of ID consultant in management |
| Chronic toxicities of antifungals given as secondary prophylaxis post-HSCT |
| Applying host disease status biomarkers to monitor for IFD relapse post-HSCT |
| Future research questions . |
|---|
| Criteria for assessing response to antifungals, role of PET/CT pre-HSCT |
| Role of isolated positive biomarkers (eg, Aspergillus GM, PCR) or serology (eg, Coccidioiodes) pre-HSCT while there is objective clinical evidence of response |
| Diagnosis of IFD relapse in HSCT patient with new fever/infiltrates |
| Immune adjunct therapy as secondary prophylaxis (eg, WBC or fungal-specific T-cell transfusions with or without cytokines) |
| Bridging antifungal strategies for patients with severe mucositis during preengraftment |
| Assessing contributable vs attributable mortality in HSCT recipient who dies post-HSCT |
| Role of new medications for AML relapse post-HSCT (eg, venetoclax) and risk of IFD relapse post-HSCT |
| Role of new medications for GVHD prevention/treatment (eg, ibrutinib) and risk of IFD relapse post-HSCT, interactions with azoles |
| Developing interventional bundles for secondary prevention of IFD relapse post-HSCT |
| Antifungal stewardship issues in HSCT recipients with history of IFD pretransplantation |
| Immunogenetics (donor/recipient) and IFD relapse |
| Clinical score cards, comorbidity indexes, and IFD relapse |
| Effective and cost-beneficial surveillance for IFD relapse in asymptomatic HSCT patients (eg, periodic CT, biomarkers) who are receiving appropriate antifungal prophylaxis |
| Role of new investigational drugs for treatment of IFD peritransplantation (to avoid toxicity, drug-drug interactions) |
| Role of ID consultant in management |
| Chronic toxicities of antifungals given as secondary prophylaxis post-HSCT |
| Applying host disease status biomarkers to monitor for IFD relapse post-HSCT |
AML, acute myeloid leukemia; GM, galactomannan; ID, infectious disease; PCR, polymerase chain reaction; WBC, white blood cell.
Back to our patient
Our patient is a 65-year-old man with acute leukemia and probable IPA who has obesity, type 2 diabetes mellitus, and chronic renal insufficiency. Although no specific clinical prediction models for IPA relapse post-HSCT exist, according to the HSCT comorbidity index, he seems to be at a higher-than-average risk for IPA relapse. However, he is in hematologic remission, which is a favorable factor. After 4 weeks of systemic VRC, the patient experiences subjective and objective improvement, including a significant decrease in the size of lung nodules on follow-up chest CT, negative serum galactomannan, and serum VRC levels within a desirable range. Although the ideal scenario would be complete resolution of all lung nodules pre-HSCT, the status of his IPA is not a contraindication to transplantation. However, peripheral blood stem cells from a matched related donor after RIC might be preferred because of the risk for IPA relapse.
Continuing VRC or another mold-active azole would be indicated for up to 1 year post-HSCT. Therapeutic monitoring of azoles, particularly VRC, would be helpful if interactions with the conditioning regimen and/or agents used to prevent GVHD are expected. The assistance of an experienced transplantation pharmacist is highly recommended. Efforts to prevent or establish early diagnosis of CMV reactivation with polymerase chain reaction surveillance would be useful. As letermovir prophylaxis is effective and safe for reducing CMV in CMV-seropositive patients undergoing HSCT, its use should be considered, because it might result in a lower risk of IFD relapse. In addition, optimal glycemic control and avoidance of nephrotoxic agents in the post-HSCT period would be helpful. Finally, particular attention should be paid to signs or symptoms of IPA relapse in the setting of severe acute or chronic GVHD requiring systemic immunosuppression. In that setting, resuming azoles, if previously stopped, would be indicated. In the event of suspicion for IPA relapse, repeat galactomannan monitoring, prompt CT imaging, early bronchoscopy with BAL, and strong consideration for infectious disease consultation should be carried out.
Acknowledgments
The authors thank Allison Gulbis for useful comments, Russell E. Lewis for useful comments and assistance with the figures, as well as Michael Worley and the Department of Scientific Publications for editorial assistance.
This work was supported, in part, by National Institutes of Health, National Cancer Institute CORE support grant 16672 (MD Anderson Cancer Center) and 4R33AI127381. D.P.K. acknowledges the Texas 4000 Distinguished Professorship for Cancer Research. P.P.-A. acknowledges the Rio Hortega grant supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (CM18/00132).
Authorship
Contribution: D.P.K. contributed to conception, design, content, writing of the manuscript, and final approval; P.P.-A. contributed to content and writing of the manuscript; and R.E.C. contributed to content and provided critical input.
Conflict-of-interest disclosure: P.P.-A. has received honoraria for talks on behalf of Pfizer. D.P.K. has received research support from Astellas and Gilead and has received honoraria from Merck, Astellas, Gilead, Cidara, and Mayne Pharmaceuticals. R.E.C. declares no competing financial interests.
Correspondence: Dimitrios P. Kontoyiannis, Department of Infectious Diseases, Infection Control, and Employee Health, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, 1400 Pressler St, Houston, TX 77030; e-mail: dkontoyi@mdanderson.org.