Key Points
Thymic ILCs and their production of IL-22 are reduced in mice with GVHD; IL-22 deficiency worsens thymic epithelial damage in GVHD.
Administration of IL-22 posttransplant can enhance thymopoiesis after experimental allogeneic bone marrow transplant.
Abstract
Graft-versus-host disease (GVHD) and posttransplant immunodeficiency are frequently related complications of allogeneic hematopoietic transplantation. Alloreactive donor T cells can damage thymic epithelium, thus limiting new T-cell development. Although the thymus has a remarkable capacity to regenerate after injury, endogenous thymic regeneration is impaired in GVHD. The mechanisms leading to this regenerative failure are largely unknown. Here we demonstrate in experimental mouse models that GVHD results in depletion of intrathymic group 3 innate lymphoid cells (ILC3s) necessary for thymic regeneration. Loss of thymic ILC3s resulted in deficiency of intrathymic interleukin-22 (IL-22) compared with transplant recipients without GVHD, thereby inhibiting IL-22–mediated protection of thymic epithelial cells (TECs) and impairing recovery of thymopoiesis. Conversely, abrogating IL-21 receptor signaling in donor T cells and inhibiting the elimination of thymic ILCs improved thymopoiesis in an IL-22–dependent fashion. We found that the thymopoietic impairment in GVHD associated with loss of ILCs could be improved by restoration of IL-22 signaling. Despite uninhibited alloreactivity, exogenous IL-22 administration posttransplant resulted in increased recovery of thymopoiesis and development of new thymus-derived peripheral T cells. Our study highlights the role of innate immune function in thymic regeneration and restoration of adaptive immunity posttransplant. Manipulation of the ILC–IL-22–TEC axis may be useful for augmenting immune reconstitution after clinical hematopoietic transplantation and other settings of T-cell deficiency.
Introduction
Allogeneic hematopoietic bone marrow transplantation (allo-BMT) is a potentially curative therapy for both benign and malignant hematopoietic diseases, but its use is restricted because of the severe morbidity and mortality associated with graft-versus-host disease (GVHD) and prolonged immunodeficiency.1 Acute GVHD occurs when alloreactive donor T cells attack tissues in the BMT recipient, and posttransplant immune function is limited by pretransplant conditioning and immunosuppressive GVHD prophylaxis.2 GVHD itself can exacerbate posttransplant immunodeficiency because of damage to the thymic stroma by donor T cells.3-5 T-cell deficiency after transplant is associated with an increased risk of infections, malignant relapse, development of secondary malignancies, and impairment in the application of immunotherapeutic strategies such as vaccination against microbes or tumors.6-11 In fact, infection and relapse account for more than 50% of mortality after BMT.12 In addition, the risk of opportunistic infections in the posttransplant period is directly correlated with impaired recovery of T cells (especially CD4 T cells) and thymic function.6,7,13 Therefore, recovery of immunity is a critical determinant of successful outcomes for patients undergoing allogeneic hematopoietic transplantation.
The thymus is the primary site of T-cell development, and intact thymic function is thus an important determinant for successfully reconstituting immunity posttransplant.14 Although the thymus is highly sensitive to acute insult, it also has a potent ability to rebound and recover. The pathways critical for thymic regeneration are poorly understood, as are the mechanisms by which this renewal can be impaired during disease states, including prolonged inflammatory conditions associated with immunodeficiency. GVHD of the thymus, a clinically relevant problem given its potential impacts on immune reconstitution, represents a potent model of immune-mediated epithelial injury for evaluating mechanisms of tissue regeneration necessary for renewal of immunocompetence.3-5
Interleukin-22 (IL-22) is an IL-10 family cytokine, and its receptor is widely expressed on epithelial cells.15 IL-22 has been shown to promote innate immunity and homeostasis of epithelial cells in the intestines, lung, and skin during acute tissue injury.16 A role for IL-22 has also been described in the endogenous regeneration of thymic epithelial cells (TECs) in response to radiation injury.17,18 IL-22 is produced primarily by T cells and group 3 innate lymphoid cells (ILC3s), which is a lymphoid-derived RAR-related orphan receptor γ(t) (RORγ(t)+) cell population that lacks rearranged adaptive immune receptors.19 ILC3s have been shown to be important for protection of the gastrointestinal (GI) tract after allogeneic hematopoietic transplantation in both experimental models and in patients undergoing clinical transplantation.20,21 Independent of IL-22 production, ILC3s present during development are important for the thymus where they interact with medullary TECs and provide signals for their maturation.22-25 However, the roles of ILCs and the IL-22 pathway in thymic recovery from GVHD are unknown, as are the mechanisms that may regulate them.
IL-21 is a T-cell–derived cytokine that signals through a common γ chain family receptor.26 Its receptor is present on numerous immune cells, including donor T cells in the setting of allo-BMT, and blockade of IL-21 posttransplant has been shown to reduce systemic and GI GVHD.27-30 Its role in thymic GVHD is unknown. The purpose of this study was to evaluate the role of intrathymic IL-22 and ILC3s after allo-BMT to understand the failure of thymic recovery and immune reconstitution during GVHD. Here we present evidence that IL-21 signaling in donor T cells contributes to the elimination of thymic ILC3s and the loss of IL-22–dependent recovery of thymopoiesis posttransplant. Elucidation of the pathophysiologic mechanisms by which thymic regeneration fails in GVHD may also be relevant for augmenting the function of thymic stroma and improving immune reconstitution in patients who have undergone repeated cycles of immune-depleting therapies or in those whose thymus has declined because of aging.
Methods
Mice and BMT
C57BL/6 (CD45.2 B6, H-2b), B6.SJL-PtprcaPepcb/BoyJ (CD45.1 B6 congenic, H-2b), B10.BR (H-2k), and BALB/c (H-2d) mice were obtained from The Jackson Laboratory. Genentech provided Il22−/− mice on B6 and BALB/c backgrounds. Allo-BMT was performed with a split dose of 850 cGy for BALB/c hosts or 1100 cGy for B6 hosts receiving bone marrow (5 × 106) T-cell–depleted with anti-Thy-1.2 antibodies and low–TOX-M rabbit complement (Cedarlane Laboratories, Homby, ON, Canada). Donor T cells (typically 0.5 to 1 × 106 B6 or 4 × 106 LP) were prepared for transplantation by harvesting donor splenocytes and enriching for T cells by Miltenyi magnetic-activated cell sorting purification of CD5 (routinely >90% purity). Syngeneic BMT was performed with a split dose of 1100 cGy for B6 hosts receiving 10 000 fluorescence-activated cell sorter–purified CD45.1 congenic Lin–Sca1+ckit+ (LSK) cells. Individual or pooled thymic single-cell suspensions were obtained after mechanical dissociation or enzymatic digestion as previously described.17 The Memorial Sloan Kettering Cancer Center Institutional Animal Care and Use Committee approved all BMT protocols.
Reagents.
To detect IL-22 and IL-23, thymic supernatants were obtained by disrupting and resuspending each thymus in defined volumes of buffer. The resulting supernatants were quantified by using cytokine-specific enzyme-linked immunosorbent assay kits purchased from BioLegend (San Diego, CA) and read on an Infinite Plate Reader (Tecan, San Jose, CA).
Surface antibodies against Ly5.1 (6C3), CD11c (HL3), CD8 (53-6.7), CD45 (30-F11), CD19 (1D3), CD11b (M1/70), Ly6G (RB6-8C5), TER-119 (TER-119), TCRβ (H57-597), CD3 (145-2C11), CD25 (PC61), CD45R (RA3-6B2), CD45.1 (A20), CD45.2 (104), and H-2Kb (AF6-88.5) were purchased from BD Biosciences. Antibodies against IL-22 (1H8PWSR), RORγt (B2D), EpCAM (G8.8), and CD44 (IM7) were purchased from eBioscience. Anti-CD90.2 (30-H12), CD4 (RM4-5), and IA/IE (M5/114.15.2) were purchased from BioLegend. Ulex europaeus agglutinin 1 (UEA-1), conjugated to fluorescein isothiocyanate or biotin was purchased from Vector Laboratories (Burlingame, CA). Flow cytometric analysis was performed on an LSRII flow cytometer (BD Biosciences), and cells were sorted on an BD FACSAria II cell sorter (BD Biosciences) using FACSDiva (BD Biosciences) or FlowJo software (Treestar). Recombinant IL-22 was purchased from GenScript and Insight Biotechnology.
Intracellular staining.
For all assays that required analysis of intracellular cytokines, cells were fixed and permeabilized in 1.6% paraformaldehyde at 37°C followed by 90% methanol at 4°C or with a Foxp3 fixation/permeabilization kit (eBioscience) per the manufacturer’s protocol. For intracellular IL-22 staining, cells were incubated for 4 hours with brefeldin A (3 μg/mL).
Statistics
Statistical analysis between 2 groups was performed with the nonparametric unpaired Mann-Whitney U test or Student t test; comparisons between more than 2 groups were performed by using 1-way analysis of variance. All statistics were calculated and display graphs were generated by using GraphPad Prism.
Results
GVHD leads to loss of thymic ILC3s and reduced intrathymic levels of IL-22
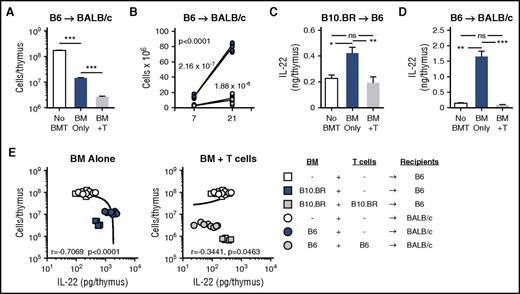
The thymus is a particularly sensitive target during acute GVHD. Consistent with published literature,3,4 conditioning followed by T-cell–depleted (TCD) allo-BMT led to a significant reduction in thymic cellularity 7 days post-BMT, and transplantation with marrow and allogeneic T cells depleted thymic cellularity even further (Figure 1A). Over time there is significant recovery of thymic cellularity in recipients of TCD allo-BMT without GVHD, but mice transplanted with allogeneic T cells to induce GVHD suffer a prolonged suppression of thymic recovery with only a little endogenous recovery even 3 weeks after transplant (Figure 1B). Given the finding that intrathymic IL-22 is critical for endogenous regeneration of the thymus after radiation injury17 and given that the recovery of thymic function after BMT is critical for posttransplant immune reconstitution, we evaluated the expression of intrathymic IL-22 after allo-BMT. In 2 distinct models of TCD allo-BMT without GVHD (B10.BR→B6 and B6→BALB/c, H-2k→H-2b and H-2b→H-2d, respectively) we found a significant increase in the absolute levels of intrathymic IL-22 compared with untransplanted controls (Figure 1C-D). However, in recipient mice undergoing allo-BMT with donor T cells to induce GVHD, the intrathymic increase in IL-22 post-BMT was completely eliminated (Figure 1C-D). Consequently, in contrast to previous findings in which multiple models of thymic injury demonstrated that loss of thymic cellularity was strongly correlated with increased intrathymic IL-22,17 this correlation was eliminated during thymic damage associated with GVHD (Figure 1E).
Thymic IL-22 levels are reduced in mice with GVHD. (A-B) BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD bone marrow (BM) cells from B6 mice (H-2b) with or without 1 × 106 BALB/c T cells to induce GVHD. (A) Total thymic cellularity on day 7 after transplant. (B) Thymic cellularity on day 7 and day 21 after transplant, analyzed by linear regression with slope comparison (1/slope for each line displayed on the graph). (C) B6 (H-2b) recipients were transplanted with 5 × 106 TCD BM cells from B10.BR mice (H-2k) with or without 2 × 106 B10.BR T cells to induce GVHD. Absolute levels of IL-22 were measured on day 7 post-BMT in B6 recipients or normal non-BMT controls (n = 10 per group). (D) BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD BM cells from B6 mice (H-2b) with or without 1 × 106 BALB/c T cells to induce GVHD. Absolute levels of IL-22 were measured on day 7 post-BMT in BALB/c recipients or normal non-BMT controls (n = 9-10 per group). (E) Correlation of thymic cellularity and IL-22 levels in BMT recipients or normal controls from panels C and D. Left panel shows values from allo-BMT without T cells (blue circles and squares), indicating that after TCD BMT, the thymic cellularity and intrathymic IL-22 levels have a strong negative correlation. Right panel shows values from mice transplanted with allogeneic T cells (gray circles and squares), indicating that GVHD causes a loss of the correlation between thymic cellularity and the compensatory IL-22 response. Untransplanted normal controls are the same in both panels (white circles and squares). Bar graphs represent mean ± standard error of the mean (SEM) of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Thymic IL-22 levels are reduced in mice with GVHD. (A-B) BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD bone marrow (BM) cells from B6 mice (H-2b) with or without 1 × 106 BALB/c T cells to induce GVHD. (A) Total thymic cellularity on day 7 after transplant. (B) Thymic cellularity on day 7 and day 21 after transplant, analyzed by linear regression with slope comparison (1/slope for each line displayed on the graph). (C) B6 (H-2b) recipients were transplanted with 5 × 106 TCD BM cells from B10.BR mice (H-2k) with or without 2 × 106 B10.BR T cells to induce GVHD. Absolute levels of IL-22 were measured on day 7 post-BMT in B6 recipients or normal non-BMT controls (n = 10 per group). (D) BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD BM cells from B6 mice (H-2b) with or without 1 × 106 BALB/c T cells to induce GVHD. Absolute levels of IL-22 were measured on day 7 post-BMT in BALB/c recipients or normal non-BMT controls (n = 9-10 per group). (E) Correlation of thymic cellularity and IL-22 levels in BMT recipients or normal controls from panels C and D. Left panel shows values from allo-BMT without T cells (blue circles and squares), indicating that after TCD BMT, the thymic cellularity and intrathymic IL-22 levels have a strong negative correlation. Right panel shows values from mice transplanted with allogeneic T cells (gray circles and squares), indicating that GVHD causes a loss of the correlation between thymic cellularity and the compensatory IL-22 response. Untransplanted normal controls are the same in both panels (white circles and squares). Bar graphs represent mean ± standard error of the mean (SEM) of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
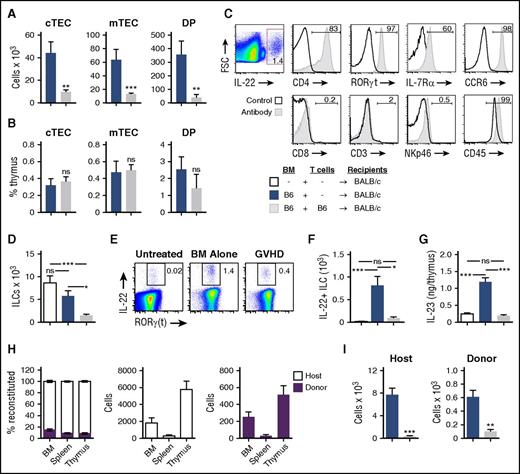
TECs are the primary target of alloreactive T cells during GVHD.4 Although TECs are a rare cell population, they are critical for supporting T-cell development,31 and loss of TECs leads to almost concurrent depletion of developing thymocytes including CD4+CD8+ double-positive (DP) thymocytes, which are the most numerous subset in the thymus. GVHD leads to a loss of total thymic cellularity as well as a loss of DP thymocytes, and loss of DP thymocytes can serve as a biomarker for thymic GVHD.3 Furthermore, there are profound reductions in the numbers of cortical TECs (cTECs), medullary TECs (mTECs), and DP thymocytes early after transplant with allogeneic T cells (Figure 2A), and these populations all contract proportionally with the overall reduction in thymic cellularity (Figure 2B).
Intrathymic ILCs are eliminated and their production of IL-22 is reduced in mice with GVHD. (A-G) BALB/c recipients (H-2d) were transplanted with 5 × 106 TCD BM cells with or without 1 × 106 T cells from B6 donors (H-2b) to induce GVHD and were analyzed on day 7 after transplant (n = 7-10 mice per group). (A) Total number and (B) frequency of cTECs, mTECs, and DP thymocytes. (C) Back-gating analysis of thymic IL-22+ cells after TCD allo-BMT; thymic harvest and incubation in vitro with brefeldin A for 5 hours, followed by intracellular and surface antibody staining. Representative flow cytometry plots were concatenated from 5 independent observations with isotype antibody (RORγt, IL-7Rα) or CD45– (CD4, CCR6, CD8, CD3, NKp46) negative controls or whole thymus positive controls (CD45). (D) Total number of intrathymic CD45+CD3–CD8–CD4+IL7R+RORγt+ ILC3s in BMT recipients or normal untransplanted controls. (E-F) Thymocytes were harvested and incubated for 5 hours with brefeldin A. Intracellular IL-22 was assessed by flow cytometry, demonstrating the frequency (E) and absolute number (F) of IL-22+ ILC3s in BMT recipients or normal untransplanted controls. (G) Absolute levels of intrathymic IL-23 on day 7 after BMT in BALB/c recipients or normal untransplanted controls. (H) B6 (CD45.2) recipients were transplanted with 10 000 Lineage–Sca1+ckit+ BM cells from congenic CD45.1+ donors, and BM, spleen, and thymus were analyzed on day 4 after transplant for ILC3 chimerism and absolute numbers of donor and host ILC3s (n = 5-15 mice per group). (I) Absolute number of host-derived (CD45.2+H-2d+) and donor BM–derived (CD45.1+H-2b+) thymic ILCs 7 days after B6→BALB/c transplant (performed as in panels A-G), gated on CD45+CD3–CD8–CD4+ cells (n = 9-10 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments.*P < .05; **P < .01; ***P < .001. FSC, forward scatter; ns, not significant.
Intrathymic ILCs are eliminated and their production of IL-22 is reduced in mice with GVHD. (A-G) BALB/c recipients (H-2d) were transplanted with 5 × 106 TCD BM cells with or without 1 × 106 T cells from B6 donors (H-2b) to induce GVHD and were analyzed on day 7 after transplant (n = 7-10 mice per group). (A) Total number and (B) frequency of cTECs, mTECs, and DP thymocytes. (C) Back-gating analysis of thymic IL-22+ cells after TCD allo-BMT; thymic harvest and incubation in vitro with brefeldin A for 5 hours, followed by intracellular and surface antibody staining. Representative flow cytometry plots were concatenated from 5 independent observations with isotype antibody (RORγt, IL-7Rα) or CD45– (CD4, CCR6, CD8, CD3, NKp46) negative controls or whole thymus positive controls (CD45). (D) Total number of intrathymic CD45+CD3–CD8–CD4+IL7R+RORγt+ ILC3s in BMT recipients or normal untransplanted controls. (E-F) Thymocytes were harvested and incubated for 5 hours with brefeldin A. Intracellular IL-22 was assessed by flow cytometry, demonstrating the frequency (E) and absolute number (F) of IL-22+ ILC3s in BMT recipients or normal untransplanted controls. (G) Absolute levels of intrathymic IL-23 on day 7 after BMT in BALB/c recipients or normal untransplanted controls. (H) B6 (CD45.2) recipients were transplanted with 10 000 Lineage–Sca1+ckit+ BM cells from congenic CD45.1+ donors, and BM, spleen, and thymus were analyzed on day 4 after transplant for ILC3 chimerism and absolute numbers of donor and host ILC3s (n = 5-15 mice per group). (I) Absolute number of host-derived (CD45.2+H-2d+) and donor BM–derived (CD45.1+H-2b+) thymic ILCs 7 days after B6→BALB/c transplant (performed as in panels A-G), gated on CD45+CD3–CD8–CD4+ cells (n = 9-10 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments.*P < .05; **P < .01; ***P < .001. FSC, forward scatter; ns, not significant.
To further assess this loss of TECs and thymocytes in GVHD, we next evaluated the thymic ILC3 compartment after allo-BMT. As in the setting of thymic radiation injury,17 IL-22 was produced by CD45+CD3–CD8–CD4+IL7R+RORγ(t)+ lymphoid tissue–inducer-like ILC3s after TCD allo-BMT (Figure 2C). There was no significant reduction in the number of thymic ILC3s after TCD allo-BMT (Figure 2D), consistent with the retained capacity to increase thymic IL-22 levels posttransplant in the absence of GVHD (Figure 1C-D). However, in mice with GVHD, there was a significant decrease in the number of intrathymic ILC3s (Figure 2D) as well as their production of IL-22 (Figure 2E-F) compared with recipients of TCD allo-BMT. Similarly, the absolute levels of intrathymic IL-23, which can induce ILC3 production of IL-22,32-34 were also increased after TCD BMT but were reduced in mice with GVHD (Figure 2G). ILC3s are long-lived and relatively radio-resistant,17,20,35 although we found that ILC3 reconstitution could begin early after syngeneic BMT, with donor ILC3s being detectable 4 days after transplant in bone marrow, spleen, and thymus (Figure 2H). Thus, the deficiency of intrathymic ILCs in mice with GVHD was the result of a significant decrease in both host-derived and donor marrow–derived ILCs (Figure 2I). GVHD thus led to a decrease in both the total number of thymic ILC3s and intrathymic IL-22 levels.
IL-22 deficiency leads to increased thymic damage as a result of GVHD
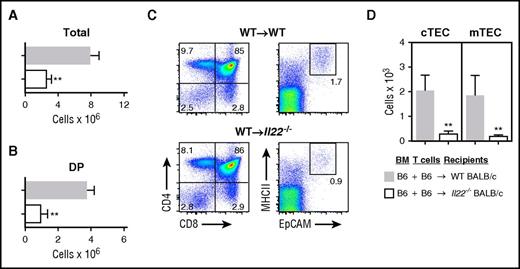
Given the reduced ILC numbers and intrathymic IL-22 levels identified in mice with GVHD (Figures 1 and 2), we next assessed the significance of IL-22 deficiency in thymic damage from GVHD. Wild-type (WT) or Il22−/− mice were used as BMT recipients in a B6→BALB/c (H-2b→H-2d) major histocompatibility complex (MHC)–mismatched GVHD model. IL-22–deficient BMT recipients transplanted with allogeneic T cells had considerably less total thymic cellularity (Figure 3A) and fewer total DP thymocytes (Figure 3B) compared with WT recipients of allo-BMT and T cells, although DP frequency was unchanged (Figure 3C), indicating that recipient-derived IL-22 contributed to maintaining overall thymopoiesis posttransplant. Because expression of the IL-22 receptor in the thymus is largely restricted to TECs,17 we hypothesized that IL-22 contributed to thymopoiesis post-BMT by helping to maintain the thymic epithelium. Indeed, IL-22–deficient recipients also had a significant reduction of TECs during GVHD (Figure 3C), including reduced numbers of both cTECs and mTECs compared with WT control recipients with GVHD (Figure 3D). Overall, IL-22 deficiency in BMT recipients with GVHD was associated with reduced thymic cellularity, loss of thymic stroma, and significantly impaired thymopoiesis.
IL-22 deficiency exacerbates thymic GVHD. WT or Il22−/− BALB/c recipients (H-2d) were transplanted with 5 × 106 B6 TCD BM (H-2b) cells and 1 × 106 WT B6 T cells. (A) Total thymic cellularity (n = 10-14 per group) and (B) number of CD4+CD8+ donor BM-derived DP cells (n = 5 per group) 21 days post-BMT. (C) Proportion of thymocyte subsets shown on the left, and MHCII+EpCAM+ TECs gated on viable CD45– cells shown on the right. (D) Total number of cTECs and mTECs (n = 5-9 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments. **P < .01.
IL-22 deficiency exacerbates thymic GVHD. WT or Il22−/− BALB/c recipients (H-2d) were transplanted with 5 × 106 B6 TCD BM (H-2b) cells and 1 × 106 WT B6 T cells. (A) Total thymic cellularity (n = 10-14 per group) and (B) number of CD4+CD8+ donor BM-derived DP cells (n = 5 per group) 21 days post-BMT. (C) Proportion of thymocyte subsets shown on the left, and MHCII+EpCAM+ TECs gated on viable CD45– cells shown on the right. (D) Total number of cTECs and mTECs (n = 5-9 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments. **P < .01.
Prevention of systemic GVHD maintains ILCs and preserves thymopoiesis in an IL-22–dependent fashion
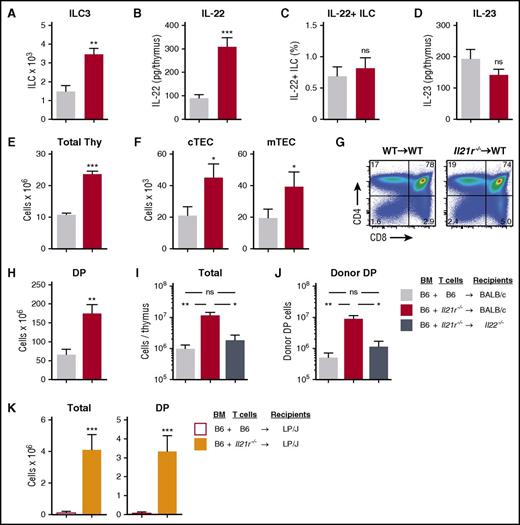
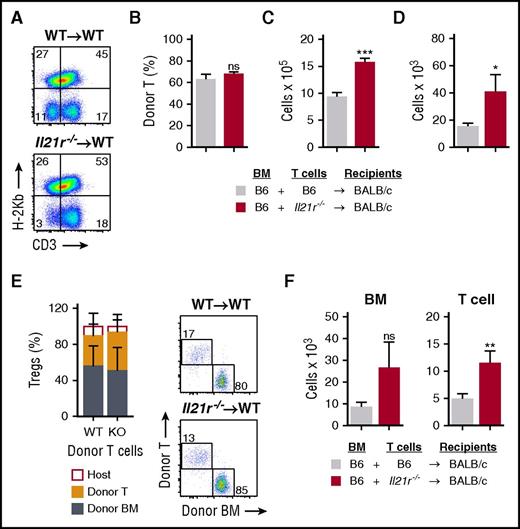
To further evaluate the function of ILCs in the transplant setting, we sought to investigate thymopoiesis in a model of attenuated GVHD. IL-21 has been shown to contribute to acute GVHD, acting directly on donor T cells to suppress regulatory T-cell expansion post-BMT and promote expression of homing molecules important for migration to the GI tract and thymus.27-30 We hypothesized that IL-21 receptor (IL-21R) signaling may be important for regulating T-cell–mediated thymic damage during GVHD as well. We thus performed allo-BMT with WT or Il21r−/− donor T cells and examined the effect on thymopoiesis and ILC function. After B6→BALB/c (H-2b→H-2d) allo-BMT, there was significant preservation of intrathymic ILC3 numbers (Figure 4A) and a significant increase in the absolute levels of intrathymic IL-22 in recipients of Il21r−/− T cells (Figure 4B). There was no change in the production of IL-22 on a per-cell basis (Figure 4C), potentially because of the stable expression of IL-23 (Figure 4D), which is known to induce IL-22 production by ILC3s.32-34 Moreover, recipients of Il21r−/− donor T cells also demonstrated increased total thymocyte numbers compared with recipients of WT T cells (Figure 4E). We thus concluded that the increased overall production of IL-22 was a result of the preservation of IL-22–producing ILCs and not because of an increase in their activation.
Abrogation of IL-21 signaling in donor T cells maintains intrathymic ILC3s and ameliorates thymic GVHD in an IL-22–dependent fashion. (A-D) WT BALB/c recipients (H-2d) transplanted with 5 × 106 CD45.1 B6 TCD BM (H-2b) cells and 1 × 106 CD45.2 B6 T cells from either WT or Il21r−/− B6 donors to induce GVHD. Thymus harvested on day 7 post-BMT. (A) Absolute number of intrathymic CD45+CD3–CD8–CD4+IL7R+RORγt+ ILC3s (n = 10 per group). (B) Absolute amount of thymic IL-22 measured by enzyme-linked immunosorbent assay (ELISA) (n = 9 per group). (C) ILC3s were isolated from thymus and incubated for 4 hours in the presence of brefeldin A and then examined for intracellular expression of IL-22 (n = 10 per group). (D) Absolute amount of thymic IL-23 measured by ELISA (n = 9 per group). (E-H) WT BALB/c recipients were transplanted with CD45.1 B6 TCD BM cells and WT or Il21r−/− CD45.2 B6 donor T cells as above; thymus was harvested 21 days post-BMT. (E) Total thymus cellularity (n = 40-44 per group). (F) Absolute number of CD45–EpCAM+MHCII+Ly51hiUEA-1lo cTECs and CD45–EpCAM+MHCII+Ly51loUEA-1hi mTECs (n = 12-14 per group). (G) Proportion of thymocyte subsets by flow cytometry gated on CD45+ cells. (H) Absolute number of BM-derived (H-2b+CD45.1+) CD4+CD8+ DP thymocytes (n = 9 per group). (I-J) B6→BALB/c BMT with WT B6 marrow, WT or Il21r−/− B6 donor T cells, and WT or Il22−/− BALB/c recipients (n = 10 per group). (I) Total thymus cellularity and (J) absolute number of CD4+CD8+ DP thymocytes at 21 days post-BMT. (K) Total thymic cellularity and absolute number of CD4+CD8+ DP thymocytes 21 days after B6→LP BMT (H-2b→H-2b) with WT B6 TCD BM and either WT or Il21r−/− B6 T cells (n = 17 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Abrogation of IL-21 signaling in donor T cells maintains intrathymic ILC3s and ameliorates thymic GVHD in an IL-22–dependent fashion. (A-D) WT BALB/c recipients (H-2d) transplanted with 5 × 106 CD45.1 B6 TCD BM (H-2b) cells and 1 × 106 CD45.2 B6 T cells from either WT or Il21r−/− B6 donors to induce GVHD. Thymus harvested on day 7 post-BMT. (A) Absolute number of intrathymic CD45+CD3–CD8–CD4+IL7R+RORγt+ ILC3s (n = 10 per group). (B) Absolute amount of thymic IL-22 measured by enzyme-linked immunosorbent assay (ELISA) (n = 9 per group). (C) ILC3s were isolated from thymus and incubated for 4 hours in the presence of brefeldin A and then examined for intracellular expression of IL-22 (n = 10 per group). (D) Absolute amount of thymic IL-23 measured by ELISA (n = 9 per group). (E-H) WT BALB/c recipients were transplanted with CD45.1 B6 TCD BM cells and WT or Il21r−/− CD45.2 B6 donor T cells as above; thymus was harvested 21 days post-BMT. (E) Total thymus cellularity (n = 40-44 per group). (F) Absolute number of CD45–EpCAM+MHCII+Ly51hiUEA-1lo cTECs and CD45–EpCAM+MHCII+Ly51loUEA-1hi mTECs (n = 12-14 per group). (G) Proportion of thymocyte subsets by flow cytometry gated on CD45+ cells. (H) Absolute number of BM-derived (H-2b+CD45.1+) CD4+CD8+ DP thymocytes (n = 9 per group). (I-J) B6→BALB/c BMT with WT B6 marrow, WT or Il21r−/− B6 donor T cells, and WT or Il22−/− BALB/c recipients (n = 10 per group). (I) Total thymus cellularity and (J) absolute number of CD4+CD8+ DP thymocytes at 21 days post-BMT. (K) Total thymic cellularity and absolute number of CD4+CD8+ DP thymocytes 21 days after B6→LP BMT (H-2b→H-2b) with WT B6 TCD BM and either WT or Il21r−/− B6 T cells (n = 17 per group). Bar graphs represent mean ± SEM of at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Given the increase in thymic ILCs and IL-22 seen in mice transplanted with Il21r−/− donor T cells, we next evaluated the thymic epithelium. Mice receiving Il21r−/− T cells demonstrated increased numbers of cTECs and mTECs (Figure 4F), both of which are known to be reduced in mice with GVHD.3,4 Consistent with the protection of TECs, there was a significant increase in the total number of, but not proportion of, donor-derived DP thymocytes (Figure 4G-H). Although abrogation of IL-21 receptor signaling in donor T cells led to protection of thymic ILCs, increased intrathymic IL-22, and improved thymopoiesis, it remained possible that the increased ILC numbers were a reflection of less thymic damage rather than a contributing factor. To formally test whether the IL-22 pathway was critical for regeneration of thymopoiesis after IL-21 blockade, we transplanted WT and Il22−/− allo-BMT recipients with marrow and Il21r−/− donor T cells. Although there was a significant increase in total thymic cellularity after transplantation with Il21r−/− T cells into WT recipients, this increase was completely abolished in Il22−/− recipient mice (Figure 4I). This total cellularity reflected the effect of IL-22 on thymopoiesis, because the increase in DP thymocytes observed in WT recipients of Il21r−/− T cells was also not present in IL-22–deficient recipients (Figure 4J). Preservation of thymopoiesis by blocking donor T-cell responses to IL-21 was thus dependent on preservation of ILCs and intact IL-22 signaling to promote thymic regeneration. Further validating the importance of IL-21 in inducing T-cell–mediated thymic damage in GVHD, allo-BMT with Il21r−/− donor T cells also led to increased thymic cellularity and improved thymopoiesis in a distinct MHC-matched B6→LP (H-2b→H-2b) transplant model (Figure 4K).
Previously, we found that IL-21 signaling can contribute to T-cell expression of homing molecules important for migration to the GI tract and that IL-21 blockade reduces intestinal infiltration with allogeneic donor T cells.27 Surprisingly, we found that these transplanted Il21r−/− T cells were able to infiltrate the thymus similar to WT T cells (Figure 5A-B), and given the increased thymic cellularity in mice receiving IL-21R–deficient donor T cells, this resulted in a larger absolute number of these donor T cells in the thymus (Figure 5C), although they were seemingly limited in their ability to mediate thymic damage. Consistent with this and with the role of IL-21 in suppressing regulatory T cells (Tregs), we found an increased total number of intrathymic Foxp3+ T cells present after BMT with Il21r−/− T cells (Figure 5D). We found no change in the proportion of Tregs in the thymus or in the proportional distribution of donor BM-derived or donor T-cell–derived Tregs (Figure 5E). The increase in total thymic Tregs was thus associated with both an expansion of donor T-cell–derived Tregs and a trend toward increased marrow-derived Tregs (Figure 5F) in a manner that was proportional to the overall increase in thymic size.
Deletion of IL-21 signaling increases the number of donor graft–derived Tregs in the thymus. (A-E) WT BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD BM cells from CD45.1+ B6 (H-2b) mice and 1 × 106 T cells derived from either WT (n = 10 recipients) or Il21r−/− (n = 10 recipients) CD45.2+ B6 mice to induce GVHD. (A) Concatenated flow cytometry plots showing transplanted donor graft–derived T cells on day 7 post-BMT. (B) Proportion of transplanted donor T cells of all thymic T cells on day 7 post-BMT. (C) Absolute number of transplanted donor graft–derived T cells in the thymus on day 7 post-BMT. (D) Absolute number of total CD3+CD8–CD25+CD4+FoxP3+ cells in the thymus on day 21 post-BMT (n = 9 per group). (E) Proportion of host-derived, graft T-cell–derived, and marrow-derived Tregs of all Tregs in the thymus on day 21 post-BMT, showing transplant with WT T cells in the left column and with IL-21R knockout (KO) T cells in right column; also shown are representative flow cytometry plots of Tregs derived from the donor marrow or from the T cells present in the donor graft. (F) Absolute number of donor BM–derived (H-2b+CD45.1+) or donor T-cell–derived (H-2b+CD45.2+) Tregs 21 days post-BMT (n = 9 per group). Dot plots concatenated from 5 independent observations from 1 experiment and gated on donor-derived (H-2b+) Tregs. Bar graphs represent mean ± SEM, combined from at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Deletion of IL-21 signaling increases the number of donor graft–derived Tregs in the thymus. (A-E) WT BALB/c (H-2d) recipients were transplanted with 5 × 106 TCD BM cells from CD45.1+ B6 (H-2b) mice and 1 × 106 T cells derived from either WT (n = 10 recipients) or Il21r−/− (n = 10 recipients) CD45.2+ B6 mice to induce GVHD. (A) Concatenated flow cytometry plots showing transplanted donor graft–derived T cells on day 7 post-BMT. (B) Proportion of transplanted donor T cells of all thymic T cells on day 7 post-BMT. (C) Absolute number of transplanted donor graft–derived T cells in the thymus on day 7 post-BMT. (D) Absolute number of total CD3+CD8–CD25+CD4+FoxP3+ cells in the thymus on day 21 post-BMT (n = 9 per group). (E) Proportion of host-derived, graft T-cell–derived, and marrow-derived Tregs of all Tregs in the thymus on day 21 post-BMT, showing transplant with WT T cells in the left column and with IL-21R knockout (KO) T cells in right column; also shown are representative flow cytometry plots of Tregs derived from the donor marrow or from the T cells present in the donor graft. (F) Absolute number of donor BM–derived (H-2b+CD45.1+) or donor T-cell–derived (H-2b+CD45.2+) Tregs 21 days post-BMT (n = 9 per group). Dot plots concatenated from 5 independent observations from 1 experiment and gated on donor-derived (H-2b+) Tregs. Bar graphs represent mean ± SEM, combined from at least 2 independent experiments. *P < .05; **P < .01; ***P < .001.
IL-22 administration can overcome the elimination of ILCs and restore thymopoiesis during GVHD
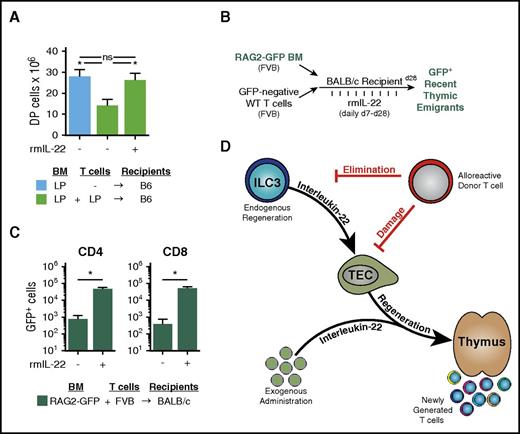
Because IL-22 deficiency led to increased thymic damage from GVHD and because preservation of IL-22 signaling was essential for maintaining thymopoiesis post-BMT, we next sought to determine whether the loss of thymic ILC3s in GVHD could be overcome by reintroduction of IL-22 via systemic administration of recombinant murine IL-22 (rmIL-22). Mice underwent clinically modeled MHC-matched LP→B6 (H-2b→H-2b) allo-BMT and were treated with 4 μg rmIL-22 or phosphate-buffered saline daily via intraperitoneal injection, starting on day 7 post-BMT. Recipients were euthanized 2 weeks later (3 weeks post-BMT) and evaluated for evidence of thymic GVHD. Administration of rmIL-22 led to enhanced thymopoiesis in mice with GVHD, with numbers of DP thymocytes after rmIL-22 treatment similar to those in mice without GVHD who had undergone TCD BMT (Figure 6A). This was despite the fact that rmIL-22–treated mice received allogeneic T cells and no immunosuppression to limit their potential to mediate GVHD.
Exogenous administration of IL-22 improves thymopoiesis and T-cell reconstitution after allo-BMT. (A) WT B6 recipients (H-2b) were transplanted with LP TCD BM (H-2b; 5 × 106) and WT LP T cells (4 × 106) (n = 9-10 recipients per group). GVHD mice were treated once per day with either phosphate-buffered saline (PBS) or rmIL-22 (200 mg/kg) starting on day 7 post-BMT; CD4+CD8+ DP thymocytes were assessed 3 weeks post-BMT. (B) Experimental model of FVB→BALB/c BMT (H-2q→H-2d) with RAG2-GFP marrow to evaluate thymic export of donor marrow–derived new peripheral T cells after IL-22 treatment. (C) BALB/c mice were transplanted with 5 × 106 RAG2-GFP (FVB background) TCD BM cells and 0.1 × 106 FVB T cells. Recipient mice (n = 5 per group) were treated once per day with either PBS or rmIL-22 (200 mg/kg) starting on day 7 post-BMT, and GFP+ T cells were enumerated in the spleen at 28 days post-BMT. (D) Thymic injury and depletion of thymocytes trigger ILC3 production of IL-22, which acts directly on TECs to induce their proliferation and survival. Through this trophic effect on TECs, IL-22 can promote endogenous immune regeneration. During GVHD, alloreactive T cells eliminate ILCs, thereby abrogating their production of IL-22 and impairing IL-22–mediated recovery of thymic epithelium. Administration of exogenous recombinant IL-22 can circumvent the loss of ILCs to enhance thymopoiesis and improve immune reconstitution after allo-BMT. Bar graphs represent mean ± SEM. *P < .05.
Exogenous administration of IL-22 improves thymopoiesis and T-cell reconstitution after allo-BMT. (A) WT B6 recipients (H-2b) were transplanted with LP TCD BM (H-2b; 5 × 106) and WT LP T cells (4 × 106) (n = 9-10 recipients per group). GVHD mice were treated once per day with either phosphate-buffered saline (PBS) or rmIL-22 (200 mg/kg) starting on day 7 post-BMT; CD4+CD8+ DP thymocytes were assessed 3 weeks post-BMT. (B) Experimental model of FVB→BALB/c BMT (H-2q→H-2d) with RAG2-GFP marrow to evaluate thymic export of donor marrow–derived new peripheral T cells after IL-22 treatment. (C) BALB/c mice were transplanted with 5 × 106 RAG2-GFP (FVB background) TCD BM cells and 0.1 × 106 FVB T cells. Recipient mice (n = 5 per group) were treated once per day with either PBS or rmIL-22 (200 mg/kg) starting on day 7 post-BMT, and GFP+ T cells were enumerated in the spleen at 28 days post-BMT. (D) Thymic injury and depletion of thymocytes trigger ILC3 production of IL-22, which acts directly on TECs to induce their proliferation and survival. Through this trophic effect on TECs, IL-22 can promote endogenous immune regeneration. During GVHD, alloreactive T cells eliminate ILCs, thereby abrogating their production of IL-22 and impairing IL-22–mediated recovery of thymic epithelium. Administration of exogenous recombinant IL-22 can circumvent the loss of ILCs to enhance thymopoiesis and improve immune reconstitution after allo-BMT. Bar graphs represent mean ± SEM. *P < .05.
We next sought to determine whether the benefit of adding back IL-22 was limited to T-cell development within the thymus or whether this resulted in successful exportation of naive T cells out of the thymus and into the periphery. We used an MHC-mismatched FVB→BALB/c (H-2q→H-2d) BMT model to take advantage of RAG2-GFP reporter bone marrow. Detection of GFP+ peripheral T cells in this transplant model indicates newly developed T cells that have undergone recent T-cell receptor gene rearrangement, a marker of recent thymic emigrants (Figure 6B). rmIL-22 or phosphate-buffered saline was injected intraperitoneally once per day starting on day 7 post-BMT, and T-cell reconstitution was assessed 4 weeks after transplant. Administration of rmIL-22 increased development of new donor marrow–derived CD4 and CD8 T cells in the periphery (Figure 6C). These findings highlight the importance of IL-22 in mediating thymic recovery posttransplant, demonstrating that IL-22 administration can improve thymopoiesis after allo-BMT and lead to development of new mature peripheral T cells (Figure 6D).
Discussion
Recent studies have made significant progress in identifying mechanisms involved in epithelial regeneration after injury, particularly in tissues that have potent endogenous capacities for regeneration such as the GI epithelium, which is in a constant state of turnover, the liver, which has considerable regenerative capacity, and the thymus, which has a remarkable ability to involute and then recover after acute stress.36 IL-22 has emerged as one such mechanism important for epithelial repair after acute damage in tissues as diverse as GI, liver, lung, skin, pancreas, and thymus.15 However, despite these growing insights into endogenous regenerative pathways, the processes leading to pathologic failure of regeneration remain poorly understood. In the thymus, this is a clinically relevant problem occurring in settings of chronic damage, such as age-associated thymic involution, chronic infection, repeated injury such as recurrent courses of cytoreductive chemotherapy, or alloreactive T-cell–mediated damage in GVHD.
There is increasing acceptance that the thymus is extremely sensitive to alloreactive damage and that this damage is a likely contributor to posttransplant immunodeficiency and chronic GVHD.4 During GVHD-related thymic injury, 3 events likely occur: (1) alloreactive T cells target TECs leading to their destruction and depletion; (2) thymocytes, including the most numerous DP population, are depleted through both direct and indirect mechanisms because of the depletion of TECs, and (3) failure to regenerate leads to prolonged suppression of T-cell reconstitution and likely contributes to the onset of chronic GVHD.3,4,37-41 In modeling the failure of thymic regeneration during GVHD, we have identified that maintenance of ILC3s and the IL-22 pathway are important for epithelial protection and recovery of thymopoiesis. ILC elimination is thus a potential mechanism whereby immune-mediated injury can abrogate the compensatory regenerative response. This pathophysiology is relevant for immune reconstitution after hematopoietic transplantation and highlights the fundamental role of the innate immune system in regulating recovery of adaptive immunity. Importantly, this pathophysiology can be ameliorated by reintroduction of IL-22 into the system.
We found that there is considerable loss of intrathymic IL-22–producing ILCs in GVHD and that IL-22 deficiency led to significantly worse thymopoiesis posttransplant. This was demonstrated by a greater loss of thymocytes and their supporting epithelial cells. Given that ILCs are not epithelial cells or traditionally appreciated targets of acute GVHD, these findings support a revised interpretation of basic GVHD pathophysiology. Thymic GVHD is not merely an alloreactive immune attack against a tissue but an abrogation of that tissue’s fundamental capacity to regenerate. Recent studies have revealed 2 putative epithelial cell progenitor populations in the adult thymus42,43 ; thus, additional insight into the epithelial cells within the thymus targeted by IL-22 will be critical for a more complete understanding of endogenous regeneration pathways and their failure in disease states. However, it should be noted that although IL-22 deficiency exacerbated loss of TECs and failure of thymopoiesis in mice with GVHD, treatment with IL-22 improved thymopoiesis posttransplant. TECs are known to express the IL-22 receptor, which activates STAT-3 and increases TEC survival and proliferation,17 so it is theoretically possible that some of the deficiency in thymopoiesis due to IL-22 deficiency and some of the improvement following IL-22 administration may be secondary either to enhanced intestinal barrier function or to improved systemic health.
Improving thymic function after allo-BMT is currently a major clinical challenge. Prolonged thymic deficiency leads to a significant delay in the recovery of the T-cell repertoire and subsequently leads to an increase in opportunistic infections and higher treatment-associated morbidity and mortality.1,44-47 We found that intrathymic ILCs contribute to restoring thymopoiesis posttransplant by producing IL-22 that acts upon the thymic epithelium. Interestingly, the finding that ILCs in the GI tract can express MHC class II48 allows for speculation that intrathymic ILCs may contribute to thymopoiesis more directly as well, perhaps via participation in T-cell selection in the thymus. Future studies should examine whether the function of intrathymic ILCs after transplant is limited to protecting thymic epithelium or whether ILCs can directly communicate with developing thymocytes.
Another important issue for thymic ILCs in the transplant setting is their ability to persist posttransplant. We have found that the ILC3 pools in the thymus and GI tract are resistant to radiation injury.17,20 Consistent with our studies in the GI tract,20 we report here that the majority of thymic ILC3s posttransplant are recipient-derived cells that persist after TCD BMT despite lethal total body irradiation. Of course, clinical conditioning regimens incorporate chemotherapy, unlike most mouse transplant models, including those evaluated here. The effect of chemotherapy on thymic ILCs is thus an important consideration. Notably, thymic ILC3s may also be relevant for epithelial protection and recovery after chemotherapy, given that ILC3s have been shown to reduce intestinal injury and increase intestinal regeneration in a mouse model of methotrexate toxicity49 and that circulating ILC3s before clinical transplantation were associated with reduced GVHD posttransplant.21
In experimental models, IL-21 produced by donor T cells contributes to acute GVHD by suppressing donor Tregs and promoting donor T-cell infiltration within the GI tract.27-30 Donor-derived IL-21 has also been shown to contribute to bronchiolitis obliterans by acting on donor B cells in experimental chronic GVHD.50 Here we have used 2 different models to show that IL-21 signaling in donor T cells also contributes to the pathogenesis of thymic GVHD. IL-21R–deficient donor T cells caused less depletion of thymic ILC3s, loss of TECs, and failure of T-cell development. Preservation of thymopoiesis in the setting of IL-21R deficiency was dependent on the presence of recipient-derived IL-22. Perhaps counterintuitively, recent studies suggest that in addition to the effect of IL-21 signaling on mature T-cell function, it can directly target positively selected thymocytes51 and can be used therapeutically to promote thymopoiesis after glucocorticoid-induced involution and in aged mice.52,53
Treating allo-BMT recipients with IL-22 significantly improved thymic function posttransplant and increased the export of recent thymic emigrants into the periphery, but the ultimate effect on functional immunity remains to be determined. Although other therapeutic strategies (such as IL-7, keratinocyte growth factor, and sex steroid ablation) have been shown to enhance thymopoiesis after radiation or cytoreductive chemotherapy,54-59 IL-22 administration is the first strategy we are aware of for reducing thymic injury in GVHD. Furthermore, its novel mechanistic approach for reducing tissue injury and stimulating the targets of immunologic attack rather than suppressing the effectors makes IL-22 an attractive therapeutic strategy for improving immune reconstitution without counterproductively increasing immunosuppressive treatments. However, although we have identified increased T-cell development with IL-22 treatment alone, given the complex multifactorial nature of impaired immune reconstitution posttransplant, we suspect that clinical translation would be optimized by a multifaceted approach that included adequate control of the pathologic alloreactive immune response and synergistic interventions such as IL-7 treatment targeting the thymocytes in addition to IL-22 targeting the thymic epithelium.
Here we show that recipient-derived IL-22 is protective in the thymus and protective in the GI tract,20,60 a pathologic potential of IL-22 that has been attributed to the effect of donor T cells.61,62 The mechanism for this donor IL-22–derived pathology is unclear, because immune cells (including donor T cells) do not express the IL-22 receptor and are not regulated by IL-22. On the basis of unbiased experiments with an anti–IL-22 neutralizing antibody eliminating both donor-derived and recipient-derived IL-22, we concluded that the overall net effect of IL-22 was a protective one.20 The etiology of the discrepancy between the function of donor-derived and recipient-derived IL-22 is not clear at present, although it may be a result of a synergistic effect with other cytokines produced at the same time and/or place. Indeed, a recent manuscript argues that the pathologic effect of donor-derived IL-22 is due to a synergistic effect with type 1 interferons.63 This combinatorial effect is at least conceptually consistent with several other examples of IL-22–related pathology, in which the pathology was dependent on the presence of another cytokine such as IL-17 or interferon-γ.15
In summary, we found that thymic ILCs were depleted during GVHD and that IL-22 deficiency led to greater loss of TECs and worsening thymopoiesis in GVHD. These findings reveal a clinically relevant pathophysiologic process that prevents thymic regeneration from immune-mediated damage and present 2 novel potential clinical strategies to improve immune reconstitution after allo-BMT: one that spares endogenous ILC numbers by IL-21 blockade and one epithelial-protective approach in IL-22 administration. The findings outlined here provide a rational approach for improving thymic function and immune reconstitution in recipients of allo-BMT and perhaps in other patients who experience a failure in endogenous thymic regeneration.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joe Sun for his expert review of the manuscript; Denzel Cole, Nury Yim, Fabiana Kreines, and Emily Levy for their technical assistance; and the Memorial Sloan Kettering Cancer Center Research Animal Resource Center for excellent animal care.
This research was supported by National Institutes of Health award numbers R00-CA176376 from the National Cancer Institute (NCI) (J.A.D.); R01-HL069929 from the National Heart, Lung, and Blood Institute (NHLBI) (M.R.M.v.d.B.); R01-AI100288, R01-AI080455, and R01-AI101406 from the National Institute of Allergy and Infectious Diseases (all to M.R.M.v.d.B.); P01-CA023766/Project 4 (NCI) (M.R.M.v.d.B.); K08-HL115355 and R01-HL125571 (NHLBI) (both to A.M.H.); and P30-CA008748 (NCI) (Memorial Sloan Kettering Cancer Center [MSKCC] Core Grant); by The Lymphoma Foundation, The Susan and Peter Solomon Divisional Genomics Program, and the MSKCC Cycle for Survival (all to M.R.M.v.d.B.); by the Cuyamaca Foundation and the Bezos Family Foundation (both to J.A.D.); and by grant agreement No. 602587 from the European Union’s Seventh Programme for Research, Technological Development and Demonstration.
J.A.D. received funding from a Scholar Award from the American Society of Hematology, and from a Mechtild Harf Award from the DKMS Foundation for Giving Life. A.M.H. received funding from the Amy Strelzer Manasevit Research Program and a Scholar Award from the American Society of Hematology.
Authorship
Contribution: J.A.D., M.R.M.v.d.B., and A.M.H. designed, analyzed, and interpreted the experiments and wrote the manuscript; A.M.M., M.H.O., R.R.J., and E.V. helped design and interpret experiments; L.F.Y. and O.M.S. provided technical assistance and helped with animal handling; R.L.B. helped interpret data; and M.R.M.v.d.B. and A.M.H. supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests. A patent application has been filed on the use of IL-22 as a thymopoietic growth factor (US 61/487 517) with J.A.D., A.M.H., and M.R.M.v.d.B. listed as inventors.
Correspondence: Jarrod A. Dudakov, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop D3-100, Seattle, WA 98109; e-mail: jdudakov@fredhutch.org; and Alan M. Hanash, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 346, New York, NY 10065; e-mail: hanasha@mskcc.org.
References
Author notes
M.R.M.v.d.B. and A.M.H. contributed equally to this study.