Abstract
The most important decision in the long-term treatment of venous thromboembolism (VTE) is how long to anticoagulate. VTE provoked by a reversible risk factor, or a first unprovoked isolated distal deep vein thrombosis (DVT), generally should be treated for 3 months. VTE provoked by a persistent or progressive risk factor (eg, cancer), or a second unprovoked proximal DVT or PE, is generally treated indefinitely. First unprovoked proximal DVT or PE may be treated for 3 to 6 months or indefinitely. Male sex, presentation as PE (particularly if concomitant proximal DVT), a positive d-dimer test after stopping anticoagulation, an antiphospholipid antibody, low risk of bleeding, and patient preference favor indefinite anticoagulation. The type of indefinite anticoagulation is of secondary importance. Low-dose oral Xa inhibitors are convenient and are thought to have a lower risk of bleeding; they are less suitable if there is a higher risk for recurrence. For cancer-associated VTE, we now prefer full-dose oral Xa inhibitors over low-molecular-weight heparin, with gastrointestinal lesions being a relative contraindication. Graduated compression stockings are not routinely indicated after DVT, but are encouraged if there is persistent leg swelling or if a trial of stockings improves symptoms. Medications have a limited role in the treatment of postthrombotic syndrome. After PE, patients should have clinical surveillance for chronic thromboembolic pulmonary hypertension (CTEPH), with ventilation-perfusion scanning and echocardiography being the initial diagnostic tests if CTEPH is a concern. Patients with CTEPH and other symptomatic patients with extensive residual perfusion defects should be evaluated for endarterectomy, balloon pulmonary angioplasty, or vasodilator therapies.
This review focuses on the long-term treatment of leg deep vein thrombosis (DVT) and pulmonary embolism (PE) and does not include arm DVT or venous thrombosis in unusual locations (eg, cerebral, splanchnic). In this review, and different from how the term has been used in the American College of Chest Physicians (ACCP or CHEST) guidelines with which the authors are closely affiliated, we use “long-term” to refer to the period after the first 3 months of treatment (often indefinitely), which has also been referred to as the “extended” or “chronic phase” of therapy (see "How long should VTE be anticoagulated?").1
The questions that we will focus on are: (1) how long should patients be anticoagulated, (2) what anticoagulant regimen should be used for indefinite therapy, (3) what long-term interventions should be used to prevent the postthrombotic syndrome (PTS), (4) how should established PTS be treated, and (5) how should residual symptoms after PE, including chronic thromboembolic pulmonary hypertension (CTEPH), be managed?
Consistent with the ACCP guidelines, we make strong (nonconditional) recommendations when treatments are expected to result in substantial benefit and apply to most patients (ie, ≥90%).2,3 We make weak (conditional) recommendation when benefits are less certain, or risks and benefits are more finely balanced, and treatments are expected to apply more selectively (ie, <90%). For weak recommendations, we indicate our preferences and the factors that influence decision making, including the importance of patient preferences.
How long should VTE be anticoagulated?
The risk for recurrent VTE (including thrombus extension) falls rapidly once anticoagulation is started, and then falls more slowly until a new baseline risk is achieved.1,4 If anticoagulants are stopped before the acute phase of therapy has been completed and the new baseline risk for recurrence has been reached, there is a higher risk for early recurrence.4 Trials that compared recurrence risk after different durations of warfarin therapy identified that it takes 3 months of treatment to reach this new baseline in most patients, although this may be achieved a bit sooner (ie, 6 weeks) after an isolated distal DVT provoked by a reversible risk factor, and a bit later (ie, 6 months) after an unprovoked proximal DVT or PE.4 Extending anticoagulation beyond the acute phase of treatment reduces recurrent VTE by more than 80% while patients are receiving therapy, but progressively longer durations of therapy do not reduce the risk for recurrence that patients will experience if they then stop anticoagulants.3-8 As a consequence, as a general rule, patients with VTE should be treated either for 3 (or 6) months or indefinitely.9 Having decided to treat indefinitely, treatment may subsequently be stopped if the risk of bleeding increases (eg, acquired renal or hepatic dysfunction, or after bleeding resulting from a nonreversible cause) or the patient changes their mind about indefinite therapy.
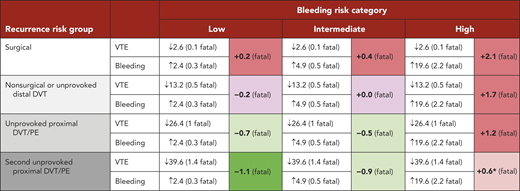
Whether treatment is stopped at 3 months or continued indefinitely mostly depends on whether the reduction in VTE with indefinite anticoagulation outweighs the increase in bleeding. This equation has to take into account that the consequences of a major bleed are generally worse than the consequences of a recurrent VTE: about 12% of major bleeds (probably lower with direct oral anticoagulants [DOACs] than with warfarin10 ), as opposed to 4% of recurrent VTE, are fatal (∼3:1 ratio).3,9,11-13 If patients do not have a high risk of bleeding (see "Influence of bleeding risk on the decision to anticoagulate indefinitely"), the long-term risk for recurrence, which differs markedly among patients, dominates the treatment decision. Risk factors for recurrent VTE after stopping anticoagulants are summarized in Table 1.
Clinical prediction rules for recurrence risk after unprovoked VTE
Four clinical prediction rules have been proposed for estimating the risk for recurrence after stopping anticoagulants in patients with unprovoked VTE.27-30 The variables that are included in each of these models, and the variables that we have used for stratification of recurrence risk, are summarized in Table 2. The HERDOO2 rule is the only one of these that provides explicit criteria for which patients should stop anticoagulation (“low risk” women) and which should continue therapy indefinitely (men and “high risk” women). However, HERDOO2 has the limitations that it does not stratify risk in men and considers women who have had VTE on estrogen as having unprovoked VTE, even though these women, who accounted for more than half of the low-risk subgroup in the HERDOO2 validation study, had a substantially lower risk for recurrence than the women who were not receiving estrogens (1.3% vs 4.9% per year).31 We believe that the approach to recurrence risk stratification that we have used, both for the full range of patients with VTE and when confined to patients with unprovoked VTE, uses the characteristics and measurements that have the greatest predictive value for recurrent VTE.
Stop anticoagulants at 3 months
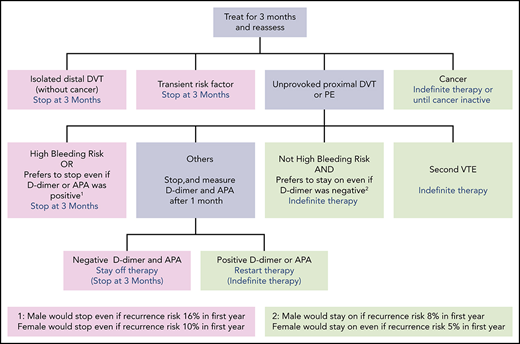
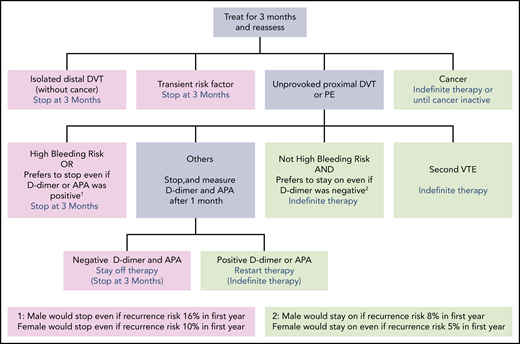
We stop anticoagulants at 3 months in patients with DVT or PE provoked by a major or minor reversible risk factor (including estrogen-associated VTE), or patents with unprovoked isolated distal DVT (Figure 1; Table 3).3,9 If the DVT or PE was very large (eg, involves most proximal veins; associated with moderate right ventricular dysfunction) or there are substantial residual symptoms, we may extend anticoagulation to 6 months, but we usually stop treatment then. If VTE was provoked by a very minor reversible risk factor (eg, flight of <8 hours, prolonged car travel, minor calf muscle strain; all suggesting a borderline unprovoked event), symptoms persist, there is a low risk of bleeding and it is the patient's preference, anticoagulants may be continued indefinitely. We do not use d-dimer or antiphospolipid antibody testing to guide duration of anticoagulation decisions in patients with VTE that was provoked by a reversible risk factor.
Patients with VTE who should be treated for 3 months or indefinitely (submitted separately). A time-limited course of anticoagulation is usually 3 months, but may be extended to 6 months (eg, DVT involving most proximal veins, PE with moderate right ventricular dysfunction, persistent symptoms, patient not ready to stop).
Patients with VTE who should be treated for 3 months or indefinitely (submitted separately). A time-limited course of anticoagulation is usually 3 months, but may be extended to 6 months (eg, DVT involving most proximal veins, PE with moderate right ventricular dysfunction, persistent symptoms, patient not ready to stop).
Continue anticoagulants indefinitely
We continue anticoagulants indefinitely in patients with VTE and active cancer or another persistent provoking factor (eg, active inflammatory bowel disease), and in patients with a second unprovoked proximal DVT or PE (Figure 1). The decision to stop anticoagulants or treat indefinitely is more difficult in patients with a first unprovoked proximal DVT or PE.
We encourage men with a first unprovoked PE (particularly with concomitant proximal DVT), or a large proximal DVT (eg, most proximal veins), to continue receiving anticoagulants indefinitely. If such men are reluctant to continue receiving anticoagulation, we measure d-dimer and antiphospholipid antibodies (APA) 1 month after withdrawal of anticoagulants, provided, after discussion of the recurrence risks with positive and negative results, the man has agreed to resume anticoagulants if either test is positive.
In most women with a first unprovoked proximal DVT or PE, we explain that the risks and benefits of indefinite anticoagulant therapy are finely balanced. If the woman has a strong preference to either continue receiving or stop anticoagulants, we generally support that decision. If the woman does not have a strong preference, we measure d-dimer and test for APA 1 month after withdrawal of anticoagulants, provided that the woman has agreed to resume indefinite anticoagulant therapy if either of these tests is positive. In women with a large unprovoked PE (particularly with concomitant proximal DVT), we encourage indefinite anticoagulant therapy without measuring d-dimer or testing for APA.
Influence of bleeding risk on the decision to anticoagulate indefinitely
At this time, there is no well-validated way to quantify the risk of major bleeding during long-term anticoagulation, and to use that bleeding risk estimate to decide whether patients with various risks for recurrence should avoid indefinite therapy.32 The ACCP guideline group proposed that risk factors for bleeding include older age (>65 and particularly >75 years), previous bleeding (particularly if the cause was not correctable), cancer (particularly if metastatic or highly vascular), renal insufficiency, liver failure, diabetes, previous stroke, thrombocytopenia, anemia, concomitant antiplatelet or nonsteroidal anti-inflammatory therapy (to be avoided), frequent falls, alcohol abuse, reduced functional capacity, and poor control of vitamin K antagonist therapy.3 They suggested that the prevalence of these factors could be used to categorize risk of bleeding as low (no risk factors), moderate (1 risk factor), or high (≥2 risk factors). However, we emphasize that severity of each factor also needs to be taken into account when assessing an individual's bleeding risk and making treatment decisions. Readers are discouraged from applying this framework in an overly simplistic way; rather, it is intended to conceptualize the decision-making process and help with application of these concepts. Similar to the approach in this review, the ACCP also categorized risk for recurrence in noncancer patients into 4 groups of ascending risk (Table 3). They then made recommendations (strong or weak) about stopping or continuing anticoagulants for each of the 12 (4 × 3) recurrence and bleeding risk combinations.3 If there is a very high risk of bleeding that cannot be corrected, even patients with a high risk for recurrence will need to stop therapy.
Role of laboratory testing (d-dimer levels, antiphospholipid antibodies, hereditary thrombophilias)
In relationship to long-term treatment decisions, we very rarely test for hereditary thrombophilias, and only measure d-dimer levels and test APA in patients with unprovoked VTE when the test result will decide whether anticoagulants are stopped or continued indefinitely (as previously discussed). As the risks and benefits of indefinite therapy are more evenly balanced in women who have had a first unprovoked proximal DVT or PE, d-dimer and APA testing is generally more helpful in women (many men decide to continue receiving anticoagulants without d-dimer and APA testing).33
What anticoagulant regimen for indefinite therapy?
We consider warfarin (target international normalized ratio [INR] 2.5), rivaroxaban (20 mg or 10 mg once daily [OD]), apixaban (5 mg twice daily [BID] or 2.5 mg BID), edoxaban (60 mg OD; 30 mg OD if creatinine clearance is 30-50 mL/min or body weight <60 kg), dabigatran (150 mg BID), and therapeutic-dose low-molecular-weight-heparin (LMWH) as the options for indefinite anticoagulant therapy. Most assessments of these anticoagulant regimens were during the acute phase of VTE treatment (often compared with warfarin, or with LMWH in patients with cancer-associated thrombosis (CAT), and usually for about 6 months),5,34-36 with a smaller number of studies focusing on extended therapy (compared with placebo, aspirin, warfarin, or the same drug in higher or lower doses).6,37-39 Network and pairwise meta-analyses help in the assessment of the relative efficacy and safety of different long-term anticoagulant regimens.5,6,40,41 The findings of these studies and analyses can be summarized as follows: (1) all regimens reduce the risk for recurrent VTE by at least 80%, with no convincing difference in the efficacy of 1 anticoagulant regimen over another; (2) the risk of major bleeding with rivaroxaban, apixaban, edoxaban, and dabigatran is about 33% lower than with warfarin (about 50% less for intracranial bleeding; more gastrointestinal bleeding with rivaroxaban and dabigatran); (3) LMWH is more effective than warfarin in CAT; (4) edoxaban is more effective than LMWH in CAT, and rivaroxaban and apixaban may be more effective than LMWH in CAT; (5) there is more bleeding with edoxaban and rivaroxaban compared with LMWH in CAT, with a suggestion that the excess is confined to those with gastrointestinal lesions; and (6) after 6 months of full-dose anticoagulation, there is little difference in the efficacy or safety of low-dose and full-dose regimens of rivaroxaban and apixaban (neither low-dose regimen has been assessed in CAT). Factors that favor use of a particular anticoagulant are summarized in Table 4.
Aspirin
After unprovoked VTE, aspirin results in a very modest reduction in recurrence (about 33%) compared with anticoagulants, and appears to have a similar risk of bleeding as low-dose oral Xa inhibitors.6,39 As a consequence, there is little role for aspirin for secondary prevention of VTE. However, in patients with an arterial indication, if aspirin was stopped when anticoagulants were started (generally encouraged), it is important to restart aspirin if the patient stops anticoagulants.
What we use for indefinite anticoagulant therapy
We usually use the same type of anticoagulant that was used for the acute phase of treatment. For patients without cancer who have a creatinine clearance of at least 30 mL/min, this is usually edoxaban, rivaroxaban, or apixaban. Nonrandomized comparisons suggesting less bleeding with apixaban than with other anticoagulants favors its use. Patients who want an OD regimen may prefer rivaroxaban or edoxaban. As it is not affected by CYP 3A4, edoxaban is preferred if patients are receiving medications that strongly inhibit (eg, clarithromycin, ketoconazole, cyclosporine) or induce (eg, carbamazepine, phenytoin, rifampin) this pathway. On the basis of a presumption that there is a reduction in bleeding with low- compared with full-dose rivaroxaban or apixaban, and that this outweighs any increase in recurrent VTE with the low-dose regimens, we generally prefer to use low-dose rather than full-dose rivaroxaban, or low-dose apixaban (full-dose apixaban is not approved for extended therapy) after the first 6 months in patients without cancer. If we are using rivaroxaban, we generally continue the full-dose regimen in patients who are both large (eg, >90 kg) and young (eg, <50 years).
For patients with CAT, we also usually use edoxaban, rivaroxaban, or apixaban. Our preference is for edoxaban, because we feel it is supported by stronger evidence and has a lower potential for interaction with cancer therapies because of its lack of CYP 3A4 interaction. We avoid edoxaban and rivaroxaban in patients with gastrointestinal lesions. We use LMWH in these patients, or apixaban if patients decline injections. We also use reduced-dose LMWH if the platelet count is expected to drop below 50 000 per microliter (eg, half-dose if 20 000-49 000 per microliter; hold if <20 000 per microliter).
What long-term interventions should be used to prevent PTS?
PTS occurs in 20% to 50% of patients after DVT, and is severe in 5% to 10%.44 As PTS can substantially reduce quality of life and is costly, its prevention and treatment are important goals of long-term therapy. Although we will consider prevention and treatment of PTS separately, there is overlap, with an important goal of treatment being to prevent PTS from worsening.
Compression therapy
There is conflicting evidence about the efficacy of elastic compression stockings (ECS) for preventing PTS.45-49 Small, single-center, unblinded, trials suggested that ECS reduced PTS by about 50%, whereas the much larger, placebo-controlled, multicenter SOX trial (by the authors) failed to show benefit from routinely wearing ECS for 2 years.50 This trial was criticized for poor adherence to ECS therapy; however, it is unlikely that poor adherence could have eliminated all benefit.
On the basis of current evidence suggesting lack of efficacy, we do not routinely use ECS to prevent PTS. However, we encourage ECS use in patients who have leg swelling, particularly if they have risk factors for PTS such as extensive proximal DVT and excess weight.51 We start with a knee-length 30 to 40 mm Hg pressure stocking, worn when out of bed. If ECS is poorly tolerated, we reduce pressure to 20 to 30 mm Hg or discontinue the stocking.
How should established PTS be treated?
The goals of PTS treatment are to relieve symptoms, prevent worsening, improve quality of life, and prevent (and treat) venous ulcers. There are few well-designed trials assessing treatment of established PTS.
Compression therapy
ECS should be used to reduce leg swelling, pain, and heaviness. This suggestion is based primarily on extrapolation from ECS use in patients with primary chronic venous insufficiency, and because ECS have a low risk for harm. Knee-length are better tolerated than thigh-length stockings, and appear to have similar efficacy. We generally start with 20 to 30 mm Hg knee-length ECS, followed by 30 to 40 mm Hg stockings if the lower pressure does not control symptoms. As with PTS prevention, the stocking is worn while out of bed, and advice is given to maximize compliance. For patients with moderate to severe symptoms that are unresponsive to ECS, we suggest a trial of a venous-return assist device or intermittent pneumatic compression.52
Exercise and lifestyle
Based on 2 small trials that suggest benefit without harm, we suggest a program of training that includes exercises to improve leg strength, joint flexibility, and overall cardiovascular fitness.53,54 Additional lifestyle suggestions include regular walking, leg elevation when seated or in bed, avoidance of prolonged heat, weight loss if obese, and use a moisturizing cream to prevent skin dryness and breakdown.52
Medications to treat PTS
There is low-quality evidence supporting the short term (eg, 3-12 weeks) use of rutosides, horse chestnut extract, diosmin, and defibrotide for treatment of PTS.52,55 We offer patients with moderate or severe PTS a 1-month trial of 1 of these medications, forewarning them that the treatment is of uncertain benefit and may cause minor adverse effects. Diuretics should not be used to try and reduce PTS-related edema.52
Should anticoagulation be continued indefinitely in patients with PTS?
Theoretical reasons for indefinite anticoagulation in patients with PTS are prevention of recurrent ipsilateral DVT, which is likely to worsen PTS, and that PTS may increase the risk for recurrent VTE in general.9,52 Although we do not consider PTS to be a strong indication for indefinite anticoagulation, we suggest that the presence of moderate or severe PTS may tilt the balance in favor of indefinite anticoagulation when other risks and benefits are finely balanced.
Venous ulcer management
About 5% to 10% of patients with DVT develop severe PTS, which may include ulceration. Ulcers are often painful, resistant to treatment, and frequently recur. Ideally, patients with postthrombotic ulcers should be managed by a wound care team that includes an internist, dermatologist, wound care nurse, and vascular surgeon.52,56,57 Ulcers should be treated with compression therapy, oral pentoxifylline (a hemorheological agent that improves blood flow and oxygen delivery to tissues), wound bed dressings, antibiotics, and debridement as necessary. Leg elevation helps to reduce edema and improve ulcer healing. Even with optimal care, venous ulcers may take months to heal. We suggest assessment for surgical or endovascular interventions in patients with ulcers that fail to heal with optimal conservative management.
Endovascular and surgical treatments for PTS
Two pathophysiologic components of PTS may respond to endovascular correction: chronic iliac vein obstruction, which may be relieved by stent placement or venous bypass surgery, and saphenous vein reflux, which may be corrected by endovenous thermal ablation, foam sclerotherapy, or stripping of the refluxing vein.58-60
Follow-up of patients with PTS
Patients with PTS should have regular follow-up to assess severity, optimize therapy, and reinforce the importance of treatment adherence.
How should residual symptoms after PE, including CTEPH, be managed?
Long-term complications of PE, which are estimated to occur in about 25% of patients, vary from mildly increased dyspnea or fatigue during exercise (“post-PE syndrome”) to severe symptoms at rest with severe pulmonary hypertension and right ventricular failure.61-66 CTEPH occurs in about 3% of patients within 2 years of symptomatic PE. We do not routinely screen patients with PE for CTEPH during follow-up, but instead, investigate those with persistent exertional symptoms (eg, breathlessness, fatigue) and those who were noted to have pulmonary hypertension or moderate right ventricular dysfunction at initial presentation. CTEPH is diagnosed on the basis of the combination of mismatched segmental perfusion defects on ventilation-perfusion scanning (and/or chronic pulmonary arterial occlusion, stenosis or webs on CT angiography) after 3 or more months of adequate therapeutic anticoagulation, with a mean pulmonary arterial pressure of 25 mm Hg or more and a pulmonary capillary wedge pressure of 15 mm Hg or less (echocardiography is a valuable initial evaluation).61
Surgical pulmonary thrombo-endarterectomy is the recommended treatment of patients with surgically accessible CTEPH. Patients with CTEPH should be assessed by a multidisciplinary expert team at a CTEPH center. For patients with CTEPH who are not candidates for, or do not wish to undergo, endarterectomy, balloon pulmonary angioplasty, which may require multiple sessions, should be considered. Riociguat, a soluble guanylatecyclase agonist, may benefit patients with inoperable CTEPH or persistent/recurrent CTEPH after endarterectomy. Indefinite anticoagulation is generally recommended. Self-directed or supervised exercise programs are encouraged for patients with post-PE syndrome, as they may be of benefit and are unlikely to cause harm.
Acknowledgments
The authors thank Jeffrey Weitz and the 3 Blood peer reviewers for suggestions on how to improve this article.
C.K. is supported by the Jack Hirsh Professorship in Thromboembolism. S.R.K. is supported by a Tier 1 Canada Research Chair. C.K. and S.R.K. are investigators of the CanVECTOR Network, which receives grant funding from the Canadian Institutes of Health Research (Funding Reference: CDT-142654).
Authorship
Contribution: C.K. and S.R.K. wrote the article.
Conflict-of-interest disclosure: C.K. reports receiving grant support from Bayer. S.R.K. reports receiving advisory board fees from BMS Pfizer, Sanofi, and Aspen.
Correspondence: Clive Kearon, Juravinski Hospital, Room A3-73, 711 Concession St, Hamilton, ON L8V 1C3, Canada; e-mail: kearonc@mcmaster.ca.