Background:
Although the JAK1/2 inhibitor RUX provides significant benefits to patients with myelofibrosis (MF), activation of non-JAK pathways could lead to therapeutic resistance, suggesting a need for rationally developed therapeutic combinations. We hypothesized that the combination of RUX and AZA would target distinct clinical and pathological manifestations of MF, resulting in synergistic efficacy. Updated phase II study results are presented in this abstract.
Methods:
This is a single-arm Phase II trial of RUX in combination with AZA for pts with MF. Eligible pts included adults >18 years with newly diagnosed or previously treated (excluding previous therapy with RUX or AZA) MF with int-1, int-2, or high risk according to the DIPSS criteria. RUX at 15-20 mg orally twice daily (BID) was given alone in 28 day cycles for the first 3 cycles. AZA was added from cycle 4 onwards at 25 mg/m2 days 1-5, and could be gradually up-titrated to 75 mg/m2 days 1-5 over subsequent cycles if clinically indicated. AZA could be initiated prior to cycle 4 in pts with proliferative disease or high bone marrow blasts. The primary objective was to determine the response rate per the IWG MRT 2013 criteria. Secondary objectives included safety and tolerability.
Results:
60 pts were enrolled (58 evaluable, 2 too early) between May 2013 and June 2019. Pt characteristics are in Table 1. The median age was 66 years (range, 46-87). Forty pts (67%) had int-2/high DIPSS score, 9 (15%) had ≥10% blasts. JAK2V617Fwas detected in 35 pts (58%). Among 44 pts with comprehensive molecular panel, 21 (48%) had additional mutations (ADD mut), with the most frequent being ASXL1 and TET2 (8 pts each, 18%; Table 1), followed by IDH, TP53 and DNMT3A.
AZA was given to 53 pts (88%), with 6 (11%) pts initiating AZA earlier than cycle 4. Median (med) follow-up was 35 months (range, 1-70+) with a med of 26 cycles (range, 1-70) administered. 20 pts (33%) are still on RUX AZA therapy, with a med of 47 cycles (range, 1-70), including 7 pts who have received >65 cycles. Twenty nine (48%) and 16 (30%) pts required RUX and AZA dose reduction and/or treatment interruption, respectively. Med dose of RUX was 15 mg BID (range, 5-25).
Among 58 evaluable pts, 43 (74%) achieved IWG-MRT 2013 objective response (OR) on study (Table 2), with a med time to response of 2.0 months (range, 0.7-19.0). Among 43 pts with baseline splenomegaly ≥5 cm, 30 (70%) and 25 (58%) had palpable spleen reduction ≥50% at any time on study and at week 24, respectively. Thirty percent of responses (13/43), including responses in spleen (10/30), occurred only after the addition of AZA (Table 2). Med time to response after the addition of AZA was 4.0 months (range, 0.5-17.0). Median overall survival (OS) was not reached, with 1-year and 3-year OS rates of 89% and 66%, respectively.
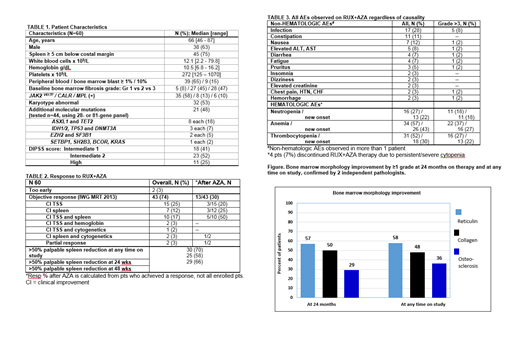
Improvement in bone marrow morphology (fibrosis, collagen and/or osteosclerosis), was noted in 21 (61%) of 35 sequentially evaluated patients (Figure 1, bone marrow improvements at 24 months and at any time on a study). The med time to response was 12.0 months (range, 6.0-18.0), and responses have been sustained in all but 1 patient. Updated results will be presented at the meeting.
The med duration of responses (DoR) was not reached for OR, ≥50% spleen length reduction, or symptom response. Patients with ≥1 ADD mut appeared to have shorter DoR spleen (≥50% spleen length reduction) than those with no ADD mut; 47 months vs NR (p=0.05). Patients with TP53 and IDH1/2 mutation had shorter DoR (DoR [OR] 18.5 and 13.0 months, respectively) than those with no ADD mut, ASXL mut, or any other single ADD mut (DoR [OR] 46.0, 45.0 months and NR).
The most common possibly therapy related non-hematological adverse events (AEs) were infections (28%) and constipation (11%). Significant hematological AEs included grade III-IV anemia (37%), neutropenia (18%) and thrombocytopenia (27%), these were transient and did not require therapy interruption in most cases. Only 4 pts discontinued therapy due to drug-related AEs (cytopenia in 4 pts). Table 3 summarizes AEs on study.
Conclusion:
Concomitant RUX with AZA appears safe and effective. Myelosuppression was manageable with only 4 pts (7%) requiring study discontinuation due to cytopenias, a similar rate to single agent RUX in phase III trials (7-9%). A 2013 IWG-MRT objective response and ≥50% spleen reduction were achieved in ≥ 70% of pts at any time on study; and responses appear durable. The study is ongoing (NCT01787487).
Verstovsek:Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Genetech: Research Funding; CTI BioPharma Corp: Research Funding; Incyte: Research Funding; NS Pharma: Research Funding; Roche: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Bose:CTI BioPharma: Research Funding; Promedior: Research Funding; NS Pharma: Research Funding; Astellas: Research Funding; Pfizer: Research Funding; Constellation: Research Funding; Kartos: Consultancy, Research Funding; Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding. Pemmaraju:affymetrix: Research Funding; sagerstrong: Research Funding; Daiichi-Sankyo: Research Funding; plexxikon: Research Funding; novartis: Consultancy, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; cellectis: Research Funding; celgene: Consultancy, Honoraria; samus: Research Funding; mustangbio: Consultancy, Research Funding; abbvie: Consultancy, Honoraria, Research Funding; incyte: Consultancy, Research Funding. Cortes:Astellas Pharma: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cyclacel LTD: Research Funding; Amgen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Borthakur:Agensys: Research Funding; Novartis: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Argenx: Membership on an entity's Board of Directors or advisory committees; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cantargia AB: Research Funding; Merck: Research Funding; Arvinas: Research Funding; Polaris: Research Funding; Strategia Therapeutics: Research Funding; Tetralogic Pharmaceuticals: Research Funding; Eisai: Research Funding; Xbiotech USA: Research Funding; PTC Therapeutics: Consultancy; AstraZeneca: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Oncoceutics: Research Funding; Cyclacel: Research Funding; Bayer Healthcare AG: Research Funding; Incyte: Research Funding; BMS: Research Funding; GSK: Research Funding; AbbVie: Research Funding; NKarta: Consultancy; Janssen: Research Funding; Eli Lilly and Co.: Research Funding; Oncoceutics, Inc.: Research Funding. Kadia:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; AbbVie: Consultancy, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. DiNardo:celgene: Consultancy, Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; medimmune: Honoraria; syros: Honoraria; jazz: Honoraria; daiichi sankyo: Honoraria; agios: Consultancy, Honoraria; abbvie: Consultancy, Honoraria. Ravandi:Menarini Ricerche: Research Funding; Selvita: Research Funding; Cyclacel LTD: Research Funding; Xencor: Consultancy, Research Funding; Macrogenix: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sasaki:Otsuka: Honoraria; Pfizer: Consultancy. Garcia-Manero:Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding; Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding. Bueso-Ramos:Incyte: Consultancy. Kantarjian:Jazz Pharma: Research Funding; Cyclacel: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; AbbVie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Agios: Honoraria, Research Funding; Astex: Research Funding; Takeda: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Ariad: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.