Abstract
Monoclonal gammopathy of undetermined significance (MGUS) is, in many ways, a unique hematologic entity. Unlike most hematologic conditions in which the diagnosis is intentional and credited to hematologists, the discovery of MGUS is most often incidental and made by nonhematologists. MGUS is considered an obligate precursor to several lymphoplasmacytic malignancies, including immunoglobulin light-chain amyloidosis, multiple myeloma, and Waldenström macroglobulinemia. Therefore, long-term follow-up is generally recommended. Despite its high prevalence, there is surprisingly limited evidence to inform best clinical practice both at the time of diagnosis and during follow-up. We present 7 vignettes to illustrate common clinical management questions that arise during the course of MGUS. Where evidence is present, we provide a concise summary of the literature and clear recommendations on management. Where evidence is lacking, we describe how we practice and provide a rationale for our approach. We also discuss the potential harms associated with MGUS diagnosis, a topic that is rarely, if ever, broached between patients and providers, or even considered in academic debate.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant, clonal plasma cell disorder, characterized by the presence of a monoclonal (M) protein, <10% clonal plasma cells in the bone marrow, and absence of multiple myeloma or related lymphoplasmacytic malignancies (LPMs).1 MGUS is present in 3% of the general population ≥50 years old, but only 0.3% among those <50 years old.2,3 There is a higher risk and earlier age of onset in blacks than in whites.3,4 It is considered a requisite precursor of multiple myeloma (MM), as well as immunoglobulin light-chain (AL) amyloidosis and Waldenström macroglobulinemia (WM), and can be detected years before the diagnosis of these particular LPMs.5-7 There are 3 subtypes of MGUS, namely, immunoglobulin M (IgM) MGUS, non-IgM MGUS, and light-chain MGUS, each with distinct rate and type of progression (Table 1).8,9
MGUS is of considerable clinical importance because of its high prevalence in the general population, the persistent risk of progression to LPM, its known causal association with several serious nonmalignant disorders, and the high frequency with which coincidental associations are detected in practice. Since its first description in 1960 by Jan G. Waldenström as “essential hyperglobulinemia” or “benign monoclonal gammopathy” and the coinage of the current term by Robert A. Kyle in 1978,10,11 there has been a remarkable amount of progress in understanding the biology, epidemiology, disease associations, and natural history of MGUS.12,13 Even though there is universal agreement on the criteria for the diagnoses of MGUS and LPMs,1 the more practical aspects, such as guidelines for the extent of initial evaluation and subsequent follow-up of MGUS, are less than uniform because of the lack of high-level evidence.14-17 In this article, we select 7 MGUS cases from our daily practice to illustrate commonly encountered clinical questions and describe how we manage them. We also summarize the relevant supporting literature and highlight controversial areas in which evidence is insufficient or absent.
Case 1. Indications for testing and disease associations
A 75-year-old man was admitted for overnight observation after presenting with a 5-day history of headache, persistent cough, severe lower-rib pain, and generalized weakness. Physical examination and chest x-ray were unremarkable. The next day, nasal swab showed the presence of influenza A. On admission, his laboratory evaluation (reference ranges provided parenthetically) was remarkable only for a hemoglobin of 12.5 g/dL (13.5-17.5). Subsequent tests included a serum protein electrophoresis (SPEP), immunofixation, and free light-chain (FLC) studies that revealed a monoclonal IgGλ of 0.5 g/dL with normal FLC values. The anemia and rib pains resolved weeks later.
When do we test for monoclonal gammopathy?
In general, we test for the presence of monoclonal gammopathy in patients who have clinical symptoms and signs concerning for the presence of MM, AL amyloidosis, or WM. However, with the exception of diffuse lytic bone lesions, macroglossia, infiltrative cardiomyopathy, and engorgement of retinal veins, most presenting symptoms and signs of LPMs are nonspecific. As a result, in most instances when testing for M-proteins is performed, an alternative explanation for the clinical presentation is typically found, and patients with positive tests are labeled as having an incidental diagnosis of MGUS. Because MGUS is common and LPMs are rare (∼35 000 new cases annually),2,18 the chance of identifying LPM in day-to-day practice is very low. In a study of 7090 patients without a history of LPM who had SPEP performed for various indications, 3% were found to have MGUS, and only 1% were diagnosed with LPM. The majority (81%) of tests were performed by nonhematologists.19 It is estimated that, on average, a hematologist-oncologist in the United States sees 2 new MM cases annually and 1 new case of AL and WM every 10 years.20 These numbers are expected to be far less in general medical practice. We also look for monoclonal gammopathy if a patient has a nonmalignant disease known to be a cause of, or associated with, monoclonal gammopathy, especially if the treatment requires control or eradication of the plasma cell clone (see the next section).
What are the nonmalignant diseases associated with monoclonal gammopathy?
Table 2 displays a list of nonmalignant diseases secondary to, or associated with, monoclonal gammopathy. The presence of any of these conditions prompts us to screen for monoclonal gammopathy, even though signs or symptoms of LPMs are absent. Because the management of these diseases is beyond the scope of this article, we have provided relevant references from recent publications. In addition, we direct the readers to case vignettes and reviews for more in-depth clinical descriptions and general management.21-25
How do we screen for monoclonal gammopathy?
We perform SPEP, serum immunofixation, and FLC as the initial screening test when looking for LPMs associated with monoclonal gammopathy. Urine protein electrophoresis is ordered subsequently when an M-protein is detected. This panel of testing is highly sensitive because it will detect an M-protein in virtually all patients with MM, AL amyloidosis, and WM.26
Case 2. Extent of evaluation
A 78-year-old woman was evaluated for chronic progressive right-shoulder pain, limiting her joint mobility. She was a farmer and engaged in heavy lifting. A shoulder x-ray showed advanced degenerative arthritis and no lytic lesion. Laboratory tests revealed a normal complete blood count, calcium, and creatinine, but total protein was elevated at 8.7 g/dL (6.3-7.9). Further testing showed IgGκ M-protein of 1.7 g/dL, κ FLC of 8.61 mg/dL (0.33-1.94), λ FLC of 0.63 mg/dL (0.57-2.63), and κ/λ ratio of 13.67 (0.26-1.65). Because the M-protein and FLC were substantially elevated, additional work-up was performed. A bone marrow biopsy showed 6% κ-restricted plasma cells. A low-dose whole-body computed tomography (CT) scan did not show lytic lesions. She was diagnosed with MGUS.
What are the minimum tests necessary during the initial evaluation of monoclonal gammopathy?
Once an M-protein is detected, the extent of further evaluation to rule out LPM depends on the pretest probability of the latter and whether or not an alternative explanation for the signs or symptoms that prompted the screening was found. We generally order the following tests, if not yet done, at the time of hematology consult: complete blood count, serum calcium, creatinine, FLC, immunofixation, and 24-h urine protein electrophoresis.
When do we perform skeletal imaging and bone marrow biopsy?
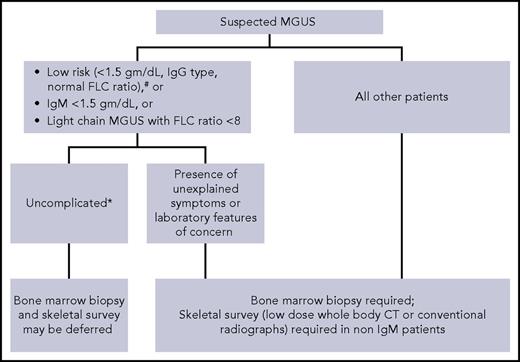
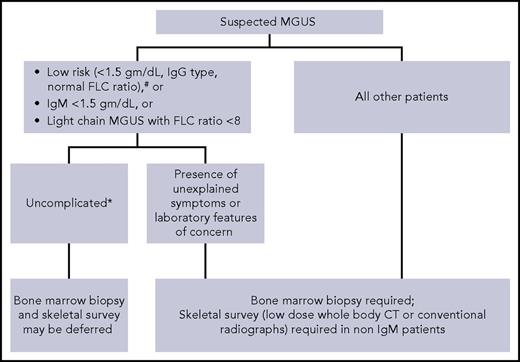
Although by definition, MGUS requires bone marrow clonal plasma cells <10% and no evidence of lytic lesions on skeletal imaging,1 not all patients with suspected MGUS need these tests (Figure 1).15,27 In patients with low-risk MGUS who do not have any unexplained clinical concerns, skeletal imaging and bone marrow biopsy can be deferred. We use the Mayo Clinic risk stratification model with low-risk defined as having all of the following: serum M-protein ≤1.5 g/dL, IgG isotype, and normal FLC ratio.28 A study of 1271 patients found that the probability of finding bone marrow plasma cells ≥10% in patients with an IgG MGUS and M-spike ≤1.5 g/dL was very low (4.7%). Similarly, the probability of finding bone lesions with M-spike ≤1.5 g/dL was also very low (2.5%).29 Moreover, in a large Mayo Clinic study, the life-time risk of progression was only 2% in patients with low-risk MGUS, despite the fact that only 10% of patients had baseline bone marrow biopsies to confirm the diagnosis.28 Thus, routine skeletal imaging and bone marrow biopsy in low-risk MGUS have a low yield. In these patients, a follow-up assessment of M-protein level in 6 months will most likely identify any patient who needs further evaluation. Because approximately 50% of MGUS patients are low risk,28 avoiding skeletal imaging and bone marrow biopsy in these patients will minimize health care costs without adversely affecting clinical outcome. Risk stratification models developed by the Spanish and Swedish groups are also available.9,30
Suggested algorithm for bone marrow biopsy and skeletal imaging in patients with monoclonal gammopathy of undetermined significance. #Mayo Clinic Risk Stratification Model. *No unexplained symptoms or laboratory features concerning for serious plasma cell disorder.
Suggested algorithm for bone marrow biopsy and skeletal imaging in patients with monoclonal gammopathy of undetermined significance. #Mayo Clinic Risk Stratification Model. *No unexplained symptoms or laboratory features concerning for serious plasma cell disorder.
Skeletal imaging can also be deferred in IgM MGUS patients without unexplained bony symptoms. IgM M-protein is associated most commonly with WM, which seldom causes lytic lesions. Although IgM MM can be encountered rarely, we feel that in the absence of bone-related symptoms, routine skeletal imaging in IgM MGUS is unnecessary. Similarly, a baseline bone marrow biopsy can also be omitted in patients with apparently asymptomatic IgM MGUS who have a small quantity of M-protein (<1.5 gm/dL) and normal blood counts because the probability of finding an LPM needing therapy is very low. Although data on light-chain MGUS are lacking, we do not recommend routine skeletal imaging and bone marrow evaluation in patients who have a low involved/uninvolved FLC ratio (<8) in whom there are no clinical concerns for LPMs.
For all other patients with MGUS (as in the patient presented in case 2), we perform skeletal imaging (either conventional radiographic survey or low-dose whole-body CT) and bone marrow biopsy at the time of diagnosis. If available, low-dose whole-body CT is preferred because it is a more sensitive test.31
Case 3. Follow-up
A 70-year-old man who moved to town was seen in the outpatient clinic to establish primary care. Initial blood tests showed normal complete blood count, calcium, and creatinine levels. He was diagnosed with IgGκ MGUS 10 years ago during the evaluation of sensory neuropathy. At diagnosis, the serum M-protein was 0.5 g/dL, but subsequent values were unavailable. On repeat testing, the M-protein was 0.7 g/dL. FLC studies were performed for the first time and showed κ 2.6 mg/dL, λ 1.8 mg/dL, and κ/λ ratio of 1.44.
What is the evidence for MGUS follow-up?
The purpose of follow-up in MGUS is to detect early progression of MGUS into LPM, with the expectation that major complications will be minimized and survival prolonged because of the initiation of timely treatment. However, prospective data supporting routine follow-up of MGUS are unavailable.32 The most relevant evidence comes from 2 population-based studies showing better overall survival (identical hazard ratios of 0.9) among MM patients who had an MGUS diagnosis or follow-up prior to the discovery of MM.33,34 In 1 study, the rates of acute kidney injury, fracture, and hypercalcemia were also decreased.34 On the basis of these data, one could argue that MM patients with a known diagnosis of MGUS do better because they are followed annually, leading to timely diagnosis and prevention of serious complications. However, without randomized trials comparing follow-up versus no follow-up, it is not possible to infer a causal relationship, and these improved outcomes may be due to lead-time bias. Furthermore, studies show that most patients with MGUS, including those with high-risk MGUS, who progress to symptomatic LPMs, are diagnosed incidentally and not as a result of follow-up.19,35 Approximately one third of progressions diagnosed during follow-up of MGUS are smoldering multiple myeloma,35 a rate double that expected in the population (∼15%).36 Despite these limitations, and the lack of data from randomized trials, annual follow-up is recommended in current clinical practice guidelines for the majority of patients with MGUS given the seriousness of certain LPM complications and the relative ease with which testing for M-proteins can be added to other routine medical tests (Table 3).14-17 Prior to the publication of these guidelines, institutional studies showed that follow-up practices varied substantially in both academic and community settings.35,37 However, years after the publications of these guidelines, evidence suggests that they have not been widely adopted. A recent MGUS pattern of care study in the United States showed a relatively low guideline concordance rate (41% to 59%) in terms of follow-up frequency, with significant disparities both geographically and demographically.38
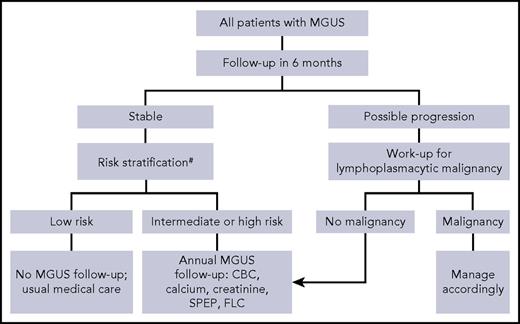
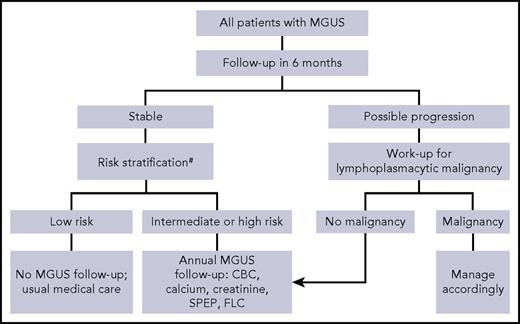
Who do we follow and for how long?
Our recommendations for follow-up largely conform to the guidelines of the International Myeloma Working Group.15 We recommend that all patients with MGUS be reassessed in 6 months with complete blood count, SPEP, FLC, calcium, and creatinine to determine clinical stability and detect rapidly evolving LPM. Although this practice is sensible and recommended by all clinical practice guidelines,14-17 evidence for this approach was unavailable until recently. A large retrospective nationwide study performed in the United States (N = 17 963) suggests that the risk of MGUS transformation is highest during the first year (2.1%) and gradually declines thereafter (1.6%, 1.2%, 1.0%, and 0.8%, for years 2-5, respectively).39 After initial follow-up, patients with low-risk MGUS need additional follow-up of MGUS only if symptoms concerning for LPMs develop.28 These patients have only a 2% risk of progression over a 20-year period. All other patients with MGUS should have an annual follow-up (Figure 2). Discontinuation of such follow-up can be considered for patients with a life expectancy of <5 years and among those >80 years old, consistent with screening guidelines for other common yet potentially curable cancers (discontinuation of screening at >65 years of age for cervical cancer, >75 years for breast and colon cancers, and >80 years for lung cancer).40 Nevertheless, evidence suggests that the majority (60%) of MGUS patients >80 years old continue to be followed regularly in current clinical practice.38
Suggested algorithm for follow-up of monoclonal gammopathy of undetermined significance.#Mayo Clinic Risk Stratification Model. CBC, complete blood count.
Suggested algorithm for follow-up of monoclonal gammopathy of undetermined significance.#Mayo Clinic Risk Stratification Model. CBC, complete blood count.
Case 4. Progression
A 50-year-old man presented with anemia and new onset of severe back pain. He had been diagnosed with MGUS 9 years ago with a serum monoclonal IgGκ of 1.7 g/dL. He was followed annually, and the M-protein level fluctuated between 1.6 and 1.9 g/dL. Over the last 2 years, there was a gradual rise in M-protein to 2.5 g/dL, but there were no clinical features to suggest transformation into LPM. Laboratory tests revealed a further increase in M-protein to 3.2 g/dL. Serum FLC assay showed κ 23.6 mg/dL, λ 1.0 mg/dL, and FLC ratio of 23.6. Skeletal survey detected multiple lytic lesions and pathologic vertebral fractures. Bone marrow biopsy revealed ∼50% κ-restricted plasma cells.
When should we suspect progression to an LPM?
A rising M-protein or serum FLC level should raise concern for progression but is seen in only about 50% of patients with MGUS prior to diagnosis of disease progression.5-7 Even when a change occurs, it is challenging to interpret such a change if it is not accompanied by symptoms or alterations in other laboratory parameters such as hemoglobin, calcium, or creatinine. A recent study in patients with smoldering multiple myeloma has identified specific changes in M-protein and hemoglobin levels associated with rapid symptomatic progression in more than 80% of patients.41 However, among those with MGUS, an “evolving” pattern (progressive rise in M-protein over 3 annual consecutive measurements) is associated with progression only in approximately 50% over a 10-year period. In contrast, a “nonevolving” pattern is associated with a low likelihood of LPM transformation (10% in 10 years).42 In addition to changes in M-protein level, progression should be considered in the presence of any unexplained signs and symptoms listed in Table 4. Any concern for progression should prompt additional testing, such as bone marrow or tissue biopsy, or imaging studies. To make a timely diagnosis, current diagnostic criteria for MM have been updated to allow a diagnosis to be made prior to end-organ damage and enable the use of advanced imaging for early detection of bone disease. In this patient, it is possible that earlier use of whole-body low-dose CT or positron emission tomography CT to work up a rising M-protein level may have led to a more timely diagnosis.
What are the other complications that can result from MGUS besides progression to LPM?
MGUS has been reported to be associated with >130 different diseases in addition to progression to malignancy.43 Because of the high prevalence of MGUS in the general population, most of these reported associations are likely coincidental. However, some associations have been verified and are now considered to be causally related to MGUS. These include monoclonal gammopathy–associated peripheral neuropathy,25 monoclonal immunoglobulin deposition disease,44 and monoclonal gammopathy–associated proliferative glomerulonephritis.45,46 In addition, some studies show that patients with MGUS may be at a higher risk of fractures (increased cortical bone porosity) and deep vein thrombosis.47-49 Awareness of these (and other) known disease associations and exclusion of other causes of these syndromes are important for accurate diagnosis (Table 2).
Case 5. Screening for monoclonal gammopathy in asymptomatic patients
A 61-year-old man was referred to hematology because of a strong family history of MM. His mother was diagnosed with active MM at the age of 70 and died 8 years later, and his sister was found to have the same diagnosis just 3 months before the visit. Neither his mother nor sister had risk factors. Prior to hematology referral, he had an SPEP and FLC performed, and no M-protein was detected.
Current practice guidelines do not recommend routine screening for MGUS in the general population because of the lack of proven benefit and absence of curative or preventive therapy.14-17 Although this rationale appears to be sound, the counterargument also has merits. It is notable that only ∼20% of prevalent MGUS cases are clinically recognized and fewer than 10% of MM, AL amyloidosis, or WM patients have a prior clinical diagnosis of MGUS.33,34,50 Therefore, approximately 80% of prevalent MGUS cases (approximately 2.8 million individuals in the United States) are not clinically recognized.51 Although this group of individuals should be at similar risk of progression to LPM, we neither seek them out nor follow their clinical course. A randomized controlled trial of MGUS screening is currently ongoing in Iceland to determine whether screening of the general population will be of clinical benefit,.
The prevalence of MGUS is increased in certain demographics (blacks, persons with occupational exposures to certain pesticides),2,4,52 immunocompromised patients (HIV infection, transplant recipients),53-55 and first-degree relatives of patients with MGUS or LPMs.56,57 These associations support a role for germline susceptibility genes and shared environmental exposures. They also raise the question of whether we should screen individuals at particularly high risk. Although there is no treatment, such individuals may benefit from periodic follow-up to prevent major complications. The patient in this case has 2 first-degree relatives affected with MM. We believe that it may be reasonable to screen for monoclonal gammopathy in high-risk patients who have 2 or more first-degree relatives with MM, AL amyloidosis, or WM, while we await data of the efficacy of screening from the Icelandic trial.
Case 6. Light-chain MGUS
A 76-year-old man was incidentally found to have hypercalcemia during an annual clinic visit. Because the review of systems elicited new-onset low-back pain, further evaluation was performed. Pertinent blood findings included a normal complete blood count and creatine, calcium at 10.9 mg/dL (8.9-10.1), parathyroid hormone at 72 pg/mL (15-65), and serum phosphorus at 2.1 mg/dL (2.5-4.5). SPEP and serum immunofixation were normal. Serum FLC studies showed κ 6.1 mg/dL, λ 1.5 mg/dL, and κ:λ 4.07. Lumbar radiographs showed degenerative changes without compression fracture or lytic lesion. The hypercalcemia was attributed to hyperparathyroidism, and the back pain resolved a week later.
What is light-chain MGUS?
The criteria for light-chain MGUS are listed on Table 1. A diagnosis of light-chain MGUS is made when a patient meeting the clinical criteria for MGUS has an M-protein consisting only of either monoclonal κ or λ light chains without an immunoglobulin heavy chain. It is a distinct entity and considered the precursor of light-chain MM (20% of all MM) and majority (65%) of AL amyloidosis.58 Unlike MGUS with intact immunoglobulin, light-chain MGUS is less common, with a prevalence of 0.8% among individuals age ≥50 years and a lower rate of malignant transformation to LPM of 0.3% per year. The risk of renal disease is increased in this population.59 As was stated earlier, we do not recommend routine skeletal imaging and bone marrow evaluation in patients who have a low involved/uninvolved FLC ratio (<8.0), in whom there are no clinical concerns for LPM. Patients should be followed-up in 6 months and then annually.
How do we interpret light-chain values in patients with chronic kidney disease?
Because serum FLCs are cleared by the kidneys, their concentrations rise as the glomerular filtration rate falls. Generally, both polyclonal κ and λ FLC levels are elevated and produce an FLC ratio that is within the normal range (0.26-1.65). In patients with chronic kidney disease, studies show that κ FLC levels tend to be higher, resulting in a revised renal reference range for FLC ratio of 0.37 to 3.1.60 Thus, to consider that an increase in the serum FLCs is a result of clonal plasma cell disorder, the serum FLC ratio must be <0.37 or >3.1 in patients with renal impairment. If the FLC ratio falls within the renal range but the index of suspicion for an underlying monoclonal gammopathy remains high, we add urine protein electrophoresis and urine immunofixation for confirmation.
Case 7. Harms of MGUS diagnosis
A 32-year-old woman was incidentally found to have an M-protein after participating in a blood donor screening held at a plasma donation center. She was healthy otherwise and given a diagnosis of MGUS. For 5 years, she was followed annually by her hematologist, and her M-protein remained stable. During the most recent visit, she confided, for the first time, that she was “scared to death” to come to follow-up visits. Every day, she felt as though she was “living on a cliff” and “could fall off anytime.” She lived in fear of hearing “the bad news” that she might not be lucky enough to “dodge the bullet” this time.
Although rarely a topic of doctor’s office conversation or even academic debate, the potential harms associated with MGUS diagnosis and subsequent follow-up deserve more attention. Several studies have now shown that psychological distress suffered by patients with nonmalignant hematologic diseases, including MGUS, is no less than it is for those with malignancies.61-63 An in-depth survey showed that among patients referred to a university cancer center for evaluation of nonmalignant hematologic diagnoses, nearly half reported increases in anxiety and stress (46% and 40%, respectively) and almost a third (30%) reported fear of having a cancer during the referral process.64 Similarly, a physician survey revealed similar concerns.65 Although this may be in part due to patients undergoing evaluation at cancer centers or being cared for by hematologists who also practice oncology, paying more attention to the psychological concerns of MGUS patients is warranted. Similar to cancer screenings, overdiagnoses of LPMs, especially the smoldering type, are inevitable.66 Harms of overtreatment and surveillance are well documented in cases of solid tumors but have yet to be studied in MGUS or monoclonal B-cell lymphocytosis, the precursor to B-cell chronic lymphocytic leukemia.67 Finally, the economic cost of MGUS follow-up is substantial and cannot be ignored. With over 500 000 individuals living with a diagnosis of MGUS in the United States and assuming once-yearly follow-up, the health care cost is estimated to be over $100 million annually.51 Although it is relatively easy to order SPEP, serum immunofixation, and serum FLC assays, clinicians need to be more judicious when ordering these tests, given the consequences of a MGUS diagnosis. These tests should be performed only in patients in whom there is clear suspicion of certain LPMs or conditions known to be associated with M-protein (Table 2).
Conclusion and future directions
Because the number of Americans ≥65 years in 2050 is projected to be more than double that in 2010, we expect that the number of living individuals diagnosed with MGUS will be well over a million in 30 years.51,68 How do we individualize MGUS follow-up care at the time of diagnosis while simultaneously accounting for life expectancy and competing comorbidities? In this article, we have outlined our approach to diagnostic work-up and management of patients with MGUS based on current data and our experience in managing these patients. We need studies targeted to populations that are at the highest risk of developing MGUS, including blacks and first-degree relatives of patients with LPMs. We must routinely engage our patients in an informed conversation incorporating the absolute risk of MGUS progression and comorbidity-adjusted life expectancy prior to recommending a follow-up program. We also need better biomarkers to predict the risk of transformation and into what type of LPM (or nonmalignant disease) MGUS will transform. In parallel, while awaiting the results of the Icelandic trial, we should also harness the power of machine-learning methods to analyze existing big data accumulated from MGUS patients followed over the past several decades. We hope that these will not only help reduce patient anxiety but also permit optimization of follow-up strategies and the development of preventive measures targeting the appropriate population.
Acknowledgments
This work was supported by research funding from the Mark A. and Elizabeth N. Binks Fund, from the Mayo Clinic Department of Medicine Innovation Award, and by research grants from the National Institutes of Health National Cancer Institute (CA107476, CA168762, and CA186781).
Authorship
Contribution: R.S.G. and S.V.R. designed and performed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald S. Go, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: go.ronald@mayo.edu.