Key Points
Expression of Hmga2 enhances megakaryopoiesis in Jak2V617F knockin mice.
Hmga2 cooperates with Jak2V617F in the development of MF.
Abstract
Myelofibrosis (MF) is a devastating blood disorder. The JAK2V617F mutation has been detected in ∼50% cases of MF. Elevated expression of high-mobility group AT hook 2 (HMGA2) has also been frequently observed in patients with MF. Interestingly, upregulation of HMGA2 expression has been found in association with the JAK2V617F mutation in significant cases of MF. However, the contribution of HMGA2 in the pathogenesis of MF remains elusive. To determine the effects of concurrent expression of HMGA2 and JAK2V617F mutation in hematopoiesis, we transduced bone marrow cells from Jak2V617F knockin mice with lentivirus expressing Hmga2 and performed bone marrow transplantation. Expression of Hmga2 enhanced megakaryopoiesis, increased extramedullary hematopoiesis, and accelerated the development of MF in mice expressing Jak2V617F. Mechanistically, the data show that expression of Hmga2 enhances the activation of transforming growth factor-β1 (TGF-β1) and Cxcl12 pathways in mice expressing Jak2V617F. In addition, expression of Hmga2 causes upregulation of Fzd2, Ifi27l2a, and TGF-β receptor 2. Forced expression of Cxcl12, Fzd2, or Ifi27l2a increases megakaryocytic differentiation and proliferation in the bone marrow of Jak2V617F mice, whereas TGF-β1 or Cxcl12 stimulation induces collagen deposition in the bone marrow mesenchymal stromal cells. Together, these findings demonstrate that expression of Hmga2 cooperates with Jak2V617F in the pathogenesis of MF.
Introduction
Myelofibrosis (MF) is the deadliest form of myeloproliferative neoplasms (MPNs), characterized by abnormal megakaryopoiesis, progressive bone marrow (BM) fibrosis, and splenomegaly.1 Median survival for patients with MF is ∼5 years.2 JAK2V617F is the most common mutation associated with MPNs. This mutation is found in ∼95% of patients with polycythemia vera (PV) and 50% to 60% of patients with essential thrombocythemia (ET) and primary MF.3-7 Mutations in calreticulin (CALR)8,9 and thrombopoietin receptor (MPL)10,11 are also found in ET and primary MF but at much lower frequencies than JAK2V617F. Mutations in epigenetic regulators such as EZH2, ASXL1, and TET2 have also been implicated in MPNs,12 and the coexistence of these mutations along with one of the MPN driver (JAK2, CALR, MPL) mutations is thought to promote clonal evolution and progression of the disease.13
Several transgenic and knockin mouse models of Jak2V617F have been established.14-19 Expression of Jak2V617F results in a PV-like MPNs in most of these animal models, including our Jak2V617F knockin mouse.16 ET-like phenotype was also observed in other Jak2V617F mice.14,19 However, a low-grade MF is seen in some of these Jak2V617F mice after a long latency.14-18 Therefore, additional genetic abnormalities might be involved in association with JAK2V617F in the pathogenesis of MF. Consistent with this, it was shown that concomitant Jak2V617F expression and loss of Ezh2 or Tet2 promote MPN disease progression and development of MF in mice.20-23
Elevated expression of high-mobility group AT hook 2 (HMGA2) has been found in patients with MF.24-27 HMGA2 is a non-histone chromatin-binding protein that regulates gene transcription.28 HMGA2 is expressed at high levels during embryogenesis, but it is expressed at low levels in normal adult tissues.28 Overexpression of HMGA2 has been found in various human tumors.28,29 It promotes tumor metastasis in breast cancer.30 Moreover, HMGA2 has been shown to play a role in the self-renewal of hematopoietic stem cells (HSCs)31 and neuronal stem cells.32 We reported that loss of Ezh2 results in increased expression of Hmga2 in hematopoietic progenitors of Ezh2-deleted Jak2V617F mice exhibiting MF.20 Increased HMGA2 expression has also been observed in association with JAK2V617F in patients with MF.26,27 However, the contribution of HMGA2 in the pathogenesis of MPNs and MF mediated by JAK2V617F remains unknown.
In this study, we determined the effects of concomitant expression of Hmga2 and Jak2V617F in the mouse hematopoietic compartments by lentiviral transduction of Hmga2 in Jak2V617F knockin mouse bone marrow followed by transplantation into lethally irradiated C57BL/6 mice. Our results show that expression of Hmga2 promotes megakaryopoiesis and accelerates the development of MF in mice expressing Jak2V617F. We also provide mechanistic insights into how Hmga2 promotes the development of MF.
Materials and methods
Mice
Conditional Jak2V617F knockin16 Mx1Cre33 mice were previously described. All mice were on a C57BL/6 background. Cre expression was induced by intraperitoneal injection of polyinosine-polycytosine at 4 weeks after birth. All animal studies were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of State University of New York (SUNY) Upstate Medical University in Syracuse, New York.
Patient samples
Peripheral blood and BM samples from MF patients were collected at SUNY Upstate Medical University. Informed consent was obtained for sample collection according to the protocols approved by the institutional review board of the SUNY Upstate Medical University and in accordance with the Declaration of Helsinki.
Supplemental reagents and protocols
Plasmids, lentiviral transduction, cell culture, colony-forming assay, blood and tissue analysis, flow cytometry, immunoblotting, immunostaining, chromatin immunoprecipitation (ChIP) assay, real-time quantitative polymerase chain reaction (RT-qPCR), and enzyme-linked immunosorbent assay (ELISA) are described in the supplemental Data, available on the Blood Web site.
RNA sequencing
Lin–Sca1+c-kit+ (LSK) cells were sorted from Jak2VF/+-vector and Jak2VF/+-Hmga2 mice at 32 weeks after bone marrow transplant using a FACSAria II cell sorter. Total RNA was extracted from LSK cells using RNeasy Mini Kit (Qiagen). RNA sequencing (RNA-seq) was performed by using NextSequation 500 High Output Kit and a NextSequation 500 next-generation sequencing instrument (Illumina). Gene set enrichment analysis (GSEA) was performed as described.34 RNA-seq data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (accession number GSE99485).
Mesenchymal stromal cell culture, coculture system, and immunofluorescence staining
Mesenchymal stromal cells (MSCs) were generated from wild-type (WT) mice as previously described.35 For the coculture system, MSCs were re-plated and cultured for 72 hours before JAK2V617F-positive megakaryoblastic SET-2 cells expressing vector or HMGA2 were placed on top of MSCs and incubated for another 72 hours. For immunofluorescence staining, MSCs were grown on cover slips. SET-2 cells were placed on top of the MSCs and incubated for 72 hours. SET-2 cells were then removed, and collagen staining was performed as described previously.36 In some cases, MSCs were incubated with transforming growth factor-β1(TGF-β1; 50 ng/mL) or Cxcl12 (100 ng/mL) for 72 hours. Collagen staining was performed with unconjugated antibodies against collagen I or collagen III (Abcam). Secondary staining was performed by using Alexa Fluor 647 donkey anti-rabbit antibody (Jackson ImmunoResearch). Fluorescence was visualized by using a Nikon Eclipse Ti microscope. Data were analyzed by using Image J software.
Statistical analysis
Results are expressed as mean ± standard error of the mean, and statistical significance was determined by using the Student t test with Prism Version 6 software (GraphPad, San Diego, CA). P < .05 was considered statistically significant.
Results
Ectopic expression of Hmga2 accelerates the development of MF in Jak2V617F knockin mice
We assessed the HMGA2 protein expression in peripheral blood mononuclear cells and BM from healthy controls and JAK2V617F-positive MF patients by immunoblot analysis. Consistent with previous reports,26,27 we observed markedly elevated HMGA2 protein expression in patients with MF compared with healthy controls (supplemental Figure 1A-B). However, we did not see any significant difference in Hmga2 messenger RNA (mRNA) expression in the total BM and LSK cells between control WT mice and heterozygous Jak2V617F knockin mice exhibiting PV (supplemental Figure 2A-B).
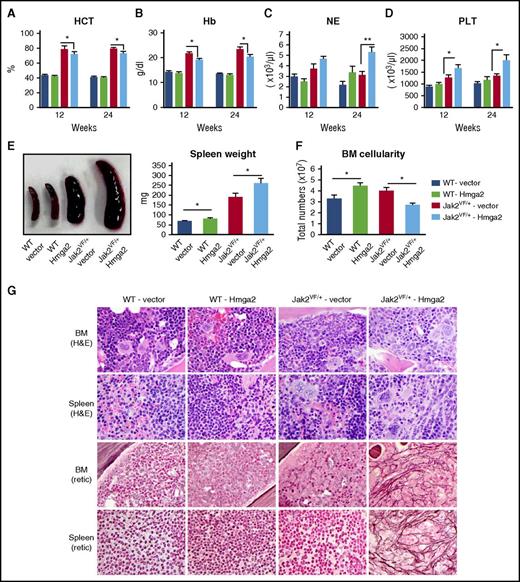
To assess the effects of HMGA2 expression on MPNs mediated by JAK2V617F, we transduced the BM cells from WT and Jak2V617F knockin mice16 using lentiviruses expressing vector or Hmga2 and transplanted them into lethally irradiated C57BL/6 recipient mice. Transduction efficiency of the lentiviral vector expressing Hmga2 was assessed in murine Ba/F3 and BM LSK cells by green fluorescent protein positivity (supplemental Figure 3A-B). Four groups of mice were analyzed: recipients of Jak2 WT BM expressing empty vector (WT-vector), recipients of Jak2 WT BM expressing Hmga2 (WT-Hmga2), recipients of Jak2V617F BM expressing vector (Jak2VF/+-vector), and recipients of Jak2V617F BM expressing Hmga2 (Jak2VF/+-Hmga2). Jak2VF/+-vector mice exhibited markedly increased hematocrit and hemoglobin levels in their peripheral blood at 12 and 24 weeks after transplantation (Figure 1A-B). Jak2VF/+-Hmga2 mice exhibited significantly increased neutrophil and platelet counts but decreased hematocrit and hemoglobin levels in their peripheral blood compared with Jak2VF/+-vector mice (Figure 1A-D). WT-Hmga2 mice did not show any significant alterations in blood parameters compared with WT-vector mice (Figure 1A-D). WT-Hmga2 mice exhibited modest increase in spleen size and weight compared with WT-vector mice (Figure 1E). Both Jak2VF/+-vector and Jak2VF/+-Hmga2 mice exhibited splenomegaly; however, Jak2VF/+-Hmga2 mice exhibited significantly larger spleen size and weight compared with Jak2VF/+-vector mice (Figure 1E), suggesting that expression of Hmga2 increases extramedullary hematopoiesis in Jak2V617F mice. BM cellularity was increased in WT-Hmga2 mice compared with WT-vector mice (Figure 1F), but Jak2VF/+-Hmga2 mice exhibited significantly reduced BM cellularity compared with Jak2VF/+-vector mice (Figure 1F) because of fibrosis in the BM of Jak2VF/+-Hmga2 mice.
Overexpression of Hmga2 accelerates the development of MF in Jak2V617Fknockin mice. (A) Hematocrit (HCT), (B) hemoglobin (Hb), (C) neutrophil (NE), and (D) platelet (PLT) counts in the peripheral blood were assessed at 12 and 24 weeks after bone marrow transplant (BMT) in WT-vector (n = 8), WT-Hmga2 (n = 9), Jak2VF/+-vector (n = 8), and Jak2VF/+-Hmga2 (n = 11) mice. (E) Spleen size and weight were significantly increased in Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice, whereas spleen size of WT-Hmga2 was modestly increased compared with WT-vector mice (n = 7-10). (F) BM cellularity was significantly increased in WT-Hmga2 mice compared with WT-vector mice, whereas Jak2VF/+-Hmga2 mice showed significantly decreased BM cellularity compared with Jak2VF/+-vector mice (n = 7-10). Student t test was used to compare 2 groups of mice. (G) Histopathologic analysis. Hematoxylin and eosin (H&E) staining of the BM and spleen sections (original magnification ×500) from WT-Hmga2 mice show normal morphology similar to that of WT-vector controls. BM sections from Jak2VF/+-vector mice show trilineage (megakaryocyte/erythrocyte/granulocyte) hyperplasia, whereas BM sections from Jak2VF/+-Hmga2 mice display fibrotic-appearing marrow with increased myelopoiesis. Spleen sections from Jak2VF/+-vector mice show trilineage extramedullary hematopoiesis, whereas spleens from Jak2VF/+-Hmga2 mice show increased myelopoiesis, increased and atypical megakaryocytes, and architectural distortion. Reticulin staining showed extensive fibrosis (grade 2 to 3) in the BM and spleens of Jak2VF/+-Hmga2 mice at 32 weeks after transplantation. BM and spleens of Jak2VF/+-vector mice exhibited little fibrosis at this stage. BM and spleens of WT-vector and WT-Hmga2 mice did not exhibit fibrosis. *P < .05; **P < .005.
Overexpression of Hmga2 accelerates the development of MF in Jak2V617Fknockin mice. (A) Hematocrit (HCT), (B) hemoglobin (Hb), (C) neutrophil (NE), and (D) platelet (PLT) counts in the peripheral blood were assessed at 12 and 24 weeks after bone marrow transplant (BMT) in WT-vector (n = 8), WT-Hmga2 (n = 9), Jak2VF/+-vector (n = 8), and Jak2VF/+-Hmga2 (n = 11) mice. (E) Spleen size and weight were significantly increased in Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice, whereas spleen size of WT-Hmga2 was modestly increased compared with WT-vector mice (n = 7-10). (F) BM cellularity was significantly increased in WT-Hmga2 mice compared with WT-vector mice, whereas Jak2VF/+-Hmga2 mice showed significantly decreased BM cellularity compared with Jak2VF/+-vector mice (n = 7-10). Student t test was used to compare 2 groups of mice. (G) Histopathologic analysis. Hematoxylin and eosin (H&E) staining of the BM and spleen sections (original magnification ×500) from WT-Hmga2 mice show normal morphology similar to that of WT-vector controls. BM sections from Jak2VF/+-vector mice show trilineage (megakaryocyte/erythrocyte/granulocyte) hyperplasia, whereas BM sections from Jak2VF/+-Hmga2 mice display fibrotic-appearing marrow with increased myelopoiesis. Spleen sections from Jak2VF/+-vector mice show trilineage extramedullary hematopoiesis, whereas spleens from Jak2VF/+-Hmga2 mice show increased myelopoiesis, increased and atypical megakaryocytes, and architectural distortion. Reticulin staining showed extensive fibrosis (grade 2 to 3) in the BM and spleens of Jak2VF/+-Hmga2 mice at 32 weeks after transplantation. BM and spleens of Jak2VF/+-vector mice exhibited little fibrosis at this stage. BM and spleens of WT-vector and WT-Hmga2 mice did not exhibit fibrosis. *P < .05; **P < .005.
BM and spleen sections from WT-Hmga2 mice showed normal morphology and were very similar to WT-vector mice with no increase in reticulin fibrosis (Figure 1G). BM of Jak2VF/+-vector mice showed trilineage hyperplasia with increased erythroid and myeloid precursors and increased megakaryocytes. Spleens of Jak2VF/+-vector mice showed extramedullary trilineage hematopoiesis. BM and spleen sections from Jak2VF/+-Hmga2 mice showed increased myelopoiesis with immature precursors, increased and atypical megakaryocytes, and architectural distortion consistent with MF. Reticulin staining demonstrated extensive fibrosis (grade 2 to 3) in the BM and spleens of Jak2VF/+-Hmga2 mice at 32 weeks after bone marrow transplant, whereas Jak2VF/+-vector mice exhibited very little fibrosis at this stage (Figure 1G). Together, these results suggest that ectopic expression of Hmga2 accelerates the development of MF in mice expressing Jak2V617F.
Effects of Hmga2 overexpression on hematopoietic precursors and progenitors expressing Jak2V617F
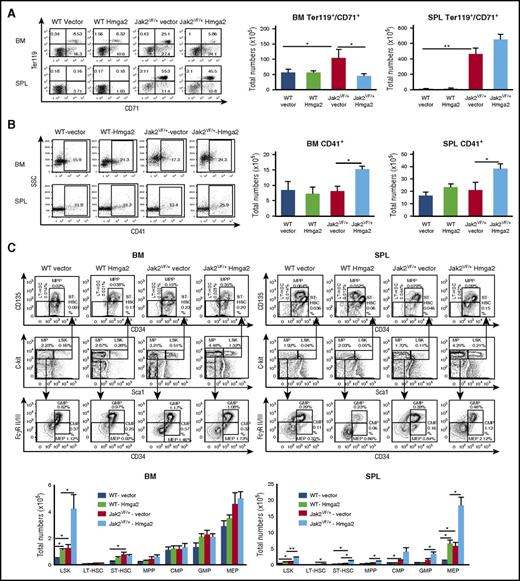
Flow cytometric analysis of the BM and spleens from Jak2VF/+-vector mice showed marked increase in erythroid precursors (CD71+Ter119+) compared with WT-vector or WT-Hmga2 mice (Figure 2A), as expected. Overexpression of Hmga2 resulted in significant decrease in CD71+Ter119+ cells in the BM of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 2A). The number of myeloid precursors (Gr-1+/Mac-1+) was increased in the BM of WT-Hmga2 compared with WT-vector mice but was significantly reduced in the BM of Jak2VF/+-Hmga2 compared with Jak2VF/+-vector mice (supplemental Figure 4A-B). However, the spleens of Jak2VF/+-Hmga2 mice showed a significant increase in Gr-1+/Mac-1+ cells compared with Jak2VF/+-vector mice (supplemental Figure 4A-B). In contrast, total numbers of CD41+ megakaryocytic precursors were significantly increased in the BM and spleens of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 2B). We also performed immunostaining for CD41 in the BM and spleen sections from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice. We observed significantly increased numbers of CD41+ cells in the BM and spleens of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector or WT mice (supplemental Figures 9 and 10). Overall, these results suggest that overexpression of Hmga2 increases megakaryopoiesis in Jak2V617F mice.
Effects of Hmga2 overexpression on hematopoietic precursors and progenitors in mice expressing Jak2V617F. (A) Representative dot plots of flow cytometric analysis of erythroid precursors in the BM and spleens (SPL) of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (at 32 weeks after BMT) using surface markers CD71 and Ter119. Total numbers of erythroid precursor cells are shown in bar graphs as mean ± standard error of the mean (SEM) (n = 5-10). (B) Representative dot plots of flow cytometric analysis of megakaryocytic precursors in the BM and spleens of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice using CD41 staining. Total numbers of CD41+ megakaryocytic precursors in the BM and spleens are shown in bar graphs as mean ± SEM (n = 5-10). (C) Representative contour plots of flow cytometric analysis of Lin–Sca-1+c-kit+ (LSK), long-term HSC (LT-HSC; Lin–Sca-1+c-kit+CD34–CD135–), short-term HSC (ST-HSC; Lin–Sca-1+c-kit+CD34+CD135–), multipotential progenitor (MPP; Lin–Sca-1+c-kit+CD34+CD135+), common myeloid progenitor (CMP; Lin–Sca-1–c-kit+CD34+FcγRII/IIlow), granulocyte/macrophage progenitor (GMP; Lin–Sca-1–c-kit+CD34+FcγRII/IIhigh), and megakaryocyte/erythroid progenitor (MEP; Lin–Sca-1–c-kit+CD34–FcγRII/III–) cells in the BM and spleens from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT. Total numbers of LSK, LT-HSC, ST-HSC, MPP, CMP, GMP, and MEP cells in the BM and spleens are shown in bar graphs as mean ± SEM (n = 8-10). *P < .05; **P < .005.
Effects of Hmga2 overexpression on hematopoietic precursors and progenitors in mice expressing Jak2V617F. (A) Representative dot plots of flow cytometric analysis of erythroid precursors in the BM and spleens (SPL) of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (at 32 weeks after BMT) using surface markers CD71 and Ter119. Total numbers of erythroid precursor cells are shown in bar graphs as mean ± standard error of the mean (SEM) (n = 5-10). (B) Representative dot plots of flow cytometric analysis of megakaryocytic precursors in the BM and spleens of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice using CD41 staining. Total numbers of CD41+ megakaryocytic precursors in the BM and spleens are shown in bar graphs as mean ± SEM (n = 5-10). (C) Representative contour plots of flow cytometric analysis of Lin–Sca-1+c-kit+ (LSK), long-term HSC (LT-HSC; Lin–Sca-1+c-kit+CD34–CD135–), short-term HSC (ST-HSC; Lin–Sca-1+c-kit+CD34+CD135–), multipotential progenitor (MPP; Lin–Sca-1+c-kit+CD34+CD135+), common myeloid progenitor (CMP; Lin–Sca-1–c-kit+CD34+FcγRII/IIlow), granulocyte/macrophage progenitor (GMP; Lin–Sca-1–c-kit+CD34+FcγRII/IIhigh), and megakaryocyte/erythroid progenitor (MEP; Lin–Sca-1–c-kit+CD34–FcγRII/III–) cells in the BM and spleens from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT. Total numbers of LSK, LT-HSC, ST-HSC, MPP, CMP, GMP, and MEP cells in the BM and spleens are shown in bar graphs as mean ± SEM (n = 8-10). *P < .05; **P < .005.
We next assessed the effects of Hmga2 overexpression on hematopoietic stem cells (HSCs) and progenitors in Jak2 WT and Jak2V617F mice by flow cytometric analysis. Total number of LSK cells was significantly increased in the BM of WT-Hmga2 mice compared with WT-vector controls (Figure 2C; supplemental Figures 5 and 6). There was also a tendency toward increased numbers of long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs) in the BM of WT-Hmga2 mice compared with WT-vector controls (supplemental Figure 6). In addition, the megakaryocyte/erythroid progenitor population was significantly increased in the spleens of WT-Hmga2 mice compared with WT-vector controls (Figure 2C; supplemental Figure 5). The frequency and total numbers of LSK cells were significantly increased in the BM and spleens of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 2C; supplemental Figure 5). Spleens of Jak2VF/+-Hmga2 mice also exhibited increased numbers of LT-HSCs, ST-HSCs, multipotential progenitors, common myeloid progenitors, granulocyte/macrophage progenitors, and megakaryocyte/erythroid progenitors compared with spleens of Jak2VF/+-vector mice (Figure 2C; supplemental Figures 5 and 6). Overall, these results suggest that Hmga2 cooperates with Jak2V617F to expand the HSC and progenitor compartment.
Expression of Hmga2 promotes megakaryocytic differentiation and enhances megakaryocytic cell proliferation in Jak2V617F mouse BM
We also performed hematopoietic progenitor colony assays to determine the effects of Hmga2 expression on hematopoietic progenitors. The burst-forming unit erythroid (BFU-E) colonies were significantly increased in the BM of Jak2VF/+-vector mice compared with control animals (Figure 3A). BM of Jak2VF/+-Hmga2 mice showed significantly reduced BFU-E colonies compared with Jak2VF/+-vector mice (Figure 3A). Colony-forming unit, granulocyte-macrophage (CFU-GM) colonies in the BM of Jak2VF/+-vector and Jak2VF/+-Hmga2 mice were comparable (Figure 3A). Erythropoietin (EPO)-independent CFU erythroid (CFU-E) colonies, a hallmark feature of PV,37 was observed in the BM of Jak2VF/+-vector mice (Figure 3B). Overexpression of Hmga2 markedly inhibited both EPO-dependent and EPO-independent CFU-E colonies in the BM of Jak2VF/+-Hmga2 mice (Figure 3B). In contrast, the number of CFU megakaryocytic (CFU-Mk) colonies was significantly increased in the BM of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 3C). The numbers of CFU-Mk colonies were also significantly increased in the BM of WT-Hmga2 mice compared with those in WT-vector BM (Figure 3C). Thus, expression of Hmga2 enhances megakaryocytic differentiation in mouse BM. We also assessed megakaryocytic cell proliferation in the BM of mice with transplants. For this purpose, we first cultured the BM cells in a megakaryocytic culture condition (StemPro-34 medium containing stem cell factor and thrombopoietin) for 4 days. Flow cytometric analysis show ∼99% cells were CD41+ after 4 days of culture (supplemental Figure 7). Then we assessed cell proliferation by using the trypan blue exclusion method every other day over 6 days. Expression of Hmga2 significantly increased megakaryocytic proliferation in both WT and Jak2V617F mouse BM (Figure 3D). We further assessed the effects of ex vivo expression of Hmga2 on megakaryocytic differentiation and proliferation in the BM of WT and Jak2V617F mice. BM cells from WT and Jak2V617F knockin mice were transduced with lentivirus expressing Hmga2 or vector. Infected cells were selected using puromycin, and CFU-Mk colony formation and megakaryocytic cell proliferation were assessed. Ectopic expression of Hmga2 significantly increased CFU-Mk colonies and megakaryocytic proliferation in the BM of both WT and Jak2V617F mice compared with vector control (Figure 3E-F). Together, these results suggest that expression of Hmga2 promotes megakaryocytic differentiation and proliferation.
Hmga2 promotes megakaryocytic differentiation and enhances megakaryocytic cell proliferation. (A) BM cells (2 × 104) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated in methylcellulose medium supplemented with cytokines. BFU-E and CFU-GM colonies were scored 7 days after plating. (B) CFU-E colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 6) were plated in methylcellulose medium in the presence of erythropoietin (3 U/mL) or absence of erythropoietin. CFU-E colonies were scored after 2 days. (C) CFU-Mk colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated into collagen-based MegaCult medium supplemented with interleukin-3 (IL-3), IL-6, IL-11, and thrombopoietin (TPO). Megakaryocytic (CFU-Mk) colonies were assessed after culturing for 8 days. (D) Expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM. BM cells from the transplanted animals were first cultured in a megakaryocytic culture condition (StemPro-34 medium containing stem cell factor [SCF] and TPO) for 4 days. Flow cytometric analysis confirmed ∼99% cells were CD41+ after 4 days of culture. Then equal numbers of megakaryocytic cells were plated, and cell proliferation was assessed by viable cell counts over 6 days. (E) Ex vivo expression of Hmga2 significantly increased CFU-Mk colonies in the BM of both WT and Jak2V617F mice compared with vector expression (n = 4-6). (F) Ex vivo expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM compared with vector expression. First megakaryocytic culture was established and then cell proliferation was assessed by viable cell counts over 6 days. All data are shown as mean ± SEM. Student t test was used for comparison between 2 groups of mice. *P < .05; **P < .005; ***P < .0005.
Hmga2 promotes megakaryocytic differentiation and enhances megakaryocytic cell proliferation. (A) BM cells (2 × 104) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated in methylcellulose medium supplemented with cytokines. BFU-E and CFU-GM colonies were scored 7 days after plating. (B) CFU-E colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 6) were plated in methylcellulose medium in the presence of erythropoietin (3 U/mL) or absence of erythropoietin. CFU-E colonies were scored after 2 days. (C) CFU-Mk colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated into collagen-based MegaCult medium supplemented with interleukin-3 (IL-3), IL-6, IL-11, and thrombopoietin (TPO). Megakaryocytic (CFU-Mk) colonies were assessed after culturing for 8 days. (D) Expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM. BM cells from the transplanted animals were first cultured in a megakaryocytic culture condition (StemPro-34 medium containing stem cell factor [SCF] and TPO) for 4 days. Flow cytometric analysis confirmed ∼99% cells were CD41+ after 4 days of culture. Then equal numbers of megakaryocytic cells were plated, and cell proliferation was assessed by viable cell counts over 6 days. (E) Ex vivo expression of Hmga2 significantly increased CFU-Mk colonies in the BM of both WT and Jak2V617F mice compared with vector expression (n = 4-6). (F) Ex vivo expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM compared with vector expression. First megakaryocytic culture was established and then cell proliferation was assessed by viable cell counts over 6 days. All data are shown as mean ± SEM. Student t test was used for comparison between 2 groups of mice. *P < .05; **P < .005; ***P < .0005.
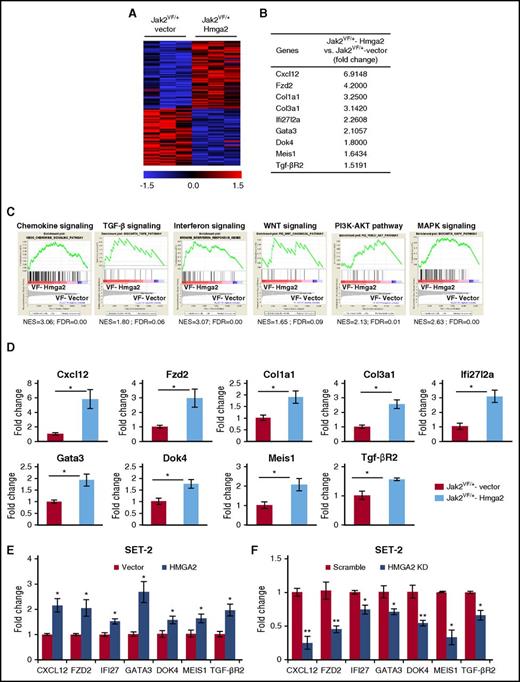
Expression of Hmga2 alters gene expression in Jak2V617F LSK cells
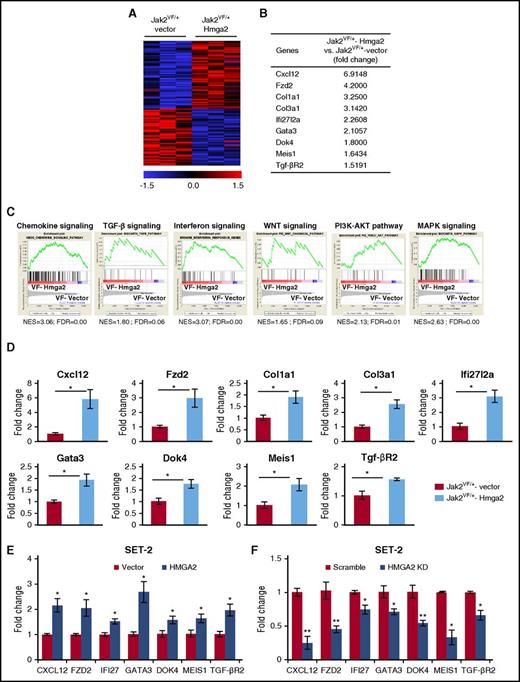
To gain insights into the mechanism by which Hmga2 overexpression promotes the development of MF in Jak2V617F knockin mice, we performed RNA-seq analyses on sorted LSK cells from Jak2VF/+-vector and Jak2VF/+-Hmga2 mice. Heat maps show significantly upregulated and downregulated genes in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells (Figure 4A). Analysis of RNA-seq data revealed that 471 genes were significantly upregulated (fold change, >1.5-fold; P < .05) in Jak2VF/+-Hmga2 LSK compared with Jak2VF/+-vector LSK cells. We also identified 90 genes that were significantly downregulated (fold change <−1.5-fold; P < .05) in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells. Several important genes were found to be upregulated in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells. These included chemokine Cxcl12, Wnt receptor frizzled 2 (Fzd2), collagen1a1 (Col1a1), collagen3a1 (Col3a1), interferon pathway-related gene Ifi27l2a, transcription factor Gata3, adapter protein Dok4, homeobox gene Meis1, and TGF-β receptor 2 (Tgf-βR2) (Figure 4B). GSEA34 revealed significant enrichment of a number of signaling pathways in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells. These include chemokine signaling, TGF-β pathway, WNT signaling pathway, interferon signaling, and PI3K-AKT and MAPK signaling pathways (Figure 4C). RT-qPCR further validated that expression of Cxcl12, Fzd2, Col1a1, Col3a1, Ifi27l2a, Gata3, Dok4, Meis1, and Tgf-βR2 was significantly upregulated in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells (Figure 4D).
Hmga2 overexpression alters gene expression in Jak2V617FLSK. (A) Heat maps showing significantly upregulated and downregulated genes in Jak2VF/+-Hmga2 Lin–Sca-1+ckit+ (LSK) cells compared with Jak2VF/+-vector LSK cells. (B) A list of selected genes that are significantly upregulated in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells. (C) Gene set enrichment analyses (GSEAs) of the RNA-seq data from Jak2VF/+-vector LSK cells and Jak2VF/+-Hmga2 LSK cells. Enrichment plots of selected gene sets with normalized enrichment score (NES) and false discovery rate (FDR) are shown. (D) Relative expression of Cxcl12, Fzd2, collagen1a1 (Col1a1), collagen3a1 (Col3a1), Ifi27l2a, Gata3, Dok4, Meis1, and Tgf-βR2 mRNA was determined in Jak2VF/+-vector and Jak2VF/+-Hmga2 LSK cells by RT-qPCR and normalized with glyceraldehyde 3-phosphate dehydrogenase (Gapdh) expression. (E) Validation of some HMGA2 targets in JAK2V617F-positive human megakaryoblastic SET-2 cells. SET-2 cells were transduced with lentivirus expressing HMGA2, and the infected cells were selected using puromycin. Relative expression of CXCL12, FZD2, IFI27 (homolog of mouse Ifi27l2a), GATA3, DOK4, MEIS1, and TGF-βR2 was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (F) Validation of the target genes by knockdown of HMGA2 in SET-2 cells. SET-2 cells were transduced with lentiviral HMGA2 short hairpin RNA (shRNA) or scramble shRNA (control), and the infected cells were selected by using puromycin. Relative expression of CXCL12, FZD2, IFI27, GATA3, DOK4, MEIS1, and TGF-βR2 was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. *P < .05.
Hmga2 overexpression alters gene expression in Jak2V617FLSK. (A) Heat maps showing significantly upregulated and downregulated genes in Jak2VF/+-Hmga2 Lin–Sca-1+ckit+ (LSK) cells compared with Jak2VF/+-vector LSK cells. (B) A list of selected genes that are significantly upregulated in Jak2VF/+-Hmga2 LSK cells compared with Jak2VF/+-vector LSK cells. (C) Gene set enrichment analyses (GSEAs) of the RNA-seq data from Jak2VF/+-vector LSK cells and Jak2VF/+-Hmga2 LSK cells. Enrichment plots of selected gene sets with normalized enrichment score (NES) and false discovery rate (FDR) are shown. (D) Relative expression of Cxcl12, Fzd2, collagen1a1 (Col1a1), collagen3a1 (Col3a1), Ifi27l2a, Gata3, Dok4, Meis1, and Tgf-βR2 mRNA was determined in Jak2VF/+-vector and Jak2VF/+-Hmga2 LSK cells by RT-qPCR and normalized with glyceraldehyde 3-phosphate dehydrogenase (Gapdh) expression. (E) Validation of some HMGA2 targets in JAK2V617F-positive human megakaryoblastic SET-2 cells. SET-2 cells were transduced with lentivirus expressing HMGA2, and the infected cells were selected using puromycin. Relative expression of CXCL12, FZD2, IFI27 (homolog of mouse Ifi27l2a), GATA3, DOK4, MEIS1, and TGF-βR2 was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (F) Validation of the target genes by knockdown of HMGA2 in SET-2 cells. SET-2 cells were transduced with lentiviral HMGA2 short hairpin RNA (shRNA) or scramble shRNA (control), and the infected cells were selected by using puromycin. Relative expression of CXCL12, FZD2, IFI27, GATA3, DOK4, MEIS1, and TGF-βR2 was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. *P < .05.
We further validated some candidate Hmga2 target genes by enforced expression or knockdown of HMGA2 in JAK2V617F-positive human SET-2 cells. We observed that overexpression of HMGA2 in SET-2 cells resulted in significantly increased expression of CXCL12, FZD2, IFI27 (homolog of mouse Ifi27l2a), GATA3, DOK4, MEIS1, and TGF-βR2 (Figure 4E). Conversely, knockdown of HMGA2 by lentiviral short hairpin RNA in SET-2 cells resulted in significantly decreased expression of these genes (Figure 4F), suggesting that these genes are bona fide targets of HMGA2.
HMGA2 binds to the promoter regions of its target genes
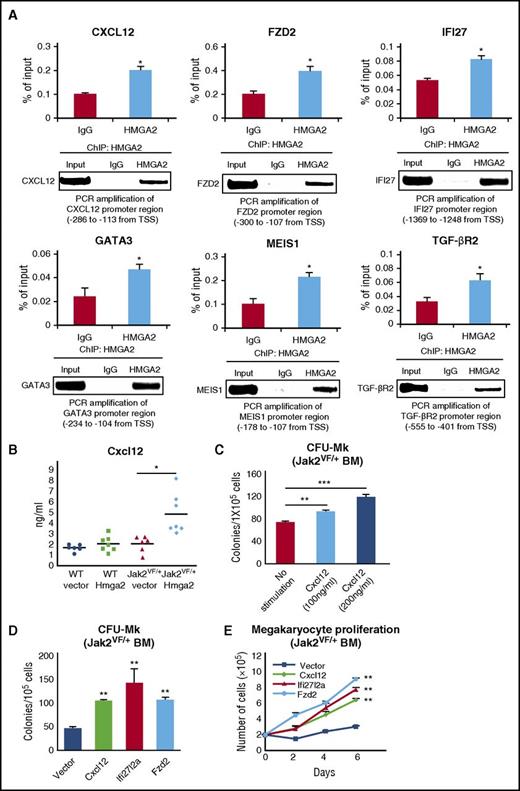
Because HMGA2 is a DNA-binding protein, we asked whether HMGA2 regulates the expression of its target genes by directly binding to their promoters. To address this question, we performed an HMGA2-specific ChIP assay in SET-2 cells and assessed the binding of HMGA2 in the promoters of target genes by RT-qPCR. We observed binding of HMGA2 to the promoter regions of CXCL12, FZD2, IFI27, GATA3, MEIS1, and TGF-βR2 in SET-2 cells (Figure 5A). Binding of HMGA2 to the histone 3 F3A promoter was used as a positive control for HMGA2 ChIP (supplemental Figure 8). The HMGA2 binding sites on these gene promoters were found adjacent to the transcription start site with AT-rich nucleotide sequence.
HMGA2 binds to the promoters of its target genes, and ectopic expression of some HMGA2 target genes increases CFU-Mk colonies and megakaryocytic cell proliferation in Jak2V617Fmouse BM. (A) HMGA2-specific ChIP followed by RT-qPCR showed binding of HMGA2 in the promoters of CXCL12, FZD2, IFI27, GATA3, MEIS1, and TGF-βR2 genes in JAK2V617F-positive SET-2 cells. Results from 3 independent experiments are presented as mean ± SEM in bar graphs. The RT-qPCR products were loaded onto 2% agarose gel. Representative pictures from agarose gel are shown in the bottom panels. (B) Total levels of Cxcl12 in the serum of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT were assessed by ELISA (n = 6-7). (C) Stimulation with Cxcl12 (100-200 ng/mL) significantly increased CFU-Mk formation in the BM of Jak2V617F mice (n = 4-8 for each concentration). (D) Ectopic expression of Cxcl12, Ifi27l2a, or Fzd2 significantly increased CFU-Mk colonies and (E) enhanced megakaryocytic proliferation in the BM of Jak2V617F mice compared with vector control (n = 4-7 for each construct). Megakaryocytic cell proliferation was assessed by viable cell counts every other day over 6 days. Data from 3 independent experiments are shown in graphs as mean ± SEM. *P < .05; **P < .005.
HMGA2 binds to the promoters of its target genes, and ectopic expression of some HMGA2 target genes increases CFU-Mk colonies and megakaryocytic cell proliferation in Jak2V617Fmouse BM. (A) HMGA2-specific ChIP followed by RT-qPCR showed binding of HMGA2 in the promoters of CXCL12, FZD2, IFI27, GATA3, MEIS1, and TGF-βR2 genes in JAK2V617F-positive SET-2 cells. Results from 3 independent experiments are presented as mean ± SEM in bar graphs. The RT-qPCR products were loaded onto 2% agarose gel. Representative pictures from agarose gel are shown in the bottom panels. (B) Total levels of Cxcl12 in the serum of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT were assessed by ELISA (n = 6-7). (C) Stimulation with Cxcl12 (100-200 ng/mL) significantly increased CFU-Mk formation in the BM of Jak2V617F mice (n = 4-8 for each concentration). (D) Ectopic expression of Cxcl12, Ifi27l2a, or Fzd2 significantly increased CFU-Mk colonies and (E) enhanced megakaryocytic proliferation in the BM of Jak2V617F mice compared with vector control (n = 4-7 for each construct). Megakaryocytic cell proliferation was assessed by viable cell counts every other day over 6 days. Data from 3 independent experiments are shown in graphs as mean ± SEM. *P < .05; **P < .005.
Ectopic expression of Cxcl12, Fzd2, and Ifi27l2a increases CFU-Mk colonies and megakaryocytic cell proliferation in Jak2V617F mouse BM
We further assessed the serum levels of Cxcl12 in the Jak2VF/+-Hmga2 mice by ELISA. We observed increased levels of Cxcl12 in the serum of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 5B). Cxcl12 was previously linked to increased megakaryopoiesis.38 We performed CFU-Mk colony assay on Jak2V617F mouse BM in the presence or absence of Cxcl12. We observed that Cxcl12 stimulation (100-200 ng/mL) significantly increased CFU-Mk colonies in the BM of Jak2V617F mice (Figure 5C). Next, we examined the effects of ectopic expression of Cxcl12 on megakaryopoiesis by lentiviral transduction in the BM of Jak2V617F mice. Vector or Cxcl12-transduced Jak2V617F BM cells were selected by using puromycin and plated for CFU-Mk colonies or cultured in megakaryocytic condition in the presence of stem cell factor and thrombopoietin. We observed that ectopic expression of Cxcl12 significantly increased the CFU-Mk colonies and enhanced the proliferation of megakaryocytic precursors in the BM of Jak2V617F mice (Figure 5D-E). We also performed CD41 and Cxcl12 immunostaining in the BM and spleen sections from the transplanted animals. Interestingly, we observed increased expression and co-localization of CD41 and Cxcl12 in the BM and spleen sections from Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (supplemental Figure 9A-B). We also observed that enforced expression of Fzd2 and Ifi27l2a, similar to that in Cxcl12, significantly increased the CFU-Mk colonies and enhanced megakaryocytic cell proliferation in the BM of Jak2V617F mice (Figure 5D-E). Together, these results suggest that increased expression of Hmga2 target genes Cxcl12, Fzd2, and Ifi27l2a may contribute to the enhanced megakaryopoiesis in Jak2VF/+-Hmga2 mice.
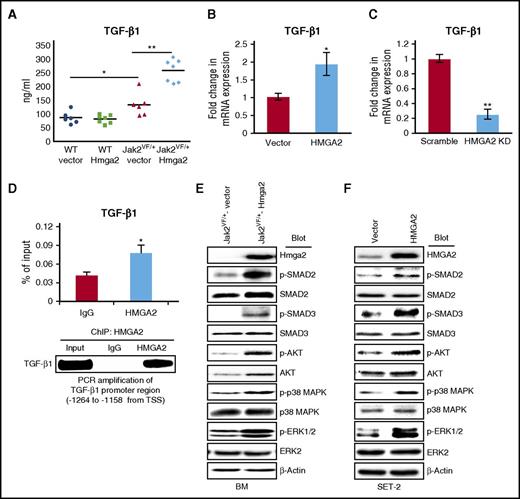
Expression of Hmga2 enhances activation of the TGF-β1 signaling pathway
TGF-β1 is thought to play a role in the pathogenesis of MF.39,40 Because expression of Hmga2 accelerated the development of MF in Jak2V617F mice, we assessed the levels of TGF-β1 in these mice by ELISA. We observed significantly increased levels of TGF-β1 in the serum of the Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 6A). We also performed CD41 and TGF-β1 immunostaining in BM and spleen sections from the transplanted mice. We observed increased expression and co-localization of CD41 and TGF-β1 in the BM and spleen sections from Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (supplemental Figure 10A-B). To further examine whether HMGA2 regulates the expression of TGF-β1, we overexpressed or knocked down HMGA2 in SET-2 cells and assessed the expression of TGF-β1 mRNA by RT-qPCR. We found that enforced expression of HMGA2 significantly increased the TGF-β1 mRNA in SET-2 cells (Figure 6B). Conversely, knockdown of HMGA2 significantly reduced the expression of TGF-β1 mRNA in SET-2 cells (Figure 6C). By using HMGA2 ChIP followed by RT-qPCR, we also found that HMGA2 binds to the promoter of the TGF-β1 gene in SET-2 cells (Figure 6D). Together, these results suggest that Hmga2 directly regulates the expression of TGF-β1 in megakaryocytic cells expressing Jak2V617F.
Expression of Hmga2 enhances activation of the TGF-β1 signaling pathway in Jak2V617Fmouse BM and human megakaryoblastic SET-2 cells. (A) Total levels of TGF-β1 in the serum of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT were assessed by ELISA (n = 6-7). (B) Overexpression of HMGA2 increases the TGF-β1 mRNA expression in JAK2V617F-positive megakaryoblastic SET-2 cells. (C) Lentiviral shRNA-mediated knockdown of HMGA2 decreases the TGF-β1 mRNA expression in SET-2 cells. The mRNA expression was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (D) HMGA2 ChIP followed by RT-qPCR showed binding of HMGA2 in the promoter of the TGF-β1 gene in SET-2 cells. Results from 3 independent experiments are presented as mean ± SEM in bar graphs. The RT-qPCR products were loaded onto 2% agarose gel. Representative picture from agarose gel is shown in the bottom panel. (E) Immunoblot analysis shows increased phosphorylation of SMAD2, SMAD3, AKT, p38 MAPK, and ERK1/2 in Jak2VF/+-Hmga2 BM compared with Jak2VF/+-vector BM. Total SMAD2, SMAD3, and AKT protein levels were also higher in Jak2VF/+-Hmga2 BM compared with Jak2VF/+-vector BM. However, total p38 MAPK and ERK1/2 levels were comparable. β-actin was used as a loading control. (F) Immunoblot analysis shows increased phosphorylation of SMAD2, SMAD3, AKT, p38 MAPK, and ERK1/2 in SET-2 cells overexpressing HMGA2 compared with vector-expressing SET-2 cells. β-actin was used as a loading control. *P < .05; **P < .005.
Expression of Hmga2 enhances activation of the TGF-β1 signaling pathway in Jak2V617Fmouse BM and human megakaryoblastic SET-2 cells. (A) Total levels of TGF-β1 in the serum of WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice at 32 weeks after BMT were assessed by ELISA (n = 6-7). (B) Overexpression of HMGA2 increases the TGF-β1 mRNA expression in JAK2V617F-positive megakaryoblastic SET-2 cells. (C) Lentiviral shRNA-mediated knockdown of HMGA2 decreases the TGF-β1 mRNA expression in SET-2 cells. The mRNA expression was assessed by RT-qPCR and normalized by GAPDH. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (D) HMGA2 ChIP followed by RT-qPCR showed binding of HMGA2 in the promoter of the TGF-β1 gene in SET-2 cells. Results from 3 independent experiments are presented as mean ± SEM in bar graphs. The RT-qPCR products were loaded onto 2% agarose gel. Representative picture from agarose gel is shown in the bottom panel. (E) Immunoblot analysis shows increased phosphorylation of SMAD2, SMAD3, AKT, p38 MAPK, and ERK1/2 in Jak2VF/+-Hmga2 BM compared with Jak2VF/+-vector BM. Total SMAD2, SMAD3, and AKT protein levels were also higher in Jak2VF/+-Hmga2 BM compared with Jak2VF/+-vector BM. However, total p38 MAPK and ERK1/2 levels were comparable. β-actin was used as a loading control. (F) Immunoblot analysis shows increased phosphorylation of SMAD2, SMAD3, AKT, p38 MAPK, and ERK1/2 in SET-2 cells overexpressing HMGA2 compared with vector-expressing SET-2 cells. β-actin was used as a loading control. *P < .05; **P < .005.
Next, we examined the effects of Hmga2 expression on TGF-β signaling. We observed increased phosphorylation and activation of SMAD2 and SMAD3 in the BM of Jak2VF/+-Hmga2 mice compared with Jak2VF/+-vector mice (Figure 6E). Similarly, we observed increased phosphorylation of SMAD2/3 upon overexpression of HMGA2 in SET-2 cells (Figure 6F). Conversely, knockdown of HMGA2 reduced the phosphorylation and activation of SMAD2/3 in SET-2 cells (supplemental Figure 11). Notably, knockdown of HMGA2 also reduced the total SMAD2/3 protein levels in SET-2 cells (supplemental Figure 11), suggesting that HMGA2 may regulate the expression of SMAD2/3. In addition to activation of the canonical TGF-β/SMAD pathway, TGF-β can also activate the p38 MAPK, ERK, and PI3K/AKT pathways.41 Indeed, we observed increased phosphorylation of AKT, p38 MAPK, and ERK in Jak2VF/+-Hmga2 mouse BM compared with Jak2VF/+-vector BM (Figure 6E). Enforced expression of HMGA2 also resulted in increased phosphorylation of AKT, p38 MAPK, and ERK in SET-2 cells (Figure 6F). Furthermore, knockdown of HMGA2 reduced the phosphorylation of these signaling molecules and pathways in SET-2 cells (supplemental Figure 11). Thus, expression of Hmga2 enhances activation of both canonical and noncanonical TGF-β1 signaling pathways.
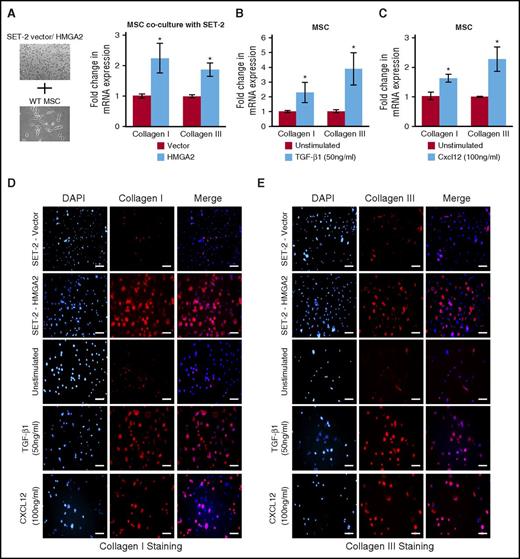
Expression of Hmga2 induces collagen deposition in the BM MSCs
It is thought that abnormal megakaryocytes produce high levels of cytokines and/or chemokines, leading to progressive BM fibrosis.40,42 Because ectopic expression of Hmga2 enhances megakaryopoiesis and accelerates the development of MF in Jak2V617F mice, we asked whether overexpression of Hmga2 in megakaryocytes produces the factors responsible for deposition of collagen in the BM MSCs. We established an ex vivo coculture method in which HMGA2 was overexpressed in the human JAK2V617F-positive megakaryoblastic cell line SET-2, and these cells were cocultured with MSCs derived from the WT mouse BM. We observed significantly increased collagen I and III mRNA expression in the MSCs after coculture with HMGA2-expressing SET-2 cells compared with vector-expressing SET-2 cells (Figure 7A). Immunofluorescence staining also revealed significant deposition of collagen I and III in the MSCs cocultured with HMGA2-expressing SET-2 cells (Figure 7D-E). Because TGF-β1 and Cxcl12 levels were significantly increased in Jak2VF/+-Hmga2 mice exhibiting MF, we also directly assessed the contribution of TGF-β1 and Cxcl12 on collagen deposition in MSCs. Stimulation with TGF-β1 or Cxcl12 significantly increased collagen I and III mRNA expression (Figure 7B-C) and deposition of collagen I and III in the BM MSCs (Figure 7D-E). These results strongly suggest that expression of Hmga2 induces collagen deposition in MSCs and promotes the development of MF by enhancing the TGF-β1 and Cxcl12 signaling pathways.
Expression of Hmga2 induces collagen deposition in the BM mesenchymal stromal cells. (A) A scheme for the coculture system is shown on the left. MSC culture was first established from the BM of WT mice. MSCs were then re-plated and cultured for 72 hours before SET-2 cells expressing vector or HMGA2 were placed on top of MSCs and cultured for another 72 hours. SET-2 cells were then removed from MSCs. RNA was isolated from the MSCs. RT-qPCR showed increased collagen I and III mRNA expression in MSCs cocultured with SET-2 cells overexpressing HMGA2. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. Stimulation with (B) TGF-β1 (50 ng/mL) or (C) Cxcl12 (100 ng/mL) showed increase in collagen I and III mRNA expression in MSCs compared with unstimulated MSCs. The mRNA expression was assessed by RT-qPCR and normalized by Gapdh. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (D-E) Immunofluorescence images showing increased expression of collagen I and collagen III in MSCs cocultured with SET-2 cells overexpressing HMGA2 compared with vector expressing SET-2 cells. Stimulation with TGF-β1 (50 ng/mL) or Cxcl12 (100 ng/mL) also increased collagen I and III expression in MSCs. Scale bars, 100 µm. *P < .05.
Expression of Hmga2 induces collagen deposition in the BM mesenchymal stromal cells. (A) A scheme for the coculture system is shown on the left. MSC culture was first established from the BM of WT mice. MSCs were then re-plated and cultured for 72 hours before SET-2 cells expressing vector or HMGA2 were placed on top of MSCs and cultured for another 72 hours. SET-2 cells were then removed from MSCs. RNA was isolated from the MSCs. RT-qPCR showed increased collagen I and III mRNA expression in MSCs cocultured with SET-2 cells overexpressing HMGA2. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. Stimulation with (B) TGF-β1 (50 ng/mL) or (C) Cxcl12 (100 ng/mL) showed increase in collagen I and III mRNA expression in MSCs compared with unstimulated MSCs. The mRNA expression was assessed by RT-qPCR and normalized by Gapdh. Data from 4 independent experiments are shown in bar graphs as mean ± SEM. (D-E) Immunofluorescence images showing increased expression of collagen I and collagen III in MSCs cocultured with SET-2 cells overexpressing HMGA2 compared with vector expressing SET-2 cells. Stimulation with TGF-β1 (50 ng/mL) or Cxcl12 (100 ng/mL) also increased collagen I and III expression in MSCs. Scale bars, 100 µm. *P < .05.
Discussion
Overexpression of HMGA2 has been frequently found in association with JAK2V617F mutation in MF26,27 but their contributions to the pathogenesis of MF remain unclear. In this study, we provide evidence for functional cooperation between Jak2V617F and expression of Hmga2 in the development of MF. We and other groups have already shown that expression of Jak2V617F in the mouse hematopoietic compartments induces a PV-like MPN.16-18 In this study, we show that forced expression of Hmga2 promotes megakaryopoiesis and accelerates the development of MF in mice expressing Jak2V617F. However, Hmga2 overexpression alone is not sufficient to give rise to MF.
Previous studies have suggested an oncogenic role for HMGA2 in various human tumors including breast, colon, pancreatic, and lung cancer.28,29 HMGA2 gene amplification and overexpression have been linked to pituitary adenomas.43 Chromosomal rearrangement and overexpression of the HMGA2 gene have also been found in patients with myeloid neoplasia.44 Increased expression of HMGA2 was observed in association with JAK2V617F in patients with MF.26,27 Overexpression of Hmga2 has been suggested to confer growth advantage to the hematopoietic progenitors in mice.45,46 In this study, we showed that ectopic expression of Hmga2 increases the LSK and megakaryocytic precursors but decreases the erythroid precursors in the BM of mice expressing Jak2V617F (Figure 2). We also showed that expression of Hmga2 increases CFU-Mk colonies and enhances proliferation of megakaryocytic precursors in the BM of Jak2V617F mice (Figure 3A-B). Thus, expression of Hmga2 promotes megakaryopoiesis in mice expressing Jak2V617F.
Recently, we and other groups showed that loss of Ezh2 causes increased expression of Hmga2 in hematopoietic progenitors of Ezh2-deleted Jak2V617F mice exhibiting MF.20-22 Loss-of-function mutations in EZH2 have been found in association with JAK2V617F mutation in MF.47 Overexpression of HMGA2 was also reported in MF patients who had a chromosomal translocation involving the HMGA2 gene.24 Chromosome 12 abnormalities have been found to be associated with Philadelphia chromosome–negative MPNs.48 It has also been suggested that patients with PV who have a chromosome 12 abnormality are more likely to progress to MF.48 HMGA2 is a possible target gene in chromosome 12,48 and the abnormalities in chromosome 12 may lead to increased expression of HMGA2. Other mechanisms involving truncation of the 3′ untranslated region of the HMGA2 gene, increased expression of Lin28b or decreased expression of let-7b microRNAs may also cause abnormal expression of HMGA2.28,45 Nevertheless, increased expression of HMGA2 induces a phenotypic switch from a PV to an MF phenotype in Jak2V617F mice.
We have identified and validated several Hmga2 target genes that are upregulated in Jak2VF/+-Hmga2 LSK cells (Figure 4B,D). Moreover, using Hmga2 ChIP, we show that HMGA2 directly binds to the promoter region of CXCL12, FZD2, IFI27, GATA3, MEIS1, and TGF-βR2 in SET-2 cells (Figure 5A). We also found that expression of these genes was upregulated in SET-2 cells upon HMGA2 overexpression (Figure 4E). Furthermore, knockdown of HMGA2 significantly reduced the expression of these genes in SET-2 cells (Figure 4F). Therefore, Cxcl12, Fzd2, Col1a1, Col3a1, Ifi27l2a, Gata3, Dok4, Meis1, and Tgf-βR2 are bona fide targets of Hmga2.
CXCL12 is a chemokine that has been shown to play a role in extramedullary hematopoiesis in the spleens of MF patients.49 It has also been reported that CXCL12 enhances the development of megakaryocytic progenitors.38 We observed increased expression of Cxcl12 in Jak2VF/+-Hmga2 mice (Figures 4D and 5B). Ectopic expression or stimulation of Cxcl12 enhanced CFU-Mk formation and megakaryocytic proliferation in Jak2V617F BM (Figure 5C-E). Furthermore, Cxcl12 stimulation induced collagen deposition in the BM MSCs (Figure 7D-E). Therefore, increased megakaryopoiesis and accelerated development of MF in Jak2VF/+-Hmga2 mice could, in part, be the result of upregulation of Cxcl12.
We also observed increased expression of Wnt receptor Fzd2 and interferon-inducible gene Ifi27l2a in Jak2VF/+-Hmga2 mouse LSK cells (Figure 4D). Fzd2 was found to be overexpressed in mesenchymal-type metastatic lung and liver cancers and was shown to play an important role in the epithelial-mesenchymal transition.50 It has been suggested that a similar epithelial-mesenchymal transition process is activated during the development of fibrosis.51 Moreover, overexpression of Fzd2 was linked to idiopathic pulmonary fibrosis.52 IFI27 (homolog of mouse Ifi27l2a) was found to be highly expressed in patients with MF.53 We show that overexpression of Fzd2 or Ifi27l2a significantly increased the CFU-Mk colonies and enhanced megakaryocytic proliferation in the BM of Jak2V617F mice (Figure 5D-E). Thus, upregulation of Fzd2 and Ifi27l2a may contribute to enhanced megakaryopoiesis in Jak2VF/+-Hmga2 mice.
Previous reports have suggested an important role for TGF-β in fibrosis40 and have shown increased levels of TGF-β1 in patients with MF.39 We also observed increased expression of Tgf-βR2 and elevated serum levels of TGF-β1 in Jak2VF/+-Hmga2 mice exhibiting MF (Figures 4D and 6A). In addition, overexpression of HMGA2 resulted in increased expression of TGF-βR2 and TGF-β1 in human SET-2 cells (Figures 4E and 6B). Interestingly, TGF-βR2 expression and TGF-β signaling were found to be significantly increased upon overexpression of HMGA2 in breast cancer cells.30 We show that HMGA2 binds to the promoter regions of TGF-βR2 and TGF-β1 (Figures 5A and 6D). Furthermore, knockdown of HMGA2 significantly reduced expression of TGF-βR2 and TGF-β1 in SET-2 cells (Figures 4F and 6C), suggesting that HMGA2 directly regulates the expression of TGF-βR2 and TGF-β1. We observed increased phosphorylation of SMAD2/3 in the BM of Jak2VF/+-Hmga2 mice as well as in SET-2 cells overexpressing HMGA2 (Figure 6E-F). Moreover, knockdown of HMGA2 significantly inhibited the phosphorylation of SMAD2/3 in SET-2 cells (supplemental Figure 11). In addition to activation of the canonical TGF-β1/SMAD pathway, expression of Hmga2 enhanced the activation of AKT, p38 MAPK, and ERK pathways in the BM of Jak2VF/+-Hmga2 mice as well as in SET-2 cells (Figure 6E-F). It has been reported that TGF-β stimulation can also lead to activation of AKT, p38 MAPK, and ERK pathways.41 Thus, expression of HMGA2 enhances activation of both canonical and noncanonical TGF-β pathways. We also show that TGF-β1 stimulation increases the deposition of collagen I and III in the BM MSCs (Figure 7D-E). Therefore, HMGA2 may contribute to development of MF by activation of the TGF-β1 signaling.
In conclusion, we demonstrate that expression of Hmga2 synergizes with Jak2V617F mutation in the development of MF. We also have provided mechanistic insights into how Hmga2 promotes the development of MF in mice expressing Jak2V617F. We show that expression of Hmga2 enhances the activation of Cxcl12 and TGF-β1 pathways, which are directly involved in the deposition of collagen in the BM MSCs. We also found that Cxcl12, Fzd2, and Ifi27l2a are direct targets of Hmga2, and expression of these genes enhances megakaryopoiesis in Jak2V617F BM. Future studies will determine whether inhibition of Hmga2, Cxcl12, or TGF-β1 in combination with JAK2 inhibition could be useful for treatment of MF.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Frank Middleton, PhD (State University of New York [SUNY] Upstate Medical University), for help with RNA-sequencing data analysis and Stephen Graziano, MD (SUNY Upstate Medical University), for providing samples from patients with myeloproliferative neoplasms; Dipmoy Nath and Yue Yang from Mohi Laboratory for helpful discussion on this project; and Lisa Phelps and Karen Gentile for assistance with fluorescence-activated cell sorting and RNA-sequencing.
This work was supported in part by grants from Worldwide Cancer Research (15-1381) and the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL095685) (G.M.). G.M. is a Leukemia & Lymphoma Society scholar.
Authorship
Contribution: A.D. performed research, analyzed data, and wrote the manuscript; R.E.H. conducted histopathologic analysis and revised the manuscript; and G.M. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Golam Mohi, Department of Pharmacology, State University of New York (SUNY) Upstate Medical University, 750 East Adams St, Syracuse, NY 13210; e-mail: mohim@upstate.edu.

![Figure 3. Hmga2 promotes megakaryocytic differentiation and enhances megakaryocytic cell proliferation. (A) BM cells (2 × 104) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated in methylcellulose medium supplemented with cytokines. BFU-E and CFU-GM colonies were scored 7 days after plating. (B) CFU-E colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 6) were plated in methylcellulose medium in the presence of erythropoietin (3 U/mL) or absence of erythropoietin. CFU-E colonies were scored after 2 days. (C) CFU-Mk colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated into collagen-based MegaCult medium supplemented with interleukin-3 (IL-3), IL-6, IL-11, and thrombopoietin (TPO). Megakaryocytic (CFU-Mk) colonies were assessed after culturing for 8 days. (D) Expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM. BM cells from the transplanted animals were first cultured in a megakaryocytic culture condition (StemPro-34 medium containing stem cell factor [SCF] and TPO) for 4 days. Flow cytometric analysis confirmed ∼99% cells were CD41+ after 4 days of culture. Then equal numbers of megakaryocytic cells were plated, and cell proliferation was assessed by viable cell counts over 6 days. (E) Ex vivo expression of Hmga2 significantly increased CFU-Mk colonies in the BM of both WT and Jak2V617F mice compared with vector expression (n = 4-6). (F) Ex vivo expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM compared with vector expression. First megakaryocytic culture was established and then cell proliferation was assessed by viable cell counts over 6 days. All data are shown as mean ± SEM. Student t test was used for comparison between 2 groups of mice. *P < .05; **P < .005; ***P < .0005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/7/10.1182_blood-2016-12-757344/4/m_blood757344f3.jpeg?Expires=1765000871&Signature=yRekETLbieQQtJW6zN5Tt7sh-820htyfGe0e34ecEcuG94nv5BjTAs2539GOK97wf8QkAPc2PyQjE6KOl2qULuzvpyFVz6xwLL2XmK~kVYW-J4LstWrB3HQx0CZjtexn6FvtD3wLVseV0s1OL4lJdJlBWZDnUbEwY663Jc7YwYQP6L8ZuCoY-b7oX22Q8keqfDBbKn5PtiRnPmpeFmHkuhOCabAhVO883R2kA0OmBtODd0tup~uxvVNnMqAHECq53ZcmGopANwXIvwlqVmyImEtGOnQeEq7DSTPrS8T2AnfZhOjj5Vx~MS9U8ihW4OX86A8ti0iyRWLmpuMfOYs-IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 3. Hmga2 promotes megakaryocytic differentiation and enhances megakaryocytic cell proliferation. (A) BM cells (2 × 104) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated in methylcellulose medium supplemented with cytokines. BFU-E and CFU-GM colonies were scored 7 days after plating. (B) CFU-E colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 6) were plated in methylcellulose medium in the presence of erythropoietin (3 U/mL) or absence of erythropoietin. CFU-E colonies were scored after 2 days. (C) CFU-Mk colonies. BM cells (1 × 105) from WT-vector, WT-Hmga2, Jak2VF/+-vector, and Jak2VF/+-Hmga2 mice (n = 5-7) were plated into collagen-based MegaCult medium supplemented with interleukin-3 (IL-3), IL-6, IL-11, and thrombopoietin (TPO). Megakaryocytic (CFU-Mk) colonies were assessed after culturing for 8 days. (D) Expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM. BM cells from the transplanted animals were first cultured in a megakaryocytic culture condition (StemPro-34 medium containing stem cell factor [SCF] and TPO) for 4 days. Flow cytometric analysis confirmed ∼99% cells were CD41+ after 4 days of culture. Then equal numbers of megakaryocytic cells were plated, and cell proliferation was assessed by viable cell counts over 6 days. (E) Ex vivo expression of Hmga2 significantly increased CFU-Mk colonies in the BM of both WT and Jak2V617F mice compared with vector expression (n = 4-6). (F) Ex vivo expression of Hmga2 significantly increased megakaryocytic cell proliferation in both WT and Jak2V617F mouse BM compared with vector expression. First megakaryocytic culture was established and then cell proliferation was assessed by viable cell counts over 6 days. All data are shown as mean ± SEM. Student t test was used for comparison between 2 groups of mice. *P < .05; **P < .005; ***P < .0005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/7/10.1182_blood-2016-12-757344/4/m_blood757344f3.jpeg?Expires=1765223763&Signature=mo4TtT~hVYsM5hx5kIs3IcSPoAzVnlSivMvbAEtShfeli6vJhGI9kiABFT2bF1Vyx0JV7vCTI3zZ-0Xl3NM9xYI4tAo6VeWoJ4OENXMC0iS2UZ3kDwIj-ax6AJzT2Vr57euWc56pUTGiS-R3L8Dn0jV1HYZA8ocb3PFMJ005DaFFgRCgsE-3G68JbbeLgpJNRwCqC5ofD7pIcs5Le104La6Qm9npb8G6lMzJ9kxp6b2TdEeffdk5f59Iw7xM1S9xYoXYfRcvtIj9BcokHRGxSaMNuiVxDiXEPEklvkozE9rJA-0M5~-5VU57bmy6CDAmCQ0d8IFqXjQL-L3bCd4QNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)