Introduction
The World Trade Center (WTC) disaster exposed first responders to high levels of aerosolized carcinogens (Lioy et. al. Env. Health Perspect 2002). Clonal hematopoiesis is associated with exposure to smoking and genotoxic stimuli (Jaiswal et. al. NEJM 2014; Genovese et. al. NEJM 2015). We sought to determine its incidence in WTC-exposed first responders. We also assessed the effect of WTC particulate matter (WTC-PM) on genome integrity in vitro, and in murine studies.
Methods
Deep targeted sequencing was performed on blood collected from 481 first responders (429 WTC-exposed firefighters, 52 WTC-exposed emergency medical service workers) and 52 non-exposed first responders. Samples were analyzed for 237 genes mutated in hematologic malignancies and interpreted using reference databases. Non synonymous somatic mutations were annotated and analyzed.
Results
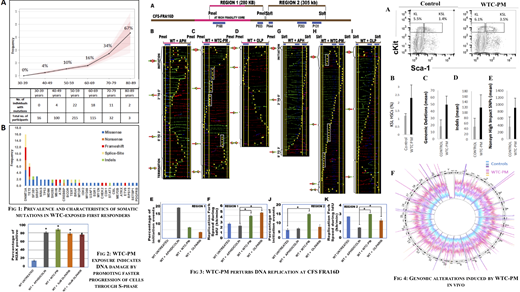
In the WTC-exposed cohort, 57 individuals with 66 somatic mutations of expected pathogenic potential were identified (overall prevalence 11.9%). In the non-exposed cohort, only one pathogenic mutation was found in the IDH2 gene (overall prevalence 1.9%). There was a strong association between increasing age and prevalence of mutations in the WTC-exposed cohort (Fig 1A). DNMT3A (16/66), TET2 (7/66), SF3B1 and SRSF2 (3/66 each) were the most common genes identified in the WTC-exposed cohort (Fig 1B). Median VAF was 12% and missense mutations were most frequent alteration. Aging, smoking, DNA repair and alkylating agent exposure related mutational signatures were observed with a cytosine to thymine (C→T) transition being most common.
Next, we assessed the effect of WTC-PM on genome integrity and replication in vitro. WTC-PM that was collected in the first three days after 9/11 was used in concentrations mimicking exposure levels. Lymphocytes exposed to WTC-PM demonstrated a significant increase in phosphorylated H2AX foci accumulation, suggesting a DNA damage response (Fig 2). Since common fragile sites (CFSs) detect basal levels of stress in the cell, and activate DNA damage response (DDR), we profiled DNA replication dynamics at CFS-FRA16D at very high resolution using the single molecule analysis of replicated DNA (SMARD) assay. Treatment with WTC-PM significantly altered replication at two common fragile sites (regions 1 and 2 of FRA16D, Fig 3A) with replication pausing being observed at multiple sites (Fig 3B-I, white rectangles). Striking increase in replication initiation was seen, characterized as dormant origins activated to rescue replication pausing (Fig 3E, J). These alterations were accompanied by a corresponding increase in replication speed, conditions that lead to DNA replication errors and mutagenesis (Fig 3F, K).
Next, we treated mice with WTC-PM via the oropharyngeal route to mimic first responder exposures, and then harvested and analyzed their bone marrow compartments. Significant expansion of hematopoietic stem cells (Kit+, Sca1+, Lineage-ve, KSL) was seen in WTC-PM treated mice (Fig 4A,B). Whole genome sequencing of sorted stem cells showed a significant increase in non-synonymous SNPs, deletions and indels in the WTC-PM treated samples when compared to control (Fig 4C-E). These genomic alterations were found to occur at low VAF throughout the whole genome, demonstrating widespread genotoxic effects of WTC-PM on hematopoietic stem cells in vivo (Fig 4F).
Discussion
We report a high burden of mutations in 11.9% (57/481) WTC-exposed first responders compared to the non-exposed cohort (1.9%, 1/52). The frequency of the somatic mutations was many fold higher than in previous studies (Jaiswal et. al. NEJM 2014; Genovese et. al. NEJM, 2015). In the 50-59 year age group, 10% of WTC-exposed individuals carried somatic mutations, compared to the frequency of 2.5% reported by Jaiswal et. al. for the same age group. Despite deeper sequencing performed in our study, the median VAF in our study was 12%, indicating that the difference in technique did not bias our study towards increased detection of small, subclinical clones when compared to previous studies. Furthermore, we demonstrate that WTC-PM can perturb DNA replication and increased genomic instability in vivo, potentially leading to higher burden of clonal hematopoiesis in WTC-exposed first responders. These results demonstrate adverse environmental exposures can be associated with a high rate of clonal hematopoiesis.
Landgren:Sanofi: Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Other: IDMC; Theradex: Other: IDMC; Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees. Fletcher:Genoptix/Neogenomics: Employment. Ebert:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.; Celgene: Research Funding; Deerfield: Research Funding. Steidl:GlaxoSmithKline: Research Funding; Celgene: Consultancy; Aileron Therapeutics: Consultancy, Research Funding; Stelexis Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Co-Founder; Pieries Pharmaceuticals: Consultancy; BayerHealthcare: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Will:Novartis Pharmaceuticals: Research Funding. Verma:Stelexis: Equity Ownership, Honoraria; Acceleron: Honoraria; Celgene: Honoraria; BMS: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.