Key Points
Ibrutinib 560 mg daily plus palbociclib 100 mg on days 1 to 21 of each 28-day cycle could be safely administered to patients with previously treated MCL.
Complete responses and duration of response (median, >2 years) were high relative to studies of single-agent ibrutinib.
Abstract
Single-agent ibrutinib is active in patients with previously treated mantle cell lymphoma (MCL); however, nearly half of all patients experience treatment failure during the first year. We previously demonstrated that prolonged early G1 cell cycle arrest induced by the oral, specific CDK4/6 inhibitor palbociclib can overcome ibrutinib resistance in primary human MCL cells and MCL cell lines expressing wild-type Bruton’s tyrosine kinase (BTK). Therefore, we conducted a phase 1 trial to evaluate the dosing, safety, and preliminary activity of palbociclib plus ibrutinib in patients with previously treated mantle cell lymphoma. From August 2014 to June 2016, a total of 27 patients (21 men, 6 women) were enrolled. The maximum tolerated doses were ibrutinib 560 mg daily plus palbociclib 100 mg on days 1 to 21 of each 28-day cycle. The dose-limiting toxicity was grade 3 rash. The most common grade 3 to 4 toxicities included neutropenia (41%), thrombocytopenia (30%), hypertension (15%), febrile neutropenia (15%), and lung infection (11%). The overall and complete response rates were 67% and 37%, and with a median follow-up of 25.6 months, the 2-year progression-free survival was 59.4% and the 2-year response duration was 69.8%. A phase 2 multicenter clinical trial to further characterize efficacy is now ongoing. The current trial was registered at www.clinicaltrials.gov as #NCT02159755.
Introduction
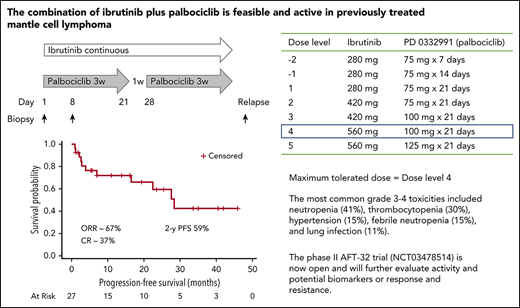
Mantle cell lymphoma (MCL) is characterized by cell cycle dysregulation. Sustained inhibition of CDK4 by palbociclib, an oral inhibitor of CDK4, induces prolonged early G1 cell cycle arrest (pG1) in Rb-positive MCL tumor cells, regardless of the p53 status.1 In a phase 1b study in patients with previously treated MCL, palbociclib yielded a response rate of 18%, with 5 of 12 patients remaining on study beyond 1 year.2 Ibrutinib is an oral inhibitor of Bruton’s tyrosine kinase (BTK) with a response rate (RR) of 68%, a complete response (CR) rate of 21%, and a median progression-free survival (PFS) of 13.9 months in patients with previously treated MCL.3 The molecular basis for resistance appears to be a result of bypassing of the BTK blockade, frequently signaling through PI3K.4 Preclinical data demonstrated that induction of pG1 by palbociclib can sensitize ibrutinib-resistant MCL cells to killing by ibrutinib.4 We conducted a phase 1 trial to evaluate the dosing, safety, and activity of palbociclib plus ibrutinib in patients with previously treated MCL.
Methods
Patients with previously treated MCL, a good performance status, adequate bone marrow/organ function, and no prior treatment with BTK or CDK4/6 inhibitors were eligible. Treatment consisted of ibrutinib administered daily and palbociclib administered for 21 days of each 28-day cycle at the following dose levels: level 1, ibrutinib 280 mg, palbociclib 75 mg; level 2, ibrutinib 420 mg, palbociclib 75 mg; level 3, ibrutinib 420 mg, palbociclib 100 mg; level 4, ibrutinib 560 mg, palbociclib 100 mg; and level 5, ibrutinib 560 mg, palbociclib 125 mg. Patients received treatment until progression, unacceptable toxicity, or withdrawal.
The primary objective of this phase 1 trial was identification of the recommended phase 2 dose, with secondary objectives including estimation of the toxicity and activity profiles. Adverse events were graded per Common Terminology Criteria for Adverse Events v4.0. Response evaluation and time-to-event outcomes were reported per International Working Group criteria3 and estimated on the basis of the Kaplan-Meier method, with 95% confidence intervals calculated using Greenwood’s formula. The final data lock was June 25, 2018.
All research was approved by institutional review boards, and participants gave written informed consent. All authors had access to primary trial data.
Results and discussion
From August 2014 to July 2016, 27 patients were enrolled (dose level 1, n = 3; dose level 2, n = 3; dose level 3, n = 6; dose level 4, n = 10; dose level 5, n = 5). Patient characteristics are described in Table 1.
The median number of cycles administered was 15 (range, 1-51), with 9 patients still ongoing (range, 16-51 cycles). Reasons for coming off study included progression (n = 9), allogeneic stem cell transplantation (n = 4), adverse event (cytopenias, n = 2; elevated liver enzymes, n = 1), refused further treatment (n = 1), and death unrelated to MCL or study treatment (n = 1).
The maximum tolerated dose identified was dose level 4, ibrutinib 560 mg and palbociclib 100 mg. The dose-limiting toxicity at dose level 5 was grade 3 rash in 2 of 5 patients. One additional dose-limiting toxicity, grade 4 neutropenia lasting more than 7 days, occurred at dose level 3. Grade 3 to 4 toxicities occurring in at least 2 patients regardless of attribution included neutropenia (41%), thrombocytopenia (30%), anemia (15%), hypertension (15%), febrile neutropenia (15%), lung infection (11%), upper respiratory tract infection (7%), fatigue (7%), increased alanine aminotransferase/aspartate aminotransferase (7%), rash (7%), hyperglycemia (7%), and myalgia (7%). Other events of interest included one of each of the following: grade 1 reactivation of viral hepatitis, grade 2 Clostridium difficile infection, grade 3 varicella zoster viral encephalitis, grade 3 influenza A infection, grade 3 atrial fibrillation, grade 3 decreased ejection fraction, grade 3 pneumonitis, and grade 4 gastric hemorrhage. Across all dose levels, 4 patients required dose interruptions and 8 patients required a dose reduction while receiving study treatment, primarily because of cytopenias.
Eighteen patients (67%) responded to treatment, including 10 (37%) CRs. Among 9 patients without a documented response to last prior therapy, 2 patients achieved a CR and 1 achieved a PR. Among 7 patients with a Ki67 higher than 30%, 5 responded, including 3 patients with a CR. Among 7 patients with a high risk Mantle Cell International Prognostic Index score, 4 responded, including 1 with a CR.
With a median follow-up in survivors of 25.6 months, the 2-year PFS was 59.4% (95% confidence interval, 37.9%-80.9%), and the 2-year overall survival was 60.6% (95% confidence interval, 41.1%-80.1%; Figure 1). There was no association between Mantle Cell International Prognostic Index score (P = .222), Ki67 (P = .359), or response to last therapy (P = .262) and PFS. Eleven patients have died, including 9 deaths related to MCL, 1 death related to complications of allogeneic stem cell transplantation, and 1 death of unknown causes unrelated to MCL or study treatment. The median survival after progression was 5 months. Among 4 responding patients who stopped treatment to undergo allogeneic stem cell transplantation, 1 died of progressive MCL, 1 died of complications of therapy, 1 experienced disease progression and graft-vs-host disease requiring re-initiation of ibrutinib, and 1 remains in remission.
Survival outcomes: median follow-up time, 25.6 months. (A) PFS. Two-year PFS was 59.4%. (B) Overall survival. Two-year overall survival was 60.6%.
Survival outcomes: median follow-up time, 25.6 months. (A) PFS. Two-year PFS was 59.4%. (B) Overall survival. Two-year overall survival was 60.6%.
Inhibition of BTK represents a major advance in the treatment of hematologic malignancies. Unfortunately, the limitations of BTK inhibition are particularly apparent in MCL, with most patients developing resistance within 10 to 14 months and with poor survival rates after treatment failure.5 Although some patients with MCL develop ibrutinib resistance because of mutations in BTK4 or in genes downstream of BTK,6 it is likely that most resistance arises through activation of signaling pathways that bypass BTK blockade. In preclinical studies, the combination of ibrutinib and palbociclib synergistically killed ibrutinib-resistant MCL cells, and the mechanism of synergy appeared to be related to inhibition of compensatory signaling pathways, principally PI3k.4 In this study, we found that the combination of ibrutinib and palbociclib was feasible and active. Despite the apparent durability of responses, the overall RR of 67% was similar to the RR reported in the phase 2 pivotal trial with single-agent ibrutinib.3 A comparable experience was noted in the trial of ibrutinib, lenalidomide, and rituximab, which reported a RR of 76% (CR, 56%),7 and the trial of ibrutinib plus venetoclax, which reported a RR of 71% (CR, 60%).8 Similar to both those studies, we found that responders to ibrutinib-based combinations had a low rate of treatment failure, but the roughly one-third of patients with primary BTK inhibitor resistance appear unlikely to be served by BTK-inhibitor-based combinations. Patients with chemotherapy-refractory or blastoid MCL are frequently resistant to BTK inhibitors, and these patients should be considered for treatment on clinical trials with alternative therapies.
Although BTK-inhibitor-based combinations appear promising, the degree to which they improve on single-agent ibrutinib is unclear. In recent analyses, ibrutinib yielded a median PFS of 25.4 months in patients with only 1 prior therapy,5 and acalabrutinib produced a CR rate of 40% in patients with a median of 2 prior therapies.9 Randomized trials will be required to define the true effect on efficacy and safety.
The ongoing phase 2 study conducted by Alliance Foundation Trials, LLC (AFT-32, NCT03478514) will evaluate ibrutinib plus palbociclib at the maximum tolerated dose of this trial. Prophylactic antimicrobials and liberal use of growth factors are recommended. The trial incorporates genetic profiling plus functional assays of gene and protein expression over time. Given the multitude of exciting combinations, it will be valuable to have a better understanding of tumor- and patient-specific factors to better tailor treatment to individual patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Pamela Harris for her insight and advocacy.
This study was sponsored by Cancer Therapy Evaluation Program (CTEP). This study was supported in part by National Institutes of Health, National Cancer Institute grant RO1CA18894 (S.C.-K.), National Institutes of Health, National Cancer Institute grant 1K24 CA201524-01 (K.A.B.), Alliance for Clinical Trials in Oncology Scholar award (P.M.), Translational Research Grants from Lymphoma Research Foundation (S.C.-K. and P.M.) and V-Foundation (S.C.-K., P.M., J.P.L., M.D.), and a Mantle Cell Lymphoma Research Initiative Award (MCL7001-18) from the Leukemia & Lymphoma Society. Funding for this project also has been provided by the Sarah Cannon Fund at the HCA Foundation (S.-C.K., P.M., J.P.L., and M.D.).
Authorship
Contribution: P.M. provided study design, contributed patients, collected and analyzed data, and prepared the manuscript; N.L.B., K.A.B., S.P., K.M., J.R., and C.D. contributed patients and data, and reviewed and edited the manuscript; L.R. contributed patients and data; Z.C. contributed to study design and performed statistical analysis; X.H. provided data generation and analysis; G.I. contributed data; M.D. provided data generation and analysis and funding support; S.C.-K. contributed to study design, data generation and analysis, manuscript preparation, and funding support; and J.P.L. contributed to study design, data analysis, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: P.M. served as a consultant for Janssen, Gilead, AstraZeneca/Acerta, Celgene, Karyopharm, and Sandoz. S.P. received research funds from Takeda, Teva, Seattle Genetics, and BMS; served as a consultant for BMS, Rafael Pharma, G1 Therapeutics, Teva, and Bayer; and served on the speakers bureau for Gilead and Seattle Genetics K.A.B. received research funds from Pharmacyclics and Janssen. K.M. received research funds from Pharmacyclics, Merck, BMS, and Novartis and served as a consultant: for Pharmacyclics, AstraZeneca/Acerta, Bayer, Novartis, and Teva. J.P.L. served as a consultant for Sutro, Bayer, MEI Pharma, Gilead, AstraZeneca, Novartis, Celgene, Biotest, Merck, Morphosys, Beigene, Nordic Nanovector, Roche/Genentech, and ADC Therapeutics. The remaining authors declare no competing financial interests.
The current affiliation for K.A.B. is Emory University, Atlanta, Georgia.
The current affiliation for S.P. is Levine Cancer Institute, Atrium Healthcare, Charlotte, North Carolina.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 5 December 2016.
Correspondence: Peter Martin, Weill Cornell Medicine, Department of Internal Medicine, 525 East 68th St, New York, NY 10021; e-mail: pem9109@med.cornell.edu.