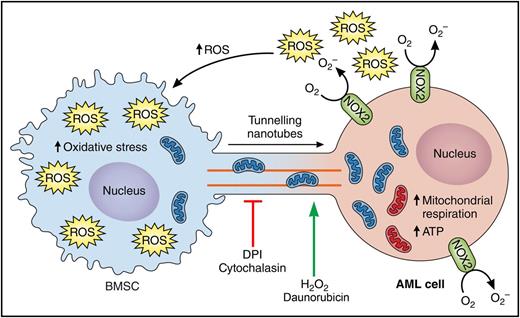
Model for NOX2-driven mitochondrial transfer from BMSCs to AML cells. Schematic representing proposed mechanism by which AML cells generates reactive oxygen species (ROS) and creates hypoxic conditions in the bone marrow that are necessary for the mitochondrial transfer from AML cells. NOX2 expressed by AML cells generates high levels of superoxide, which increases the oxidative stress in the BMSCs and stimulates the formation of TNTs from the membrane of AML cells to the BMSCs. The increased oxidative state in the BMSC triggers the mitochondrial transfer from BMSCs to AML cells, which will in turn increase mitochondria respiration and adenosine triphosphate (ATP) generations in the AML cells. Cytochalasin (inhibitor of actin polymerization) and diphenyleneiodonium (DPI, an inhibitor of NOX2) block mitochondrial transfer. Hydrogen peroxide (H2O2) and the chemotherapy agent daunorubicin increase mitochondrial transfer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model for NOX2-driven mitochondrial transfer from BMSCs to AML cells. Schematic representing proposed mechanism by which AML cells generates reactive oxygen species (ROS) and creates hypoxic conditions in the bone marrow that are necessary for the mitochondrial transfer from AML cells. NOX2 expressed by AML cells generates high levels of superoxide, which increases the oxidative stress in the BMSCs and stimulates the formation of TNTs from the membrane of AML cells to the BMSCs. The increased oxidative state in the BMSC triggers the mitochondrial transfer from BMSCs to AML cells, which will in turn increase mitochondria respiration and adenosine triphosphate (ATP) generations in the AML cells. Cytochalasin (inhibitor of actin polymerization) and diphenyleneiodonium (DPI, an inhibitor of NOX2) block mitochondrial transfer. Hydrogen peroxide (H2O2) and the chemotherapy agent daunorubicin increase mitochondrial transfer. Professional illustration by Patrick Lane, ScEYEnce Studios.
The treatment of AML, the most common type of leukemia in adults, has not improved over the past several decades, with outcomes remaining poor and most patients dying of relapse.2,-4 The bone marrow microenvironment, which consists of different noncancerous, nonhematological cell types labeled as BMSCs, plays a vital role in the development and progression of AML and contributes to chemotherapy resistance.5 This suggests that understanding the metabolic cross-talk between AML cells and BMSC could give rise to more effective therapeutic approaches directed not only at the malignant cells but also at microenvironment in which they live and thrive.
AML cells have an increased mitochondrial mass compared with their normal counterparts6 and display more robust mitochondrial activity and higher oxygen consumption, consistent with the finding that AML cells are mainly dependent on oxidative phosphorylation for survival.7 This introduces the important question of how these extra mitochondria arise: are they actively generated within the AML cells or are they taken up from neighboring cells, as suggested by earlier studies?8 This is a fascinating and timely topic because mitochondrial transfer from bone marrow cells to AML cells was recently documented as a mechanism to rescue respiration and cell survival after exposure to chemotherapy.9 However, the molecular mechanisms facilitating mitochondrial transfer and the therapeutic relevance of blocking this process remain largely unknown.
Marlein et al present additional evidence in support of the previously documented mitochondrial transfer from bone marrow cells to AML cells9 and take this concept 1 step further by providing mechanistic evidence for how this occurs. The authors begin by assessing mitochondrial transfer between patient-derived AML cells and BMSC. They demonstrate that, unlike their normal counterparts, AML cells uptake mitochondria from the stromal cells under coculture conditions in vitro. However, this is not just an in vitro phenomenon; the authors further confirm the acquisition of murine mitochondrial DNA but not murine genomic DNA in patient-derived AML cells isolated from the marrow of NOD.Cg-Prkdcscid IL2rgtm1Wjl/SzJ (NSG) mice that had been engrafted with these cells. The newly acquired mitochondria are functional and provide AML cells with a metabolic advantage as shown by an increase in oxidative phosphorylation and ATP production. Although these experiments nicely confirm what has been previously shown by others, the authors dug deeper into the dynamic and molecular mechanisms stimulating this transfer and their therapeutic potential. Although mitochondrial transfer in AML was recently described to occur via endocytosis,9 the authors show that, in human AML cells, this occurs primarily by direct cytoplasmic transport through protrusions that extend from the plasma membrane of the AML cells called tunneling nanotubes (TNTs). Cytochalasin B, an inhibitor of actin polymerization, was indeed able to prevent TNT formation and mitochondrial transfer, further supporting this hypothesis. Through a small pharmacological screen, the authors found that mitochondrial transfer was also inhibited by N-acetyl cysteine, DPI, and glutathione, and was enhanced by H2O2 and the hypoxia-mimetic agent cobalt chloride. Similar to what was previously described with cytarabine, the authors also found that the chemotherapy agent daunorubicin increases mitochondrial transfer. These observations led to the hypothesis that the existence of an altered redox state was at the basis of the mitochondrial transfer and, ultimately, resistance to therapy. The authors identified that coculture of BMSC with AML cells causes a contact-dependent increase of ROS and oxidative stress in BMSC, and that this occurs in a NOX2-dependent fashion. Interestingly, NOX2 knockdown (NOX2 KD) via short hairpin RNA in AML cells considerably reduces the production of ROS in BMSC, the BMSC-to-AML directional transfer of mitochondria, and the basal and maximum mitochondrial respiration in AML cells. To evaluate the therapeutic potential of NOX2 inhibition in AML, the authors engrafted parental or NOX2 KD AML cell lines into NSG recipient mice. Mice transplanted with NOX2 KD AML cells exhibited reduced engraftment and disease progression as well as an extended overall survival.
Overall, these results identify an oncogenic role of active, NOX2-driven mitochondrial transfer in AML. This study represents a significant step forward in the understanding of metabolic crosstalk between AML cells and BMSC, which offers new opportunities to deliver improved AML treatments alone or in combination with current therapies. Hopefully, the data provided by Marlein et al will bring us closer to being able to manipulate the AML microenvironment to the benefit of patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.