Abstract
While we are now able to diagnose inherited thrombophilias in a substantial number of patients with venous thromboembolism (VTE), the initial hope that their presence would inform recurrence risk and thus decisions on anticoagulation duration has largely been disappointing. Indeed, the presence or absence of transient provoking risk factors has proven to be the most important determinant of VTE recurrence risk. Thus, particular attention to transient acquired risk factors for VTE remains paramount, as they have generally been shown to carry more prognostic weight than inherited thrombophilias. The presence of other acquired risk factors may require additional management considerations, whether pertaining to anticoagulant choice, as in antiphospholipid antibody syndrome, or to addressing a new predisposing medical condition, as in malignancy. Antithrombin deficiency or the presence of ≥1 thrombophilic defect may be exceptions that can have a role in prognostication; however, as illustrated in this review through several case vignettes, interpretation and clinical application of the results of inherited thrombophilia testing is nuanced. We have chosen to focus on cases in which patients have been identified as having thrombophilic defects rather than the indications for undertaking testing in the first place or the extent of investigation. Management decisions in such cases ultimately hinge on individualized consideration of the benefits and risks of anticoagulation along with patient preference rather than on an algorithmic pathway based on thrombophilia status.
Introduction
Venous thromboembolism (VTE) is a multicausal disease influenced by a variety of acquired and inherited risk factors. Considerable effort has been made to elucidate states of hypercoagulability that might better inform decision making regarding anticoagulation duration and prophylaxis strategies to mitigate the occurrence of VTE. These efforts have led to the discovery of multiple inherited thrombophilic defects, including the factor V Leiden (FVL) and prothrombin G20210A (PT20210A) mutations, along with deficiencies of antithrombin (AT), protein C (PC), and protein S (PS). However, despite the ability to identify a thrombophilic defect in a substantial proportion of VTE patients, there are limited data to support the clinical utility of testing in patient management. Thus, the initial hope that such defects would serve as an actionable risk-stratification tool for deciding which patients may benefit most from long-term anticoagulation has not been fulfilled. In fact, misinterpretation of tests can actually cause harm if too much predictive value is assumed from the results.
For these reasons, most available guidelines either specifically minimize the role of inherited thrombophilia testing or do not provide concrete guidance, and there are currently no published validated testing guidelines.1-3 Despite efforts at reducing widespread and inappropriate testing, it remains all too common for the consulting hematologist to encounter patients in whom inherited thrombophilia testing has already been performed. In the genomic era, it is also becoming increasingly common for patients to undergo self-testing through commercially available genetic screening platforms. It then becomes the task of the physician (who may not have even ordered the testing) to determine what impact, if any, these results should have on management. Given this, we will not discuss in detail which patient populations should undergo thrombophilia testing.
In this review, we will instead discuss both the inherited thrombophilias and antiphospholipid antibody syndrome (APLS) through several clinical cases that illustrate the limitations and nuances of these risk factors on management. We will also place a particular emphasis on the importance of identifying acquired risk factors given their prognostic implications in the recurrence of VTE along with their separate clinical implications on patient outcomes (see Table 2). We will also provide cases in which thrombophilia testing can influence management decisions with regard to duration of anticoagulant therapy or choice among agents. Through this discussion, we hope to demonstrate the limited role that thrombophilia testing has in impacting patient management.
FVL
A 78-year-old woman with a history of osteoarthritis of the hip and hypertension self-refers to a hematology clinic for perioperative recommendations regarding a diagnosis of FVL heterozygosity prior to undergoing total hip replacement. While no record of FVL testing could be identified in her medical chart, the patient relays that her diagnosis stemmed from a commercial ancestry testing platform, which included hereditary thrombophilia testing for the FVL andPT20210Agene mutations. She has no personal history of VTE, despite challenges of 1 abdominal surgery and 3 successful pregnancies, nor any family history of VTE.
This case reflects a phenomenon that is becoming increasingly common with the growing availability of direct-to-consumer diagnostics and hype regarding the potential benefits of personalized genomic testing. Regardless of how one feels about such consumer tests, the results are likely to fall to the health care provider when patients seek advice and interpretation of them.4-6 Our patient obtained testing that led to the finding of FVL heterozygosity. This mutation represents the most common inherited thrombophilia in those of European ancestry with an allele frequency of ∼4% to 6% in whites.7,8 Its thrombophilic mechanism results from increased resistance to factor Va degradation by activated PC, as the mutation occurs at its first enzymatic cleavage site. In unselected white patients with a first episode of VTE, ∼12% to 20% will be heterozygous for FVL. While the relative risk for incident VTE is ∼4, the risk of recurrent VTE has been shown to be low (1.4-fold).9,10
These statistics provide a rational basis for recommending both against screening for FVL in the general population and also against testing unselected patients with VTE. With regard to screening, despite its prevalence in those of European descent, only ∼11% of those affected will ever experience VTE; thus the vast majority of those carrying 1 copy of the mutation will never go on to experience VTE in their lifetime.11 Furthermore, while patients who have experienced VTE are enriched for a higher prevalence of FVL, the very modest increase in risk for recurrent VTE is insufficient to routinely influence medical decisions regarding anticoagulation duration.
Our patient had lived nearly 79 years without ever experiencing VTE and had no family history of VTE. Based on all available guidelines, she would never have been screened for hereditary thrombophilia had it not been for direct-to-consumer genetic testing. Not surprisingly, there is data suggesting that the FVL and PT20210A gene mutations are overrepresented in patients who experience VTE after joint replacements.12 That stated, it can be argued that the risk of VTE after knee or hip replacement is high enough that all patients without excess bleeding risk should be provided pharmacologic anticoagulant prophylaxis in the postoperative period. Nonetheless, there is widespread use of aspirin alone in North America as pharmacologic prophylaxis in low-risk patients undergoing total knee or hip replacement. We therefore recommended to our patient and her surgical team that they employ pharmacologic prophylaxis with an anticoagulant for 30 days. Low-dose rivaroxaban at 10 mg daily for 5 days, followed by low-dose aspirin as per the EPCAT II trial may be an acceptable alternative, though it was not our preferred option for this patient.13
Had her results instead shown heterozygosity for PT20210A, our discussion with her and recommendations would have been the same. PT20210A is a mutation within the 3′-untranslated region of the gene leading to increased mRNA transcription and ultimately translated protein levels that are ∼30% higher than average.14,15 Less prevalent than FVL, this is the second most common inherited thrombophilia and is present in ∼2% of whites, and up to 6% of those who have VTE. Presence of this mutation confers an approximately threefold increased risk for a first VTE, though its presence has not consistently demonstrated any increased risk of recurrent VTE.10,16 Thus, as for FVL, there is no indication to screen for this mutation in the general population, as the vast majority of those affected will never experience VTE. Similarly, it does not carry any significant predictive value in VTE recurrence and therefore should not be used to inform decisions regarding anticoagulation duration.
Homozygous PT20210A and pregnancy
A 34-year-old woman in good health is referred to hematology clinic by her gynecologist for management of homozygous PT20210A. Her mother has a history of pulmonaryembolism (PE) while on a combined oral contraceptive and was found to be heterozygous for PT20210A. Her father was tested and found to be homozygous for PT20210A, and the patient was subsequently tested and similarly found to be homozygous. She has no personal VTE history and has not previously been on oral contraceptives, though she is now planning for pregnancy.
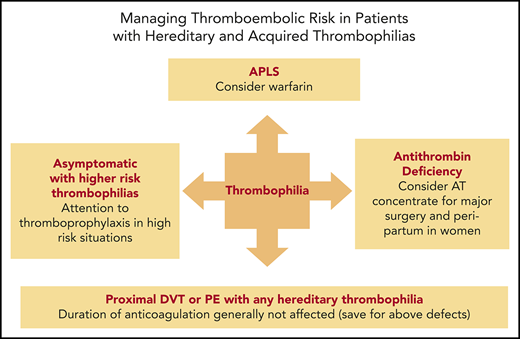
This case illustrates 2 challenging areas within thrombophilia management: mutation homozygosity and hormone-related synergism of risk. Homozygosity of either FVL or PT20210A is obviously far less common than their heterozygous counterparts and may represent ∼1% of patients with VTE.17 Given the low incidence of homozygosity, VTE risk estimates have been more difficult to capture and have varied significantly over time. Recent studies suggest an odds ratio for first VTE of 6.7 for homozygous PT20210A and of 11 for homozygous FVL.10 It is worth noting that the odds ratio of 11 for homozygous FVL is substantially lower than early estimates of ∼80 derived from the Leiden Thrombophilia Study.17 Despite the increased risk of VTE in homozygous patients, the absolute risk of VTE is still sufficiently low enough that patients should generally not be routinely anticoagulated in the absence of prior VTE or transient risk factors. As an example, one series of 36 patients with homozygous PT20210A demonstrated that the majority of these patients remained asymptomatic despite the presence of additional prothrombotic risk factors.18 Exemplifying this point is that fact that our patient and her father were found to be homozygous despite both having an absent VTE history. More notable is her mother’s history of VTE in association with oral contraceptive use and our patient’s own plans for pregnancy. Due to limited data and probable selection bias in prior family studies, estimation of VTE risk in pregnant patients with inherited thrombophilia is challenging, and there is variable guidance on thromboprophylaxis in this population. Recent studies have provided better estimates of gestational VTE risk and generally demonstrate a high risk (>2% to 3%) in homozygous FVL, compound heterozygous FVL and PT20210A, and severe AT deficiency.19,20 In our view, these data and the known synergism between the inherited thrombophilias and high estrogen states pose a sufficient antepartum VTE risk to merit anticoagulation in homozygous FVL, compound heterozygous FVL and PT20210, and AT deficiency. While data are more limited for homozygous PT20210A, we would treat this similarly as a high-risk thrombophilia in pregnancy. Thus, in line with American Society of Hematology guidelines, we managed our patient with prophylactic anticoagulation in the ante- and postpartum state without complications.21
Deficiencies of the natural anticoagulants
The 3 remaining inherited thrombophilias include deficiencies of the natural anticoagulants AT, PC, and PS. Unlike FVL and PT20210A, these conditions are caused by a multitude of different mutations in the 3 genes resulting in either reduced synthesis (type 1) or reduced function (type 2); thus, genetic testing is generally not feasible or readily available. Rather, these are detected most often through functional assays (Table 1). Notably, the normal range for PS is wider than for the other natural anticoagulants, and functional assays for PS have technical characteristics that make them less robust in establishing a diagnosis of hereditary PS deficiency. Determination of free PS antigen level is therefore the best assay to diagnose PS deficiency.22-24 The causative mutations are heterozygous, and homozygosity is extremely rare. Below are case vignettes of AT deficiency that illustrate the heterogeneity of such cases.
Inherited thrombophilias and preferred approach to diagnostic workup
| Inherited thrombophilia . | Workup . |
|---|---|
| FVL | APC resistance, genetic testing |
| Prothrombin G20210A | Genetic testing |
| AT deficiency | Functional assay |
| PC deficiency | Functional assay |
| PS deficiency | Free PS antigen |
| Inherited thrombophilia . | Workup . |
|---|---|
| FVL | APC resistance, genetic testing |
| Prothrombin G20210A | Genetic testing |
| AT deficiency | Functional assay |
| PC deficiency | Functional assay |
| PS deficiency | Free PS antigen |
A 57-year-old woman with hereditary AT deficiency (baseline antigen and activity ∼40%) well known to our clinic presents for an upcoming total hip replacement. She has an extensive family history of VTE, including an older sister who tragically died in adolescence from a PE, and she has been anticoagulated since childhood with warfarin that was initially for primary prophylaxis. She has had multiple pregnancies requiring cessation of warfarin and use of heparin products and at times while off of anticoagulation has experienced objectively confirmed venous thrombotic events.
AT deficiency was the first of the inherited thrombophilias to be identified and has the highest odds ratio for a first episode of VTE of ∼16.25 However, it is the least common of the inherited thrombophilias, with a prevalence of ∼0.02% to 0.2% in the general population. In comparison, deficiencies of PC and PS confer odds ratios for VTE of ∼7.5 and 5.4, respectively.25 The prevalence of PC deficiency is 0.2% to 0.4%, while the prevalence of PS deficiency has been estimated to be 0.03% to 0.5%. Additionally, among patients with unprovoked VTE, AT deficiency appears to carry a greater risk of recurrent VTE, with an odds ratio of 3.6, compared with 2.9 from PC deficiency and no apparent increase from PS deficiency.25 Despite being the most clinically penetrant of the single defect inherited thrombophilias, its presence does not necessarily change clinical management, as patients with a first unprovoked proximal deep vein thrombosis or PE are often anticoagulated indefinitely given the high risk of recurrence. However, the presence of AT deficiency can support this decision in the setting of a younger patient with a preference not to be committed to long-term anticoagulation, particularly in the presence of a strong family history.
Despite having the highest risk of VTE of the inherited thrombophilias, the clinical penetrance can still be quite variable; some patients develop severe or life-threatening VTE at a young age, while some may not develop VTE in their lifetime. Our patient presented from a particularly prothrombotic family with 6 first-degree relatives having sustained VTE, including her father, 3 siblings, and 2 children. When she was diagnosed at a young age after her sister’s death from PE, the decision was made to anticoagulate her with warfarin indefinitely in the absence of having sustained VTE. This represents a rare situation in which it is reasonable to consider long-term anticoagulation for a patient in the absence of a personal VTE history after a discussion of its benefits and risks. This differs from many other situations in which screening asymptomatic family members of a patient known to have an inherited thrombophilia only results in a decision to provide appropriate or perhaps more aggressive thromboprophylaxis with an anticoagulant during temporary states of increased risk (ie, pregnancy or surgery). She has since remained on warfarin outside of pregnancies, during which time she received therapeutic anticoagulation with LMWH or heparin.
Her total hip replacement surgery in addition to her AT deficiency necessitated aggressive antithrombotic management in the perioperative period. We opted to treat her with a therapeutic enoxaparin bridge after warfarin cessation preoperatively along with an infusion of AT concentrate just prior to surgery and postoperative day 1 amid concern over full therapeutic dosing in the immediate postoperative days; warfarin was reinitiated on the evening of the day of surgery, and prophylactic doses of enoxaparin were started 12 hours after surgery. Enoxaparin was increased to therapeutic doses several days after surgery, and it was discontinued when her international normalized ratio was in the therapeutic range. This management plan was successful without bleeding or thrombotic complications.
As many patients with a first unprovoked or recurrent VTE are either started on or switched to direct oral anticoagulants (DOACs), the question arises as to whether our patient should be switched to one from warfarin. There is no mechanistic reason to believe that DOACs would not be as effective as warfarin in patients with any of the hereditary thrombophilias; small case series support this premise.26 However, it is not our practice to push or even strongly recommend that highly thrombosis-prone patients transition to a DOAC from warfarin; this is provided that they have not had a recurrence in years and their international normalized ratios are well controlled.
A 29-year-old woman is referred at 18 weeks’ gestation with a recent diagnosis of AT deficiency (initial activity 73% with subsequent level of 65%). She has no personal VTE history but underwent screening for hereditary thrombophilia based on her father having died at the age of 30 years from a massive PE; he had not been diagnosed with thrombophilia prior to his untimely death. There was no other family history of VTE.
As discussed earlier, there is a synergistic relationship between inherited thrombophilic defects and high estrogen states. AT deficiency is a higher risk defect with an antepartum risk of VTE of 6% to 9%.19 However, this risk was restricted to those with a definite diagnosis of hereditary AT deficiency with levels that are generally between 40% to 60% (reference range ∼80% to 120%). Overall, the absolute risk of pregnancy-associated VTE without anticoagulation may be as high as 16%.20 Even in the absence of a definite diagnosis of AT deficiency, though, there is evidence that milder deficiency in the range of 60% to 80% can lead to an increased risk of VTE.27 There are also data that pregnancy can reduce AT levels by as much as 20%, though prior studies suggested that normotensive pregnancies may be less impacted.28,29
This adds further complexity to the interpretation of these results and nuances to decision making regarding anticoagulation. We repeated her levels, and her AT antigen and activity levels were 51% and 61%, respectively. Given her father’s fatal PE at a young age and her diagnosis of type 1 AT deficiency, she was managed with intermediate-dose enoxaparin (1 mg/kg body weight once daily) ante- and postpartum after discussion of the benefits and risks. In such cases, AT concentrate can also be administered at the onset of labor when anticoagulation is held.
The normal reference ranges of PC and PS are wider than AT; diagnoses of deficiency can therefore be more difficult to establish with certainty, as some heterozygotes with disease-causing mutations can have levels as high as 65% to 80% of normal. Additionally, functional levels for PC, PS, and AT can be impacted by certain clinical factors; acute thrombosis may potentially lower levels, heparin will lower AT, DOACs can lead to overestimation of each in clot-based assays, and warfarin will lower PC and PS while rarely elevating AT. Lastly, women on oral contraceptives and during pregnancy have a substantial reduction of PS levels; thus, a diagnosis of hereditary PS deficiency cannot be reliably made in these settings.
Acquired risk factors
A 68-year-old man presents to the emergency department with flank pain and is found to be hypoxemic; computed tomography pulmonary angiography shows bilateral segmental pulmonary emboli. He has no provoking factors, underlying illnesses, or any personal or family history of VTE. He is started on rivaroxaban therapy and discharged from the hospital. As an outpatient, he is evaluated for the presence of antiphospholipid antibodies (APAs), which show very elevated levels of anti-β-2-glycoprotein I (anti-B2GPI) immunoglobulin M (IgM) antibodies (>150) and anticardiolipin (aCL) IgM antibodies (74); these are persistently elevated at similar levels 12 weeks later. His partial thromboplastin time was not elevated on presentation, and it is decided not to discontinue anticoagulation to perform testing for a lupus anticoagulant (LA).
While we have focused thus far on the inherited thrombophilias, identification of acquired risk factors is paramount in the evaluation of patients with VTE and in decision making regarding the need for long-term anticoagulation. Transient or reversible acquired risk factors generally include surgery, trauma, immobility, hospitalization, indwelling catheters, and high estrogen states (Table 2). Indeed, the presence of such factors defines episodes of VTE as “provoked,” which confers a relatively low recurrence risk, whereas “unprovoked” events are associated with a high recurrence risk in the absence of continued anticoagulation. The recurrence rates of unprovoked, nonsurgically provoked, and surgically provoked VTE at 1 year are ∼10%, 5%, and 1%, whereas the annual rates thereafter are lower at 5%, 2.5%, and 0.5%.30 Thus, at 4 years, the recurrence rates of unprovoked compared with surgically provoked differ by an order of magnitude (25% vs 2.5%). These significant differences in recurrence risk form the basis of recommending a finite anticoagulation course of 3 to 6 months in provoked VTE compared with long-term anticoagulation in unprovoked VTE. It is worth emphasizing, though, that there is heterogeneity with regard to the strength of different provoking factors, which is reflected by different recurrence rates between surgical and nonsurgical risk factors.31,32 These differences, the multifactorial nature of VTE, and the presence of other persistent risk factors at presentation (such as obesity) highlight the need to individualize management decisions. Patient sex can also impact decision making as to the duration of anticoagulation following a first unprovoked episode of VTE, as males have been shown to have a higher recurrence risk than women.33
Acquired thrombophilias and preferred approach to diagnostic workup
| Acquired thrombophilia . | Workup . |
|---|---|
| Surgery, trauma, immobility, hospitalization, indwelling catheter, high estrogen state | History (transient/reversible risk factors) |
| Myeloproliferative neoplasm | Mutation analysis for JAK2, CALR, MPL |
| Malignancy, SLE/collagen vascular disease, nephrotic syndrome, inflammatory bowel disease, obesity | History/examination, basic laboratory tests (CBC, renal/ hepatic panels, urinalysis for protein), chest radiograph, age-appropriate cancer screening |
| Paroxysmal nocturnal hemoglobinuria | If suspected, CBC, haptoglobin, LDH, total/direct bilirubin, iron studies, urinalysis; peripheral blood flow cytometry |
| APLS | Revised Sapporo criteria (both required): clinical, vascular thrombosis and/or pregnancy morbidity; laboratory, 1 of the following on ≥2 occasions at least 12 weeks apart: IgG or IgM anti-cardiolipin antibodies (>40 U); IgG or IgM anti-β2-glycoprotein I antibodies (>40 U); LA |
| Acquired thrombophilia . | Workup . |
|---|---|
| Surgery, trauma, immobility, hospitalization, indwelling catheter, high estrogen state | History (transient/reversible risk factors) |
| Myeloproliferative neoplasm | Mutation analysis for JAK2, CALR, MPL |
| Malignancy, SLE/collagen vascular disease, nephrotic syndrome, inflammatory bowel disease, obesity | History/examination, basic laboratory tests (CBC, renal/ hepatic panels, urinalysis for protein), chest radiograph, age-appropriate cancer screening |
| Paroxysmal nocturnal hemoglobinuria | If suspected, CBC, haptoglobin, LDH, total/direct bilirubin, iron studies, urinalysis; peripheral blood flow cytometry |
| APLS | Revised Sapporo criteria (both required): clinical, vascular thrombosis and/or pregnancy morbidity; laboratory, 1 of the following on ≥2 occasions at least 12 weeks apart: IgG or IgM anti-cardiolipin antibodies (>40 U); IgG or IgM anti-β2-glycoprotein I antibodies (>40 U); LA |
CBC, complete blood count; LDH, lactate dehydrogenase; SLE, systemic lupus erythematosus.
There is little evidence that the presence of inherited thrombophilias provide such recurrence risk stratification in the setting of a first unprovoked episode of VTE, except arguably for AT deficiency.34-37 This remains true despite the ability to detect a high prevalence of inherited thrombophilias in those with VTE, which may be as high as 30% to 40% in some populations. Guidelines for anticoagulation duration therefore rely on the presence or absence of provoking factors rather than on that of inherited thrombophilias. Provoking factors are therefore the most important determinant of prognosis with respect to recurrence risk; they arguably trump the results of thrombophilia testing and perhaps the presence of markers of APLS. However, there is a paucity of data on the prognostic impact of persistently positive APAs in patients with provoked VTE, and the clinical criteria for a diagnosis of APLS does not distinguish between provoked vs unprovoked VTE.
While provoking factors have proven to be the most important determinant of recurrence risk, identification of other acquired risk factors is also likely to alter patient management. The occurrence of VTE is therefore an opportunity to identify conditions that both influence VTE risk and may have an impact on overall health outcomes; these include but are not limited to APLS, malignancy, rheumatologic diseases including systemic lupus erythematosus, inflammatory bowel disease, myeloproliferative neoplasms, obesity, nephrotic syndrome, and paroxysmal nocturnal hemoglobinuria (Table 2). Most of these conditions can be diagnosed with a thorough history, physical exam, basic laboratory evaluation including complete blood count, chemistries including renal and hepatic panels along with urinalysis for protein, chest radiograph, and age-appropriate cancer screening. Extensive evaluation for cancer is not generally recommended, as it has not convincingly shown to improve survival; limited cancer screening identifies most cases reasonably well.38-40 This approach to testing is fairly straightforward and inexpensive and can generally be accomplished within a single clinic appointment. The importance of identifying these acquired conditions cannot be overstated, although in discussions of hypercoagulability they often are underemphasized for the sake of the nuanced testing and interpretation of the inherited thrombophilias.
Of both the acquired and inherited risk factors, APLS in patients with a first unprovoked VTE is unique in that it is associated with an increased risk of recurrence, can lead to arterial thrombosis, and may also have specific treatment implications with regard to anticoagulant choice. APLS is an autoimmune disorder, diagnosed by the required presence of VTE, arterial thrombosis, or obstetric complications alongside persistently positive APAs over 12 weeks. A proportion of healthy individuals (∼3% to 9%) may have elevated levels of APAs; however, they are generally of low titer, transient, and often without any clear clinical relevance.41-43 While most patients with VTE can be effectively managed with DOACs, APLS is a condition that merits consideration of warfarin as first-line therapy.26 The recent TRAPS trial compared rivaroxaban to warfarin in “triple-positive” APLS patients (positive for LA, anti-B2GPI, and anti-aCL antibodies) and was terminated early due to more thromboembolic events in the rivaroxaban arm (12% vs 0% in those on warfarin), all of which were arterial in nature (except for 1 bilateral deep vein thrombosis that developed while off drug due to bleeding). There was also more major bleeding in the rivaroxaban arm.44 It should be noted, however, that this was a particularly high-risk APLS population including many patients with underlying connective tissue disease.
Another study suggested a higher risk of recurrent thromboses in APLS on DOACs as compared with warfarin, while the earlier RAPS trial did not45,46 ; the primary end points of the RAPS trial, however, were coagulation markers. Additionally, in a cohort of patients with unprovoked VTE who stopped anticoagulation in response to a low d-dimer, the presence of no APAs (though the cutoff for positivity was less than the standard of 40), a 1-time positive APA, and a persistent positive APA were associated with a recurrent VTE event rate of 5.4%, 10.5%, and 16.2%, respectively.47 These findings highlight the unique therapeutic implications and high recurrence risk of a diagnosis of APLS.
Our patient in this case experienced an unprovoked VTE episode with findings of persistently positive anti-aCL and anti-B2GPI of the IgM isotype, consistent with a diagnosis of APLS. While he did not have LA testing performed due to being maintained on anticoagulation, his partial thromboplastin time at baseline was normal, potentially reducing the likelihood that a LA was present. Although triple-positive APLS patients are deemed to be at particularly high recurrence risk, there is less strong evidence for the recurrence risk among patients who are single or double positive. Whereas the presence of a persistently positive LA is felt to be the single strongest marker of recurrence risk, there are data to suggest that patients with presence of elevated IgM levels alone may be less prothrombotic than other laboratory profiles.48-50
A discussion of risks, benefits, and differences in anticoagulant drugs was held with our patient. He strongly preferred to remain on rivaroxaban as opposed to switching to warfarin and understood the risks. His lack of any prior VTE history, the presence of venous as opposed to arterial thrombosis, and the potentially lower-risk IgM isotype as opposed to IgG suggest that he may be in a lower recurrence risk subgroup of APLS, for which non-warfarin therapy may be effective; data, however, are unfortunately limited, and other trials of DOACs as compared with warfarin in APLS are ongoing. Fortunately, he has tolerated rivaroxaban therapy well without any complications for several years. Another caveat, however, is that we would not consider reducing him to a lower dose of rivaroxaban (10 mg as opposed to 20 mg), which is an option for many patients with a first VTE based on the results of the EINSTEIN Choice trial.51
In contrast, if a patient presents with arterial thrombosis in association with APLS, warfarin would be strongly recommended as first-line therapy given the absence of data to support the efficacy of DOACs in such patients. Furthermore, if a patient with unprovoked VTE has been diagnosed with APLS and is also at increased bleeding risk or has sustained a bleed, the presence of APLS denotes a high-risk thrombophilia and constitutes a strong reason to attempt continued long-term anticoagulation. Indeed, individualized management including patient preference with respect to long-term anticoagulation is paramount.
Authorship
Contribution: This article was written by J.M. and K.A.B.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenneth A. Bauer, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02115; e-mail: kbauer@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal