Key Points
After HSCT with in vivo T-cell depletion using ATG, HLA-DPB1 nonpermissive mismatches at the GVH direction increase the risk for aGVHD.
HLA-DPB1–matched pairs have an increased risk for disease progression if intermediate risk by the Disease Risk Index.
Abstract
We investigated the impact of donor-recipient HLA-DPB1 matching on outcomes of allogeneic hematopoietic stem cell transplantation with in vivo T-cell depletion using antithymocyte globulin (ATG) for patients with hematological malignancies. All donor-recipient pairs had high-resolution typing for HLA-A, HLA-B, HLA-C, HLA-DRB1, HLA-DQB1, HLA-DPB1, and HLA-DRB3/4/5 and were matched at HLA-A, HLA-B, HLA-C, and HLA-DRB1. HLA-DPB1 mismatches were categorized by immunogenicity of the DPB1 matching using the DPB T-cell epitope tool. Of 1004 donor-recipient pairs, 210 (21%) were DPB1 matched, 443 (44%) had permissive mismatches, 184 (18%) had nonpermissive mismatches, in graft-versus-host (GVH) direction, and 167 (17%) had nonpermissive mismatches in host-versus-graft (HVG) direction. Compared with HLA-DPB1 permissive mismatched pairs, nonpermissive GVH mismatched pairs had the highest risk for grade II to IV acute graft-versus-host disease (aGVHD) (hazard ratio [HR], 1.4; P = .01) whereas matched pairs had the lowest risk (HR, 0.5; P < .001). Grade III to IV aGVHD was only increased with HLA-DPB1 nonpermissive GVH mismatched pairs (HR, 2.3; P = .005). The risk for disease progression was lower with any HLA-DPB1 mismatches, permissive or nonpermissive. However, the favorable prognosis of HLA-DPB1 mismatches on disease progression was observed only in peripheral blood stem cell recipients who were in the intermediate-risk group by the Disease Risk Index (HR, 0.4; P = .001) but no other risk groups. Our results suggest avoidance of nonpermissive GVH HLA-DPB1 mismatches for lowering the risk for grade II to IV and III to IV aGVHD. Permissive or nonpermissive HVG HLA-DPB1 mismatches may be preferred over HLA-DPB1 matches in the intermediate-risk patients to decrease the risk for disease progression.
Introduction
HLA-mismatch antigens are strongly immunogenic, and disparity at these loci is associated with an increased risk for graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT).1-4 There is agreement that donor-recipient HLA matching should consider allele-level HLA match at HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci (8/8 matched) to lower the risks for GVHD and mortality after HSCT.5-7 The clinical significance of mismatches at other HLA loci, DQB1 and DPB1 on HSCT outcomes, and their role in donor identification algorithm is not clearly defined.
Various studies have shown that among HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1–matched recipient donor pairs, HLA-DPB1 mismatching is seen in approximately 85% of pairs and is associated with an increased risk for GVHD that is counterbalanced by a decreased risk for leukemia relapse.2,8-11 HLA-DPB1 classification according to T-cell epitope grouping can identify mismatches that might be tolerated (permissive) and others that appear to increase the risks for GVHD and mortality (nonpermissive).8,12-14
Investigating the prognostic role of specific HLA loci on transplant outcomes is a complex process due to different factors influencing transplant outcomes, including disease type and status, patient and donor sex, age, and cytomegalovirus (CMV) serostatus, as well as transplant-conditioning regimen and GVHD prophylaxis. The number and/or functionality of alloreactive T cells are important and are influenced by the graft, the use of ex vivo or in vivo T-cell depletion (ie, antithymocyte globulin [ATG] or related agents), the conditioning regimen, and the type of GVHD immune prophylaxis.
In this single-center analysis, we investigated the role of HLA-DPB1 in addition to other low-expression alleles, HLA-DRB3/4/5 and HLA-DQB1, on HSCT outcomes in a population of transplant recipients with “8/8” matched unrelated donors (MUDs). In our analysis, all patients received ATG for in vivo T-cell depletion in addition to tacrolimus and methotrexate for GVHD prophylaxis.
Methods
Study population
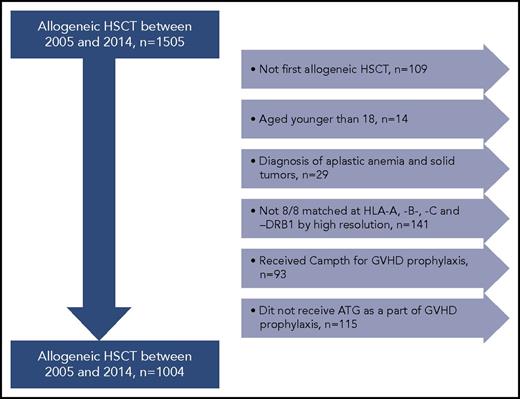
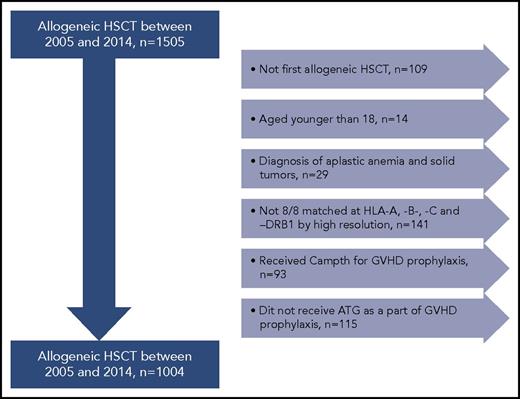
Adult patients with hematological malignancies who received their first allogeneic HSCT with MUDs between 2005 and 2014 were included (n = 1505). Patients eligible for this study had donor and recipient HLA typing performed at the HLA-A, HLA-B, and HLA-C; and HLA-DRB1, HLA-DRB3/4/5, HLA-DQB1, and HLA-DPB1 loci using sequenced-based typing methods at high resolution. We included patients older than 18 years who received ATG as part of their preparative regimen. The final study cohort included 1004 patients transplanted with 8/8 MUDs at HLA-A, HLA-B, HLA-C, and HLA-DRB1 by high-resolution typing (Figure 1). This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board of the University of Texas MD Anderson Cancer Center.
Study cohort. Patients with hematological malignancies who received their first allogeneic HSCT with MUDs between 2005 and 2014 were included (n = 1505). Patients eligible for this study had donor and recipient HLA typing performed at the HLA-A, HLA-B, HLA-C and HLA-DRB1, HLA-DRB3/4/5, HLA-DQB1, and HLA-DPB1 loci using sequenced-based typing methods at high-resolution levels. Patients with second allogeneic HSCT, diagnosis of aplastic anemia and solid tumors, age younger than 18 years, and those who did not receive ATG for GVHD prophylaxis were excluded from the analysis. Therefore, the final study cohort included 1004 patients transplanted with 8/8 MUDs at HLA-A, HLA-B, HLA-C, and HLA-DRB1.
Study cohort. Patients with hematological malignancies who received their first allogeneic HSCT with MUDs between 2005 and 2014 were included (n = 1505). Patients eligible for this study had donor and recipient HLA typing performed at the HLA-A, HLA-B, HLA-C and HLA-DRB1, HLA-DRB3/4/5, HLA-DQB1, and HLA-DPB1 loci using sequenced-based typing methods at high-resolution levels. Patients with second allogeneic HSCT, diagnosis of aplastic anemia and solid tumors, age younger than 18 years, and those who did not receive ATG for GVHD prophylaxis were excluded from the analysis. Therefore, the final study cohort included 1004 patients transplanted with 8/8 MUDs at HLA-A, HLA-B, HLA-C, and HLA-DRB1.
This heterogeneous group of patients with various hematological malignancies was risk stratified using the Disease Risk Index (DRI) defined by Armand et al.15 Disease and disease status at HSCT are presented in supplemental Table 1, available on the Blood Web site.
The impact of conditioning regimens on outcomes was analyzed by their dose intensity, using Center for International Blood and Marrow Transplant Research criteria.16 Details of conditioning regimens are presented in supplemental Table 2. All patients received rabbit ATG (thymoglobulin, Genzyme) given as 0.5 mg/kg on day −4, 1 mg/kg on day −3, and 2 mg/kg on day −2 as a part of their GVHD prophylaxis in addition to tacrolimus and methotrexate.
Classification based on HLA typing
HLA-DPB1 mismatches were classified into permissive and nonpermissive mismatches according to the latest version (2.0) of the DPB1 T-Cell Epitope Algorithm available at the IPD-IMGT/HLA web site (https://www.ebi.ac.uk/ipd/imgt/hla/dpb.html).17 Because our primary outcome of interest was acute graft-versus-host disease (aGVHD), we also took the direction of mismatch into consideration while generating comparison groups. We had 4 comparison groups: HLA-DPB1 matched (n = 210), HLA-DPB1 mismatched and permissive (n = 443), mismatched and nonpermissive in a graft-versus-host (GVH) direction (n = 184), and mismatched and nonpermissive in a host-versus-graft (HVG) direction (n = 167).
End points and outcome definitions
The primary end point was grades II to IV acute GVHD (aGVHD). Secondary end points included primary graft failure, grade III to IV aGVHD, transplant-related mortality (TRM), disease progression, and progression-free survival (PFS). Patients who had no engraftment of neutrophils by day 42 were treated as primary graft failures. AGVHD was graded according to the consensus criteria.18,19
Statistical methods
We estimated the incidence rates of TRM, disease progression, and aGVHD using the cumulative incidence method to account for competing risks. Disease progression and death attributable to persistence disease were considered competing risks for TRM, death before progression was considered a competing risk for disease progression, and disease progression and death before GVHD were considered competing risks for GVHD. We evaluated prognostic factors for TRM, disease progression, and aGVHD using the Fine and Gray regression model to account for competing risks. Actuarial PFS was estimated with the Kaplan-Meier method. Prognostic factors for PFS were evaluated with Cox proportional hazards regression analysis.
First-degree interaction effects were evaluated and accounted for in multivariate analyses when indicated. Statistical significance was defined at the .05 level for univariate and multivariate analyses. We did not apply Bonferroni correction (P = .001) for multiple comparisons because that would have excluded clinically relevant confounding factors from multivariate analysis. Statistical analyses were primarily performed with STATA 14.0 (StataCorp, https://www.stata.com/stata14/).
Results
Patient characteristics
The median age of the study cohort was 55 years (age range, 19-77 years) as presented in Table 1. DRI was very high risk in 62 patients (6%), high risk in 259 (26%), intermediate risk in 497 (50%), and low risk in 142 patients (14%). The conditioning regimen was myeloablative in 649 patients (65%), and the hematopoietic stem source was peripheral blood stem cells (PBSCs) in 571 patients (57%). The donor age was >30 years in 514 patients (51%). Of 1004 pairs, 566 (56%) donor-recipient pairs had ABO mismatches and 440 (44%) had sex mismatches. CMV serostatus was nonreactive in both the donor and recipient in 130 pairs (13%).
Similar to previous reports, only 210 donor-recipient pairs (21%) were HLA-DPB1 matched. Matching at HLA-DQB1 and HLA-DRB3/4/5 was more common and observed in 955 pairs (95%) and 925 pairs (92%), respectively. There was no correlation between mismatches at HLA-DPB1 and mismatches at other loci-like HLA-DRB3/4/5 and HLA-DQB1. Only 11 donor-recipient pairs were mismatched for all 3 loci at HLA-DPB1, HLA-DQB1, and HLA-DR3/4/5.
HLA-DPB1 mismatch at the HVG direction did not increase the risk for primary graft failure
Only 12 primary graft failures were observed during the study period: 6 with bone marrow (BM) and 6 with PBSCs used as the hematopoietic stem cell source. Of the 12 graft failures, 5 were observed in nonpermissive mismatched HLA-DPB1 pairs at the HVG direction; 1 in nonpermissive GVH mismatched pairs; 3 in permissive mismatched pairs; and 3 in matched pairs.
Nonpermissive GVH HLA-DPB1 mismatches represent the highest-risk group for grade II to IV and grade III to IV aGVHD after HSCT
The cumulative incidence of grade II to IV aGVHD by day 100 after HSCT was 34% in the entire cohort. The lowest risk for grade II to IV aGVHD was observed in HLA-DPB1–matched donor-recipient pairs compared with permissive HLA-DPB1–mismatched pairs as presented in Table 2 and Figure 2A (hazard ratio [HR], 0.5; 95% confidence interval [CI], 0.4-0.7; P < .001). The risk for grade II to IV aGVHD was different in HLA-DPB1–mismatched pairs with the highest risk observed in nonpermissive GVH HLA-DPB1–mismatched pairs (HR, 1.4; 95% CI, 1.1-1.8; P = .01), but there was no difference for pairs mismatched permissive and nonpermissive HVG (HR, 1.1; 95% CI, 0.8-1.5; P = .5).
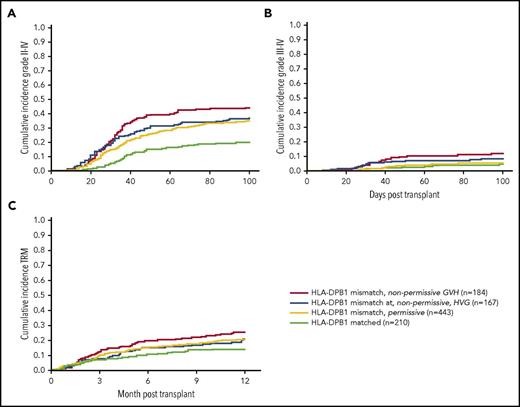
Incidence of grade II to IV aGVHD, grade III to IV aGVHD, and TRM by matching at HLA-DPB1 locus. Nonpermissive GVH HLA-DPB1 mismatches increased the risks for grade II to IV aGVHD (A), grade III to IV aGVHD (B), and 1-year TRM (C). The highest risk for grade II to IV aGVHD was observed in HLA-DPB1–mismatched nonpermissive GVH pairs with an incidence of 44% followed by pairs mismatched nonpermissive HVG and permissive HVG, with incidences of 37% and 35%, respectively. The incidence of grade III to IV aGVHD was also the highest in the nonpermissive GVH HLA-DPB1–mismatched pairs with an incidence of 12%. Pairs that are matched, mismatched permissive and mismatched nonpermissive HVG had similar incidences of grade III-IV aGVHD with 5%, 5%, and 9%, respectively. TRM at 1 year was 25% in nonpermissive GVH HLA-DPB1–mismatched pairs. Mismatched pairs that are nonpermissive HVG and permissive had similar cumulative incidences of 1 year TRM with 21% and 20%, whereas matched pairs had 14% at 1 year.
Incidence of grade II to IV aGVHD, grade III to IV aGVHD, and TRM by matching at HLA-DPB1 locus. Nonpermissive GVH HLA-DPB1 mismatches increased the risks for grade II to IV aGVHD (A), grade III to IV aGVHD (B), and 1-year TRM (C). The highest risk for grade II to IV aGVHD was observed in HLA-DPB1–mismatched nonpermissive GVH pairs with an incidence of 44% followed by pairs mismatched nonpermissive HVG and permissive HVG, with incidences of 37% and 35%, respectively. The incidence of grade III to IV aGVHD was also the highest in the nonpermissive GVH HLA-DPB1–mismatched pairs with an incidence of 12%. Pairs that are matched, mismatched permissive and mismatched nonpermissive HVG had similar incidences of grade III-IV aGVHD with 5%, 5%, and 9%, respectively. TRM at 1 year was 25% in nonpermissive GVH HLA-DPB1–mismatched pairs. Mismatched pairs that are nonpermissive HVG and permissive had similar cumulative incidences of 1 year TRM with 21% and 20%, whereas matched pairs had 14% at 1 year.
As expected, the use of PBSCs as the hematopoietic stem cell source led to a higher incidence of grade II to IV GVHD (HR, 1.3; 95% CI, 1.04-1.6; P = .02) compared with the use of BM. The incidence of grade II to IV GVHD was 30% (95% CI, 26%-35%) in BM recipients and 36% (95% CI, 33%-41%) in PBSC recipients. Considering the clinical and statistical significance of hematopoietic stem cell source on grade II to IV aGVHD, we performed stratified analyses according to stem cell source to assess the impact of HLA-DPB1 mismatches. The results showed that the favorable impact of HLA-DPB1 matches on grade II to IV GVHD was consistent in BM and PBSC recipients. In both groups, HLA-DPB1–matched pairs had a lower risk for grade II to IV aGVHD compared with permissive mismatched pairs, whereas the highest risk was observed in nonpermissive GVH HLA-DPB1–mismatched pairs as presented in supplemental Table 3.
Among other donor, recipient, and transplant-related variables, major ABO mismatch was also found to increase the risk for grade II to IV aGVHD by day 100 after HSCT, but that effect was limited to PBSC recipients (HR, 1.4; 95% CI, 1.02-1.9; P = .04) and not observed in BM recipients (HR, 0.9; 95% CI, 0.6-1.3; P = .5). In a similar manner, HLA-DQB1 mismatches increased the risk for grade II to IV aGVHD in PBSC recipients (HR, 1.9; 95% CI, 1.1-3.3; P = .02), but there were very few patients with an HLA-DQB1 mismatch (n = 22) to investigate its impact on aGVHD further.
We performed multivariate regression analyses assessing the impact of HLA-DPB1 mismatches after adjusting for hematopoietic stem cell source and major ABO mismatch. The results confirmed that nonpermissive GVH HLA-DPB1 mismatches were independently associated with the highest risk for grade II to IV aGVHD by day 100 after HSCT (HR, 1.4; 95% CI, 1.05-1.8; P = .02) as presented in Table 3.
The cumulative incidence of grade III to IV aGVHD was 7% by day 100. The results were similar to grade II to IV aGVHD that the donor-recipient pairs with nonpermissive GVH HLA-DPB1 mismatches represented the highest-risk group (HR, 2.3; 95% CI, 1.3-4.1; P = .005) compared with permissive mismatched pairs as presented in Table 2 and Figure 2B. However, a major difference was observed that HLA-DPB1–matched donor-recipient pairs did not experience a lower risk for grade III to IV aGVHD compared with permissive HLA-DPB1–mismatched pairs (HR, 0.9; 95% CI, 0.4-1.8; P = .7). As expected, the use of PBSCs as the hematopoietic stem cell source also increased the risk for grade III to IV aGVHD (HR, 2.6; 95% CI, 1.5-4.6; P = .001). When we performed stratified analyses for grade III to IV aGVHD by hematopoietic stem cell source, we observed that the increased risk for grade III to IV aGVHD with nonpermissive GVH HLA-DPB1 mismatches was limited to PBSC recipients (HR, 2.7; 95% CI, 1.4-5.2; P = .003) but had no significant impact in BM recipients (HR, 0.8; 95% CI, 0.2-3.6; P = .7) (supplemental Table 3). Among the other recipient, donor, and transplant-related characteristics, only HLA-DR3/4/5 was associated with an increased risk for grade III to IV aGVHD in PBSC recipients (HR, 2.5; 95% CI, 1.2-5.4; P = .02). However, the sample size was not big enough to pursue further investigation of the independent impact of HLA-DR3/4/5.
Recipients of HLA-DPB1 mismatches did not have a higher risk for 1-year TRM after HSCT
The cumulative incidence of TRM at 1 year was 20% in the study cohort. Univariate analyses showed that HLA-DPB1–matched pairs had lower 1-year TRM compared with permissive mismatched pairs, although that difference did not reach statistical significance (HR, 0.7; 95% CI, 0.1-1.02; P = .06). On the other hand, the 1-year incidence of TRM was not different for HLA-DPB1–mismatched nonpermissive GVH (HR, 1.3; 95% CI, 0.9-1.8; P = .2) and nonpermissive HVG (HR, 0.9; 95% CI, 0.7-1.5; P = .9) compared with permissive mismatched pairs as presented in Table 2 and Figure 2C.
The use of PBSC as the hematopoietic stem cell source was associated with an increased risk for TRM (HR, 1.3; 95% CI, 0.9-1.8; P = .06), but that did not reach statistical significance. The incidence of TRM was 17% (95% CI, 14%-21%) vs 22% (95% CI, 19%-26%) at 1 year after HSCT in BM and PBSC recipients, respectively. Other prognostic variables for 1-year TRM were recipient age >55 years (HR, 1.7; 95% CI, 1.3-2.2; P < .001) and major ABO mismatch (HR, 1.5; 95% CI, 1.1-2.1; P = .01). The impact of major ABO mismatch on 1-year TRM was only significant in PBSC recipients (HR, 1.7; 95% CI, 1.1-2.6; P = .01) but not in BM recipients (HR, 1.3; 95% CI, 0.7-2.1; P = .4) as presented in supplemental Table 4.
When multivariate regressions were performed, as shown in Table 3, major ABO mismatch with the use of PBSCs, and recipient age >55 years were found to be poor prognostic factors for 1-year TRM.
Any HLA-DPB1 mismatch decreases the risk for disease progression after HSCT in intermediate-risk patients by DRI
The cumulative incidence of disease progression at 3 years was 33% in the study cohort. Prognostic factors for a lower risk for disease progression were the use of PBSCs as the hematopoietic stem cell source (HR, 0.7; 95% CI, 0.6-0.9; P = .007), in addition to low- and intermediate-risk groups by DRI (HR, 0.3; 95% CI, 0.2-0.4; P < .001 and HR, 0.5; 95% CI, 0.4-0.6; P < .001, respectively).
There was no significant difference for the incidence of disease progression between donor-recipient pairs based on HLA-DPB1–matching status as presented in Table 2 and Figure 3A.
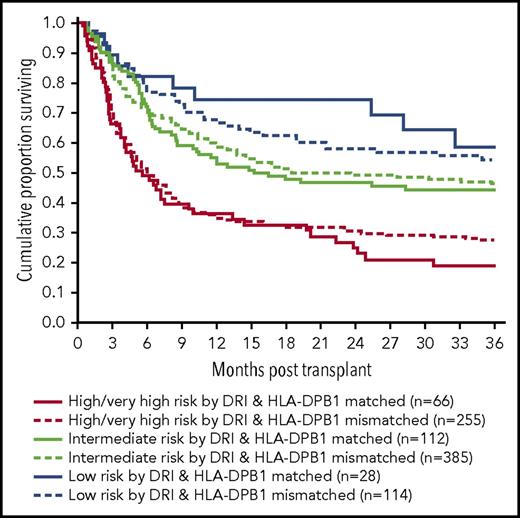
Incidence of disease progression by HLA- DPB1 matching and disease risk by DRI. There was no statistically significant difference regarding the risk for disease progression among donor-recipient pairs by HLA-DPB1 mismatching (A). The cumulative incidences at 3 years were 26%, 29%, and 34% for mismatched nonpermissive GVH, mismatched nonpermissive HVG, and mismatched permissive pairs, but the incidence was 41% for HLA-DPB1–matched pairs. However, there was a significant interaction between HLA-DPB1 mismatches and disease risk groups by DRI (B). Lower risk for disease progression was observed with any HLA-DPB1 mismatch in the intermediate-risk group by DRI but not others. Intermediate-risk patients by DRI with any HLA-DPB1 mismatch had a lower risk for disease progression with an incidence of 25%, which was similar to 19% observed in patients with low-risk DRI. However, intermediate-risk patients without any HLA-DPB1 mismatch had an increased risk for progression with an incidence of 41%, which was similar to 46% observed in patients with high/very high-risk DRI.
Incidence of disease progression by HLA- DPB1 matching and disease risk by DRI. There was no statistically significant difference regarding the risk for disease progression among donor-recipient pairs by HLA-DPB1 mismatching (A). The cumulative incidences at 3 years were 26%, 29%, and 34% for mismatched nonpermissive GVH, mismatched nonpermissive HVG, and mismatched permissive pairs, but the incidence was 41% for HLA-DPB1–matched pairs. However, there was a significant interaction between HLA-DPB1 mismatches and disease risk groups by DRI (B). Lower risk for disease progression was observed with any HLA-DPB1 mismatch in the intermediate-risk group by DRI but not others. Intermediate-risk patients by DRI with any HLA-DPB1 mismatch had a lower risk for disease progression with an incidence of 25%, which was similar to 19% observed in patients with low-risk DRI. However, intermediate-risk patients without any HLA-DPB1 mismatch had an increased risk for progression with an incidence of 41%, which was similar to 46% observed in patients with high/very high-risk DRI.
However, there was a significant interaction between the HLA-DPB1 mismatches and disease risk groups by DRI for disease progression, indicating that the impact of mismatches on disease progression was not uniform among DRI risk groups (supplemental Table 5). HLA-DPB1–mismatched donor-recipient pairs had a significantly lower risk for disease progression compared with HLA-DPB1–matched pairs, but this effect was limited to intermediate-risk patients by DRI (HR, 0.5; 95% CI, 0.4-0.8; P = .001) as presented in Figure 3B. Intermediate-risk patients by DRI had a lower risk for progression similar to patients with low-risk DRI (HR, 1.4; P = .1) if they had HLA-DPB1–mismatched donors. On the other hand, intermediate-risk patients by DRI had a higher risk for disease progression comparable with high/very high-risk group if they had HLA-DPB1–mismatched donor (HR, 0.8; P = .1) as presented in Figure 3B. Stratified analyses showed that this effect was limited to PBSC recipients.
Multivariate regression analyses confirmed that PBSC recipients with HLA-DPB1–matched donors that were intermediate risk by DRI, very high/high-risk patients by DRI, and BM recipients of any disease risk category by DRI had a high risk for disease progression after HSCT as presented in Table 3.
Nonpermissive and permissive HLA-DPB1 mismatches did not have an impact on PFS after HSCT
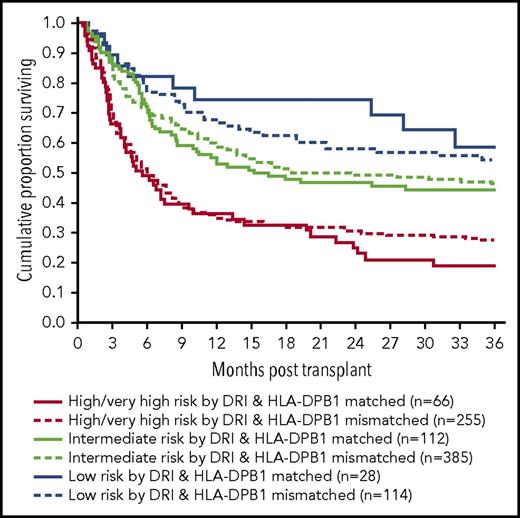
The 3-year estimate of PFS was 46% (95% CI, 40%-50%) in the study population. Neither HLA-DPB1 nonpermissive GVH mismatches nor nonpermissive HVG mismatches were found to be prognostic for PFS at 3 years (HR, 0.9; 95% CI, 0.7-1.2; P = .6 and HR, 0.9; 95% CI, 0.7-1.2; P = .5) as presented in Figure 4. When multivariate regression analyses were performed, recipient age >55 years and high-risk disease groups by DRI were found to negatively influence 3-year PFS as presented in Table 3.
PFS by HLA-DPB1 matching and disease risk by DRI. HLA-DPB1 mismatch had no impact on PFS. This remained the same for each DRI risk group.
PFS by HLA-DPB1 matching and disease risk by DRI. HLA-DPB1 mismatch had no impact on PFS. This remained the same for each DRI risk group.
Discussion
In this study of patients with HLA–matched donors at HLA-A, HLA-B, HLA-C, and HLA-DRB1 that had in vivo T-cell depletion with ATG, we showed that HLA-DPB1 mismatches nonpermissive GVH are associated with a significantly increased risk for grade II to IV and III to IV aGVHD within 100 days after allogeneic HSCT. HLA-DPB1 mismatches permissive and nonpermissive HVG also increased grade II to IV aGVHD but not grade III to IV. Decrease for the risk of disease progression was observed in patients with any HLA-DPB1 mismatches but only in PBSC recipients with intermediate risk by DRI defined by Armand et al.15
The current consensus for unrelated donor selection requires matching for HLA-A, HLA-B, HLA-C, HLA-DRB1, and preferably HLA-DQB1 alleles between the donor and the recipient. Based on large, multicenter studies,8,20 matching for HLA-DPB1 and avoidance of nonpermissive mismatches are recommended because of the association with decreased treatment-related mortality. The largest study to date with 5428 donor-recipient pairs with “10/10 match” (HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1) had BM as the graft source, and T-cell depletion was used in 16% of the patients.8 That study showed significant differences between nonpermissive mismatched recipient-donor pairs and matched ones for nonrelapse mortality, overall mortality, and aGVHD. There was also a highly significant difference for relapse between the HLA-DPB1 allele-matched group and the permissively mismatched group, whereas the difference for relapse between the nonpermissive and the permissive groups did not reach statistical significance. A CIBMTR study20 with 5449 “8/8” matched pairs showed increased aGVHD and decreased relapse with HLA-DPB1 mismatches, whereas nonpermissive mismatches were associated with higher TRM and lower overall mortality compared with permissive HLA-DPB1 mismatches. In that study, approximately half of the patients had BM as the graft source, and in vivo T-cell depletion was performed in 28% of “8/8” matched pairs.
Our findings were similar to previous reports with a decreased risk for disease progression for HLA-DPB1–mismatched pairs, even when HLA-DPB1 mismatches were permissive or nonpermissive HVG, compared with HLA-DPB1–matched pairs. Those clinical findings have been supported by strong polyclonal immune responses observed with both permissive and nonpermissive HLA-DPB1 mismatches, indicating the potential for immunogenicity of permissive mismatched HLA-DPB1 alleles.21,22 Therefore, one may assume that HLA-DPB1 mismatches permissive and nonpermissive GVH may induce mild alloreactivity sufficient for a graft-versus-tumor effect but not strong enough to mediate grade III to IV aGVHD. However, the graft-versus-tumor effect induced by HLA-DPB1 mismatches may not overcome the poor prognosis of high-risk groups because a decrease in disease progression was only observed in the intermediate-risk group by DRI in our study cohort. This unique finding, which is different from previous reports, can be explained by the use of DRI,15 the risk classification schema modeled to classify the heterogeneous group of patients with various hematological malignancies at different disease stages for their risk groups and validated for its prognostic value in different independent cohorts.
Consistent with previously published reports,20,23 HLA-DQB1–mismatched pairs, although small in number, had increased the risk for grade II to IV aGVHD in PBSC recipients, but not in BM recipients, compared with HLA-DQB1–matched pairs. We did not observe any impact of HLA-DQB1 mismatch on TRM and PFS. However, it was noteworthy that 38 of 42 donor-recipient pairs with HLA-DQB1 mismatches also had HLA-DPB1 mismatches. Because of relatively small sample size, we could not evaluate the additive impact of HLA-DQB1 and HLA-DPB1 for grade II to IV aGVHD, but avoiding the presence of double mismatches among “8/8” matched pairs may improve the aGVHD outcomes especially in PBSC recipients.
Our cohort is smaller than that in previously published studies but is unique with homogeneity for in vivo T-cell depletion with ATG and standard GVHD prophylaxis, as well as stratified analyses by the graft source and the use of DRI for specific outcomes of interest. Stratified analyses confirmed that the impact of HLA-DPB1 mismatch on transplant outcomes is not independent of the stem cell source. We also took the matching vector into consideration and showed that the vector of mismatch may have an impact on specific outcomes as GVHD. Exclusion of the patients who did not have in vivo T-cell depletion with the use of ATG allowed us to investigate the impact of HLA-DPB1 mismatch in a uniform setting. The cumulative incidences of grade II to IV and III to IV aGVHD was 34% and 7%, respectively. Previously published studies with various conditioning regimens had shown that the addition of ATG to standard GVHD prophylaxis in patients with MUDs is an independent prognostic factor decreasing the incidence of aGVHD without compromising other outcomes, with incidences in the range of 21% to 57%.24-26
The concept of choosing HLA-A, HLA-B, HLA-C, and HLA-DRB1 MUDs based on their permissiveness at HLA-DPB1 has been evolving, and different models have been proposed to this regard.11 The proposed Expression-model,27 which was found to be predictive of aGVHD when there is 1 mismatch at least in the GVH direction, was based on the observation that the risk for aGVHD was determined by the HLA-DPB1 rs9277534 expression. In our analyses, we used the model based on structural T-cell-episodes (3-group algorithm), which was based on the observation of less vigorous T-cell alloreactivity if mismatches between HLA-PB1 alleles were from the same T-cell epitope groups. Recently, studies have shown that modification of the T-cell epitope model, such as designing a 4-group algorithm with HLA-DPB1*02 kept as a separate group12 or refining the model by the use of “functional distance” score reflecting the combined impact of amino acid polymorphism in HLA-DPB1 on T-cell alloreactivity,28 may increase the predictive value of the 3-group algorithm. It would be of great interest to compare the T-cell epitope and Expression models for their prognostic value of posttransplant outcomes, especially aGVHD.
HLA-DPB1 mismatches have a high frequency with more than 80% of all MUD transplants due to low degrees of linkage disequilibrium with the other HLA loci. In this context, our results are important and support the notion to avoid nonpermissive GVH HLA-DPB1 mismatches for MUD HSCT after in vivo T-cell depletion with ATG. On the other hand, patients with HLA-DPB1–mismatched donors permissive or nonpermissive HVG may be preferred vs HLA-DPB1–matched donors in patients with intermediate-risk disease by DRI while taking other transplant-related prognostic factors into account for choosing the unrelated donor. Generalization of our findings in the absence of in vivo T-cell depletion with ATG remains to be determined. Novel approaches should be studied in recipients mismatched for HLA-DPB1 in the GVHD direction, with a goal to control GVHD while retaining the enhanced graft-versus-leukemia activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by an ACC research fund (grant #110560) (K.C.).
Authorship
Contribution: B.O., R.E.C., and K.C. designed and performed the research, analyzed data, and wrote the paper; R.M.S. performed the research, analyzed data, and wrote the paper; M.A., Y.C., and P.C. performed the research; and M.d.L., G.R., S.A., A.A., B.S.A., P.A., Q.B., S.C., M.F.-V., C.H., P.K., M.K., I.K., D.M., Y.N., A.O., U.P., K.R., M.Q., and E.J.S. performed the research and wrote the paper.
Conflict-of-interest disclosure: B.O. has received research funding from Astex and Arog pharmaceuticals. B.S.A. has a patent or intellectual property interest (Company: MD Anderson Cancer Center). C.H. has received honoraria and travel, accommodations, or expenses from Cardinal Health and Seattle Genetics, in addition to research funding from Celgene. Q.B. is on the advisory board of Takeda, Amgen, and Spectrum Pharma, and has received research funding from Celgene and Takeda Pharma. P.A. has received honoraria from Shire. S.C. has stock or other ownership interests with CytoSen Therapeutics, and consulting or advisory roles at Spectrum Pharmaceuticals and Molimed Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Betül Oran, Departments of Stem Cell Transplantation and Cell Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd Unit #423, Houston, TX 77030; e-mail: boran@mdanderson.org.
REFERENCES
Author notes
R.E.C. and K.C. contributed equally to this study.