Key Points
Germ line biallelic loss-of-function THPO mutations cause BMF.
Marrow failure due to THPO mutations is characterized by poor graft function after transplantation but responds to THPO receptor agonists.
Abstract
We report 5 individuals in 3 unrelated families with severe thrombocytopenia progressing to trilineage bone marrow failure (BMF). Four of the children received hematopoietic stem cell transplants and all showed poor graft function with persistent severe cytopenias even after repeated transplants with different donors. Exome and targeted sequencing identified mutations in the gene encoding thrombopoietin (THPO): THPO R99W, homozygous in affected children in 2 families, and THPO R157X, homozygous in the affected child in the third family. Both mutations result in a lack of THPO in the patients’ serum. For the 2 surviving patients, improvement in trilineage hematopoiesis was achieved following treatment with a THPO receptor agonist. These studies demonstrate that biallelic loss-of-function mutations in THPO cause BMF, which is unresponsive to transplant due to a hematopoietic cell-extrinsic mechanism. These studies provide further support for the critical role of the MPL-THPO pathway in hematopoiesis and highlight the importance of accurate genetic diagnosis to inform treatment decisions for BMF.
Introduction
Bone marrow (BM) failure (BMF) is a potentially life-threatening inherited or acquired disorder characterized by low blood counts and an empty marrow. Mutations in key intracellular pathways are responsible for the development of inherited BMF syndromes.1,2 Replacement of the impaired hematopoietic system with a hematopoietic stem cell transplant constitutes the standard curative treatment approach for BMF disorders.
Here, we report 5 children from 3 families presenting with early-onset thrombocytopenia evolving into BMF. Four of the children received hematopoietic stem cell transplants and all showed poor graft function even after repeated transplants with different donors. Genetic investigation was pursued and identified a hematopoietic cell-extrinsic factor deficiency causing BMF.
Methods
Patient samples
This study was conducted in accordance with a protocol approved by the institutional review boards of the University of Washington, Seattle Children’s Hospital, Boston Children’s Hospital, Rabin Medical Center (Israel), and Ulm University (Germany) and in accordance with the Declaration of Helsinki. Informed consent was obtained from all study subjects.
Genetic analysis
Targeted gene capture panel sequencing and analysis were performed as previously described.3 Whole-exome capture was performed with the Agilent SureSelect V5 enrichment capture kit. The enriched library was then sequenced on an Illumina HiSeq 4000 (100-bp end). Filtering for single-nucleotide variants and small insertions/deletions excluded sequences with a read depth <5 and a quality score <20. We included only homozygous variants (variant frequency ≥0.85) on exons ± 2 bp, with nonexistent dbSNP annotation and no entry or a frequency of <0.0001 in the ExAC database of 60 706 exomes. Only single-nucleotide variants with a Combined Annotation Dependent Depletion (CADD) score >10 were considered.
Cell culture and transfections
HEK-293T cells (CRL-1573; ATCC) and Baf3-Mpl cells (kind gift of Kenneth Kaushansky, Stony Brook University) were cultured as described (supplemental Methods, available on the Blood Web site). Transfections were performed using TransIT-LT1 (Mirus Bio).
Plasmids
The thrombopoietin (THPO) wild-type (WT) and R99W (295C>T) complementary DNAs (cDNAs) were purchased from Genscript and cloned into the pcDNA3.1/myc-His expression vector (Genscript).
THPO enzyme-linked immunosorbent assay quantification
THPO levels from patient serum and secreted THPO from cell culture supernatant, respectively, were quantitated using the human thrombopoietin quantikine enzyme-linked immunosorbent assay kit (R&D Systems) in triplicate (coefficient of variation <10%).
Quantitative polymerase chain reaction
Quantitative polymerase chain reaction for THPO gene expression was performed using iTaq universal SYBR Green (Bio-Rad). Primer sequences are available in the supplemental Methods.
Proliferation/survival assay
BaF3-Mpl cells were plated, in triplicate, in complete media ± 20 ng/mL murine interleukin-3 or 1-5 ng/mL THPO (no murine interleukin-3). Cells were counted in triplicate for each condition for 3 independent experiments using Trypan Blue (Life Technologies). Cell viability was measured by Alamar Blue (Bio-Rad).
Cell culture and transfections
HEK-293T cells (CRL-1573; ATCC) were cultured with Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and penicillin/streptomycin. BaF3-Mpl cells (kind gift of Kenneth Kaushansky, Stony Brook University) were cultured in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin/streptomycin, and 20 ng/mL murine interleukin-3 (Peprotech).
Structural modeling
Results
Clinical features
Five children from 3 families (Figure 1A; supplemental Table 2) presented with thrombocytopenia which progressed to severe pancytopenia with marrow hypocellularity. No dysmorphic features were noted on examination. Evaluation for known causes of inherited or acquired thrombocytopenia or BMF failed to reveal a diagnosis (see supplemental Methods for details of clinical presentation). Four of the children received hematopoietic stem cell transplants and all showed poor graft function even after repeated transplants with different donors.
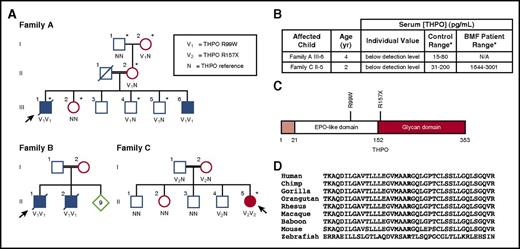
Family pedigrees. (A) The arrow indicates the proband. Family A and Family B: Asterisks indicate individuals evaluated with targeted gene capture panel/high-throughput sequencing. The variant allele (295C>T; R99W) is indicated with V1, and reference alleles are indicated with an N. Family C: Asterisk indicates individual evaluated with whole-exome sequencing. The variant allele (469C>T; R157X) is indicated with V2, and reference alleles are indicated with an N. (B) Serum THPO levels in affected individuals. Serum THPO concentrations (picograms per milliliter) are given for the affected child along with the testing laboratory’s control ranges. (C) THPO protein domains. The signal peptide (amino acid residues 1-21) is shaded in light red. The positions of the THPO mutations are indicated on the structure. (D) THPO amino acid conservation across vertebrate species. Bold font indicates the arginine residue at position 99 of the human THPO sequence. Twenty–amino acid residues flanking position 99 are shown (NCBI RefSeq). EPO, erythropoietin; N/A, not applicable.
Family pedigrees. (A) The arrow indicates the proband. Family A and Family B: Asterisks indicate individuals evaluated with targeted gene capture panel/high-throughput sequencing. The variant allele (295C>T; R99W) is indicated with V1, and reference alleles are indicated with an N. Family C: Asterisk indicates individual evaluated with whole-exome sequencing. The variant allele (469C>T; R157X) is indicated with V2, and reference alleles are indicated with an N. (B) Serum THPO levels in affected individuals. Serum THPO concentrations (picograms per milliliter) are given for the affected child along with the testing laboratory’s control ranges. (C) THPO protein domains. The signal peptide (amino acid residues 1-21) is shaded in light red. The positions of the THPO mutations are indicated on the structure. (D) THPO amino acid conservation across vertebrate species. Bold font indicates the arginine residue at position 99 of the human THPO sequence. Twenty–amino acid residues flanking position 99 are shown (NCBI RefSeq). EPO, erythropoietin; N/A, not applicable.
Family A.
A Bedouin boy with consanguineous parents presented at the age of 9 months with a platelet count of 40 × 109/L (family A III-1) (Figure 1A; supplemental Table 2). The remainder of his blood counts and his physical examination were unremarkable. Macrocytosis (mean corpuscular volume [MCV] of 100 fL) was first noticed at 3 years of age followed 1 year later by pancytopenia with a white blood cell count (WBC) of 2.45 × 109/L, an absolute neutrophil count (ANC) of 0.5 × 109/L, hemoglobin (Hb) of 9.9 g/dL, MCV of 98 fL, and a platelet (PLT) count of 9 × 109/L. The BM appeared hypocellular (5%-10% cellularity) with an absence of megakaryocytes. He soon became transfusion-dependent and underwent BM transplantation (BMT) from an HLA-A allele mismatched unrelated female donor. Due to failure of engraftment with persistence of the Y chromosome in 100% of BM cells, a second transplant using granulocyte colony-stimulating factor–mobilized peripheral blood–derived stem cells from the same donor was performed. He remained profoundly pancytopenic and a BM aspirate on day +70 revealed empty spicules but 100% XX cells by fluorescence in situ hybridization. Immune suppression was discontinued without improvement in the blood counts. Four months after the second transplant, severe gastrointestinal and pulmonary hemorrhage led to his demise.
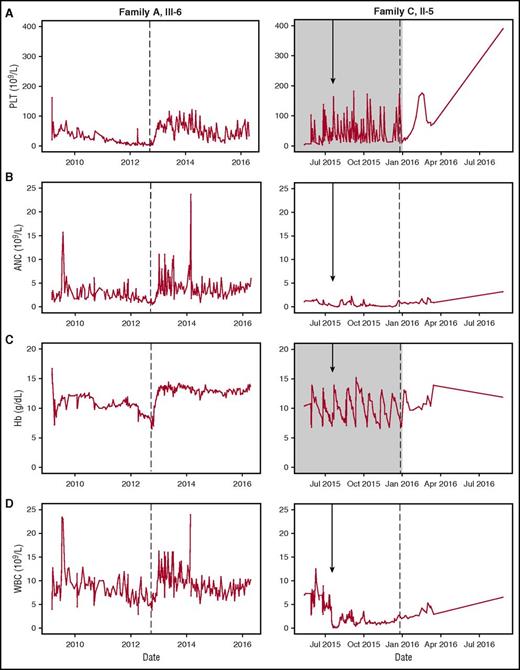
The younger brother (family A III-6) (Figure 1A; supplemental Table 2) of patient III-1 in family A was thrombocytopenic at birth (PLT, 21 × 109/L). Around 3 years of age, macrocytosis with pancytopenia developed (Hb, 6.5 g/dL; WBC, 4.5 × 109/L; ANC, 0.51 × 109/L; PLT, 3 × 109/L). Empiric therapy with romiplostim (5 µg/kg body weight per week) was followed by improved trilineage hematopoiesis with increased PLT, WBC, ANC, and Hb levels (Figure 2). THPO levels, drawn after he was no longer requiring platelet transfusions, were undetectable.
Clinical response to romiplostim. Trilineage response to romiplostim therapy. (A) PLT, (B) ANC, (C) Hb, and (D) WBC counts are shown for affected individuals: Family A, III-6 and Family C, II-5. Arrow indicates date of BMT. Gray shading indicates timeframe of platelet and erythrocyte transfusion dependence. Dotted line indicates initiation of romiplostim therapy. Note difference in time scale (x-axis) between left and right panels.
Clinical response to romiplostim. Trilineage response to romiplostim therapy. (A) PLT, (B) ANC, (C) Hb, and (D) WBC counts are shown for affected individuals: Family A, III-6 and Family C, II-5. Arrow indicates date of BMT. Gray shading indicates timeframe of platelet and erythrocyte transfusion dependence. Dotted line indicates initiation of romiplostim therapy. Note difference in time scale (x-axis) between left and right panels.
Family B.
A 2.5-year-old boy (family B II-1) (Figure 1A; supplemental Table 2) from a second family was referred to our clinic on April 1996 for evaluation of cutaneous bleeding. He was born after normal pregnancy and delivery to Arabic parents who were first-degree cousins. No other abnormalities were noted on physical examination. His blood count revealed a platelet count of 18 × 109/L, Hb of 10.4 g/dL, and MCV of 92.3 fL, which progressed to pancytopenia and an aplastic marrow. As there was no HLA-matched related donor, 2 courses of antithymocyte globulin and cyclosporine A were administered without improvement. Therefore, at age 4.5 years, he underwent an unrelated HLA-matched (low-resolution HLA-A and HLA-B, high-resolution HLA-DRB1) cord blood transplant. The patient remained pancytopenic with no hematopoietic recovery for 60 days. There was no evidence of donor cell engraftment by restriction fragment length polymorphism analysis of the patient’s blood. A subsequent transplant was performed with the patient’s haploidentical father as the donor, but the patient remained pancytopenic. The patient died with multiorgan system failure 3 months after the first stem cell graft.
During the search for an HLA-matched family donor, the patient’s 2-year-old brother (family B II-2) was found to be thrombocytopenic (PLT, 22 × 109/L) with mild neutropenia (ANC, 1.4 × 109/L), which progressed to pancytopenia with a hypoplastic marrow. He had no physical anomalies on examination. At the age of 5.5 years, the patient was transplanted with haploidentical paternal peripheral blood–derived stem cells. Two months later, in the absence of any sign of hematopoietic engraftment, he received a second transplant with maternal haploidentical CD34+ enriched stem cells, but a disseminated infection with Pseudallescheria boydii led to multiorgan failure and death after 3 weeks.
Family C.
In a third family, the patient (family C II-5) (Figure 1A; supplemental Table 2) was the fifth child of a consanguineous family of Saudi Arabian origin. Following spontaneous vaginal delivery, the girl presented with isolated thrombocytopenia (16 × 109/L) unresponsive to IV immunoglobulin or steroids. Maternal alloimmune thrombocytopenia was ruled out. She required platelet transfusions 1 to 2 times per week but had no severe bleeding complications. At the age of 3 months, she developed moderate neutropenia with an ANC of 0.5 × 109/L to 0.9 × 109/L but no recurrent or severe infections. At the age of 10 months, a BM biopsy revealed a hypocellular marrow without clonal cytogenetic aberrations. BMT was performed at the age of 19 months with a graft from an HLA-identical brother who had normal blood counts. He remained pancytopenic and transfusion-dependent for platelets and red cells. The BM biopsy performed on day +88 post-BMT was hypocellular. Persistent complete donor chimerism was documented in her BM and peripheral blood. Serum thrombopoietin levels were undetectable, drawn 6 days after the last platelet transplant when the platelet count was 21 × 109/L. Serum thrombopoietin levels of the donor were normal. Treatment with the thrombopoietin receptor (MPL) agonist romiplostim was initiated with a subsequent rise in platelet counts to >100 × 109/L; reticulocytes rose above 20% and ANC rose above 1 × 109/L (Figure 2). BM biopsy now showed regeneration trilineage hematopoiesis with only mild morphologic signs of dysplasia. The last follow-up was 13 months after BMT with romiplostim being administered every 2 weeks with a normal full blood count.
Gene discovery
Targeted capture followed by next-generation sequencing of candidate genes (MarrowSeq)3 was performed on family A (supplemental Table 1; Figure 1A). Sequencing revealed 1 homozygous germ line variant in THPO cosegregating with the phenotype in an autosomal-recessive pattern. The THPO variant 295C>T (cDNA sequence, NM_000460.36 ) resulted in THPO R99W (NP_000451.1), at chr3:184 091 304 (hg19). THPO R99W was not present in public databases including dbSNP141, the Exome Variant Server, the 1000 Genomes Project, or the ExAC database of 60 706 exomes, and has not been seen in our in-house databases (1419 individuals). In silico analyses predict THPO R99W to be damaging (PolyPhen-2, 1.00; SIFT, 0.001). Arg99 lies in the erythropoietin-like receptor-binding domain (Figure 1C) and is highly conserved in vertebrate species (Figure 1D). Sanger sequencing of the entire THPO gene in family B identified the same homozygous THPO R99W variant in affected individuals. Whole-exome sequencing of the proband, II-5, in family C identified a homozygous nonsense mutation THPO 469C>T (NM_000460.3; chr3:184 090 894 [hg19], R157X [NP_000451.1]). THPO R157X cosegregated with the marrow failure phenotype and was also not present in the public databases listed earlier.
Functional characterization
The THPO gene encodes a 353-aa protein including the 21-aa signal peptide. To test whether THPO R99W affects THPO function, normal and R99W THPO were obtained in the supernatant of HEK-293T cells transfected with the corresponding expression vectors. Both normal and R99W THPO supported MPL-dependent cell survival and proliferation (supplemental Figure 2). Coupled with the absence of THPO in serum of patients homozygous for THPO R99W (Figure 1B), these data suggest that THPO R99W severely reduces THPO levels without affecting THPO function. R157X introduces a stop codon in the middle of the protein, resulting in the absence of the C-terminal glycan domain. Previously published studies have shown that THPO C-terminal deletion of the glycan domain markedly impaired THPO production through reduced THPO secretion.7-9
Discussion
We identified 5 individuals in 3 families with early-onset thrombocytopenia evolving into hypoplastic BMF unresponsive to transplant and caused by homozygous THPO mutations cosegregating with clinical findings in an autosomal-recessive fashion. BMT of 3 patients resulted in engraftment failure or persistent BMF despite donor cell engraftment, consistent with the cause of the marrow failure being extrinsic to hematopoietic cells. Although serum THPO levels are typically elevated with BMF, THPO R99W and R157X resulted in low or absent endogenous THPO levels. THPO is produced primarily in the liver, kidney, BM stroma, spleen, and nervous system,10-13 so transplantation of hematopoietic cells failed to cure the BMF. The combination of absent endogenous THPO and discovery of these THPO mutations led us to treat the 2 surviving patients with romiplostim, a thrombopoietin receptor agonist, resulting in trilineage improvement in blood counts. The long-term effects of romiplostim therapy remain to be determined for this patient population. Future studies are needed to address whether romiplostim treatment is associated with the development of clonal hematopoiesis in patients with THPO deficiency where the goal is to replace a missing factor rather than to deliver a supraphysiologic stimulus. Because the liver is a major source of THPO production, liver transplant might offer a potential alternative therapy for this disorder.
A previous study reported homozygosity for THPO R38C (NP_000451.1) in a child with hypoplastic BMF and in a sibling with clinically asymptomatic cytopenias.14 THPO R38C did not affect endogenous THPO levels but impaired the ability of THPO to support the growth of a THPO-dependent cell line. It was hypothesized that THPO R38C might affect the receptor-binding domain of THPO. Another report described 3 unrelated patients with thrombocytopenia and de novo 3q26.33-3q27.2 microdeletions that encompassed THPO.15 None of these prior reports comment on therapies for these patients.
The THPO-MPL pathway is important early in hematopoietic development and is critical for hematopoietic stem cell function and trilineage hematopoiesis. The interaction of thrombopoietin with its receptor, MPL, is essential for production and function of megakaryocytes, as well as for the maintenance of hematopoietic stem cells.16 Heterozygous gain-of-function mutations in THPO result in a myeloproliferative syndrome characterized by thrombocythemia,17-20 whereas autosomal-recessive loss-of-function mutations in MPL, which is expressed on the surface of hematopoietic stem cells, result in congenital amegakaryocytic thrombocytopenia (CAMT).21-23 Patients with CAMT typically present with thrombocytopenia at birth and later develop trilineage BMF.24 Knockout of either mpl or thpo in murine models results in reduced trilineage hematopoiesis.25-27
Additional studies of the clinical features and outcomes of patients with THPO mutations are needed. Despite similarities in clinical presentation, BMF due to mutations in THPO vs MPL differs dramatically in their treatment.21 Although hematopoietic stem cell transplantation is curative for CAMT, patients with THPO mutations do not benefit from hematopoietic stem cell transplantation even with full donor engraftment. This difference is analogous to the differences in the hematopoietic stem cell-intrinsic vs cell-extrinsic defects associated with mutations in another hematopoietic cytokine/receptor pair, stem cell factor and c-kit.28-31 THPO levels provide an important clue to the underlying diagnosis. This report implicates the importance of accurate diagnosis for optimal management of patients with inherited BMF and specifically points to the importance of investigation of THPO mutations to guide treatment of inherited BMF syndromes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and their families for participating in this research study. The authors thank K. Kaushansky (Stony Brook University) for generously sharing the Baf3-Mpl cells.
This work was supported by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases grants R24DK099808 (A. Shimamura and M.-C.K.) and F30DK103462 (A. Seo); by an Israel Cancer Association grant (H.T.); by the Ghiglione Aplastic Anemia Fund and Julian’s Dinosaur Guild from Seattle Children’s Hospital (A. Shimamura); by the NIH National Institute of General Medical Sciences Medical Scientist Training Program Training grant T32GM007266 (A. Seo); by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development Training grant T32HD007183 (A. Seo); and by an ARCS Foundation Fellowship (A. Seo). K.S. was supported by the Center for Personalized Immunology (supported by National Health and Medical Research Council of Australia), The Australian National University, Canberra, Australia.
Authorship
Contribution: A. Seo, M.S., A. Schulz, M.-C.K., H.T., and A. Shimamura conceived and designed the experiments; A. Seo, M.S.-B., T.W., M.L., K.S., C.S. and O.D. performed the experiments; A. Seo, T.W., S.G., M.K.L., M.S., M.H., A. Sendamarai, A. Schulz, U.P., M.L., K.S., M.-C.K., C.S., A.E.K., and A. Shimamura analyzed the data; M.B.-H., J.S., J.K., M.S., M.H., H.C., K.-M.D., and A. Schulz identified study subjects and collected clinical data and samples; and A. Seo, M.B.-H., M.S., M.S.-B., A. Schulz, H.T., and A. Shimamura wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akiko Shimamura, 300 Longwood Ave, Karp 8210, Boston, MA 02115; e-mail: akiko.shimamura@childrens.harvard.edu.
References
Author notes
A. Seo, M.B.-H., and M.S. contributed equally to this work.
A. Schulz, H.T., and A. Shimamura contributed equally to this work.