Key Points
Zoonotic P cynomolgi switches red cell tropism for reticulocytes expressing Trf1 (CD71+) and DARC (CD234+).
In the human host, P cynomolgi displays an almost identical rheopathobiology to P vivax.
Abstract
Two malaria parasites of Southeast Asian macaques, Plasmodium knowlesi and P cynomolgi, can infect humans experimentally. In Malaysia, where both species are common, zoonotic knowlesi malaria has recently become dominant, and cases are recorded throughout the region. By contrast, to date, only a single case of naturally acquired P cynomolgi has been found in humans. In this study, we show that whereas P cynomolgi merozoites invade monkey red blood cells indiscriminately in vitro, in humans, they are restricted to reticulocytes expressing both transferrin receptor 1 (Trf1 or CD71) and the Duffy antigen/chemokine receptor (DARC or CD234). This likely contributes to the paucity of detectable zoonotic cynomolgi malaria. We further describe postinvasion morphologic and rheologic alterations in P cynomolgi–infected human reticulocytes that are strikingly similar to those observed for P vivax. These observations stress the value of P cynomolgi as a model in the development of blood stage vaccines against vivax malaria.
Introduction
Plasmodium cynomolgi was first observed in 1907 in a Macaca fascicularis collected in Java and described in detail a year later,1 although its specific status was not confirmed until 1935.2 The striking similarity to P vivax, and in particular its ability to cause relapse infections, has and still makes P cynomolgi a model of choice for chemotherapeutic and biological investigations.3-6 Indeed, the exoerythrocytic cycle of mammalian Plasmodium parasites was first uncovered by using P cynomolgi,7 as was the discovery of the dormant liver stages responsible for relapses, the hypnozoites.8 Early attempts to inoculate P cynomolgi sporozoites or infected blood failed to yield an infection in neurosyphilis patients (paretics) undergoing malaria therapy,9,10 but in the early 1960s, accidental infections with P cynomolgi through infected mosquito bites were recorded in staff members from 2 distinct groups in the United States (1group from Tennessee and the other from Ohio).11,12 This prompted a series of further experimental infections of volunteers and paretics that confirmed the susceptibility of humans to different strains of this parasite (summarized in Coatney et al13 ). In these recipients, the parasitemia rarely exceeded a few hundred parasites per microliter, with probable relapses noted in some sporozoite-induced infections, and the clinical picture was one of relatively mild self-resolving symptoms with no signs of anemia. These observations raised the possibility of zoonotic transmission of simian malaria, a concern that was reinforced by the first record of a naturally acquired infection with another parasite prevalent in macaques, P knowlesi, in a US Army surveyor after a visit to Malaysia.14 This species, first isolated in the 1930s,2 was shown to infect humans readily through experimental inoculation.15 It was then assessed for use in malariotherapy of paretics, but this was tempered by observations of high parasitemias and severe clinical symptoms in some of the recipients.16,17
The specter of zoonotic transmission that waned over the next 4 decades (during which no further cases were recorded) became a reality with the discovery of a major epidemic centered in Malaysian Borneo.18 Indeed, zoonotic P knowlesi infections now predominate in Malaysia, and in the last decade, they have also been recorded in the neighboring countries.19-21 By contrast, to date, only 1 case of zoonotic P cynomolgi has been reported recently from Malaysia,22 and it came to light solely through careful molecular analysis. Three interrelated factors might account for the disparity in the observed prevalence of zoonotic infections for these 2 species that have similar high prevalence in their natural hosts.23 First, P cynomolgi could be easily misdiagnosed as P vivax to which it bears a remarkable morphologic similarity. Second, the relatively mild self-limiting clinical symptoms and the very low peak parasitemias of P cynomolgi in humans would reduce the likelihood of identifying infected patients and reaching the correct diagnosis. Finally, variations in the tropism of the merozoite to invade the erythrocytes of different host species would limit multiplication and thus the course of infection. It is this last factor that we have explored in this study.
Methods
Sample collection and DNA extraction
P cynomolgi B-strain–infected blood samples (5 mL in heparin) were collected from 6 different Rhesus monkeys (Macaca mulatta) and from naïve monkeys at the Armed Forces Research Institute of Medical Science, Bangkok, Thailand, and the Sing Health Experimental Medicine Centre Animal Facility, Singapore (Institutional Animal Care and Use Committee–Approved Application #2015/SHS/1024). An aliquot of 200 µL was stored at −20°C for subsequent DNA extraction (DNAeasy Blood Kit, Qiagen, Hilden, Germany) from which the Duffy antigen/chemokine receptor (DARC) sequence was obtained. White blood cells were removed by using nonwoven fabric filters (NWFs) before using the blood samples. Collection of the clinical P vivax isolates that were used as positive control in this study was approved by the ethics committees of the Faculty of Tropical Medicine, Mahidol University, the Thai Ministry of Public Health, the Institute for the Development of Human Research Protection, and by the Oxford Tropical Research Ethics Committee (OxTREC; No.17-11). For researchers in the United States, various strains of P cynomolgi (including the B strain) are available through the Malaria Research and Reference Reagent Resource Center repository (https://www.beiresources.org/MR4Home.aspx).
Concentration of P cynomolgi and P vivax schizonts by magnetic sorting
P cynomolgi- or P vivax–infected red blood cells (RBCs) were placed in McCoy’s 5A medium supplemented with 2.4 g/L d-glucose and 20% heat-inactivated O serum (P cynomolgi) or human AB serum (P vivax) and matured to schizonts by incubation in an atmosphere of 5% O2 at 37.5°C. After maturation, the infected RBCs were suspended in McCoy’s 5A medium at 50% hematocrit and passed through an LD column (Miltenyi Biotec, Singapore.) on a magnetic sorter, from which the concentrated schizonts were subsequently eluted.
Reticulocyte enrichment using CD71 sorting
Human cord blood was collected in heparinized tubes immediately after delivery, and the white blood cells were removed by using an NWF filter. Transferrin receptor 1 (Tfr1 [CD71])–positive cells (reticulocyte fraction) were obtained by passing blood through an LS column (Miltenyi Biotec). For the blood collected from naïve monkeys, the white blood cells were also removed by using an NWF filter, and the reticulocytes were obtained by Percoll gradient centrifugation: 5 mL at 50% hematocrit naïve were overlaid on a 6 mL 70% Percoll cushion and centrifuged at 1200g for 15 minutes. The fine band of concentrated reticulocytes at the interface was collected, and the cells were washed twice with McCoy’s 5A medium. Cells in the negative fraction in the lower layer were also collected and washed twice with McCoy’s 5A medium.
Invasion assay
The invasion assay methodology used was based on methods in Russell et al.24 The concentrated P vivax or P cynomolgi schizonts were diluted in McCoy’s 5A medium and mixed with uninfected human or macaque RBCs. They were then maintained at 2% hematocrit in 200 µL of McCoy’s 5A medium (supplemented with 2.4 g/L d-glucose and 20% heat-inactivated human AB (P vivax) or O (P cynomolgi) serum. Cultures were incubated at 37°C for at least 24 hours, which allowed the merozoites to reinvade fresh RBCs. Blockade of RBC invasion was assayed by using different antibodies and a chemokine against human DARC: anti-FY6 2C325 at 20 µg/mL, anti-FyB (Abcam) at 25 µg/mL, and the chemokine interleukin-8 (CXCL-8/IL-8) (Peprotech Asia) at 1000 nM. The overall experimental scheme is shown in Figure 1.
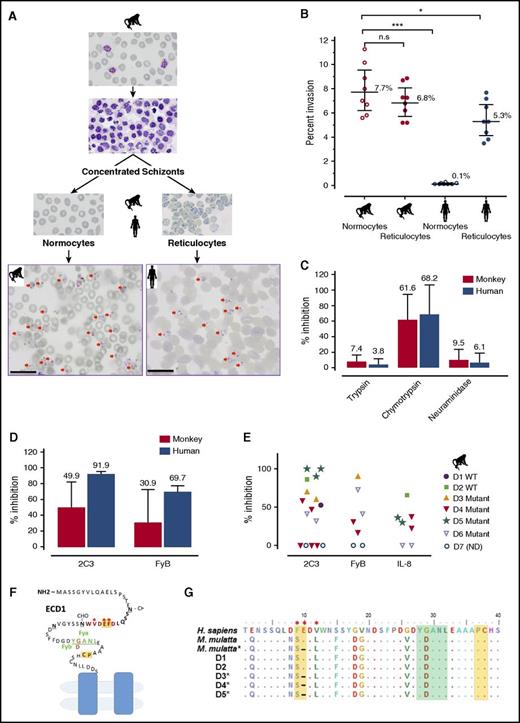
P cynomolgi RBC tropism and specificity for DARC. (A-B) Invasion efficiencies of P cynomolgi into macaque or human RBCs, reticulocytes, and normocytes (CD71–). Two representative photomicrographs of P cynomolgi invasions in monkey normocytes and human reticulocytes (scale bar = 20 μm). The invasion data were derived from 8 independent experiments, and significance was determined by one-way analysis of variance (ANOVA) and post hoc analysis. (C) Inhibition of P cynomolgi invasion by enzymatic treatment of RBCs. (D) Inhibition of macaque or human RBC invasion by anti-Fy6 (2C3 antibody) or anti-FyB. (E) In-depth analysis of inhibition of macaque RBC invasion by anti-Fy6 (2C3 antibody), anti-FyB, or the chemokine IL-8. (F) The extracellular domain 1 (ECD1) of DARC showing the region targeted by IL-8 and the antibodies and used in this study. Residues in red represent the 2C3 antibody recognition region, with the key binding residues marked by an asterisk; residues in green represent the position of the Fy group (FyB in macaque, G D); residues in yellow indicate the IL-8 binding sites.26 (G) Alignment of partial sequences of DARC from 5 of the monkey donors (D1-5) used in this study. The color notations correspond to the ECD1 diagram of DARC. *P < .05; ***P < .001. n.s., nonsignificant.
P cynomolgi RBC tropism and specificity for DARC. (A-B) Invasion efficiencies of P cynomolgi into macaque or human RBCs, reticulocytes, and normocytes (CD71–). Two representative photomicrographs of P cynomolgi invasions in monkey normocytes and human reticulocytes (scale bar = 20 μm). The invasion data were derived from 8 independent experiments, and significance was determined by one-way analysis of variance (ANOVA) and post hoc analysis. (C) Inhibition of P cynomolgi invasion by enzymatic treatment of RBCs. (D) Inhibition of macaque or human RBC invasion by anti-Fy6 (2C3 antibody) or anti-FyB. (E) In-depth analysis of inhibition of macaque RBC invasion by anti-Fy6 (2C3 antibody), anti-FyB, or the chemokine IL-8. (F) The extracellular domain 1 (ECD1) of DARC showing the region targeted by IL-8 and the antibodies and used in this study. Residues in red represent the 2C3 antibody recognition region, with the key binding residues marked by an asterisk; residues in green represent the position of the Fy group (FyB in macaque, G D); residues in yellow indicate the IL-8 binding sites.26 (G) Alignment of partial sequences of DARC from 5 of the monkey donors (D1-5) used in this study. The color notations correspond to the ECD1 diagram of DARC. *P < .05; ***P < .001. n.s., nonsignificant.
Sequence of the M mulatta DARC
The primers to amplify DARC were designed on the basis of the sequence from M mulatta (accession No. AF311921.1, HQ285849.1): DARC forward primer (5′ CGGAACTCTCCCCCTCAACTC 3′) and DARC reverse primer (5′ GAGCTGCGGGTGTTACCTAGCCC 3′). Briefly, the 30-µL amplification reaction mixture contained 100 to 200 ng of DNA, each primer at 0.5 µM, 0.2 mM deoxynucleotide triphosphates, 0.02 U Phusion Taq polymerase, and 6 µL of high-fidelity buffer. The amplification cycling conditions were 98°C for 30 seconds, 30 cycles of 60°C for 30 seconds, 72°C for 30 seconds, and 98°C for 10 seconds, followed by 60°C for 2 minutes and a final extension at 72°C for 10 minutes. The amplified fragments were then purified by MinElute polymerase chain reaction purification Kit (Qiagen), sequenced at AITbiotech, Singapore
Micropipette aspiration
Micropipette aspiration was modified from a previously published protocol.27 Briefly, aspiration was performed at 37°C and observed by using an oil immersion objective (magnification ×1000 ) with an Olympus research inverted microscope IX73. Borosilicate glass micropipettes (diameter 1.5 ± 0.2 µm) were used to hold or aspirate RBCs. Individual RBCs were aspirated at a pressure drop rate of 0.5 Pa⋅s for 100 seconds. The cell membrane deformation was recorded by using the Dual CCD Digital Camera DP80 (Olympus) at an image-taking rate of one frame per second. Images were processed by cellSens Dimension (Olympus). Hemispherical cap model was used to calculate the membrane shear elastic modulus as a quantitative surrogate measure of the rigidity of the RBC membrane skeleton.27
Atomic force microscopy
At least 10 infected RBCs from each isolate and cell type were scanned by using atomic force microscopy. Thin smears were scanned by using a Dimension 3100 model with a Nanoscope IIIa controller (Veeco, Santa Barbara, CA) using tapping mode. The probes used for imaging were 125 µm long by 30 µm wide single-beam shaped cantilevers (Model PPP-NCHR-50, Nanosensors) with tip radius of curvature of 5 to 7 nm. Images were processed and measurements were performed by using Nanoscope 5.30 software (Veeco).
Statistical analysis
Nonparametric analysis of data was performed by using GraphPad Prism 6. The DARC sequences were analyzed and aligned by Multiple Sequence Comparison by Log-Expectation (MUSCLE; www.ebi.ac.uk/Tools/msa/muscle).
Results
Host-specific tropism of P cynomolgi merozoites for RBC types
The efficiency of the invasion of RBCs by P cynomolgi merozoites was assessed in an in vitro assay. P cynomolgi schizonts purified from an infected Rhesus monkey were incubated with normocytes or reticulocytes from humans or Rhesus monkeys. Whereas both the mature and immature macaque RBCs are indiscriminately invaded, invasion of the human RBCs was almost totally restricted to the reticulocytes (Figure 1A-B). The efficiency of invasion of human normocytes was significantly lower than that of the 2 Rhesus RBC fractions (Figure 1B).
Duffy-dependence for the invasion of human reticulocytes
It is known that P vivax invasion is dependent on the presence of the DARC on human reticulocytes. To ascertain whether this is also the case for P cynomolgi, invasion efficiencies of human and macaque RBCs were assessed. Chymotrypsin treatment of the RBCs, which removes DARC from the surface (Figure 1C), significantly inhibited invasion, but trypsin or neuraminidase treatment, which does not affect DARC, did not inhibit invasion.
In contrast to the clear-cut inhibition of invasion observed for human reticulocytes, this was highly variable when Rhesus RBCs were tested (Figure 1D), which might have been the result of polymorphisms in the residues critical for the anti-Fy6 (2C3) binding (Figure 1E).25,26 Although sequencing of the relevant DARC fragment from the animals used revealed such polymorphisms (Figure 1F), their pattern did not correlate with the variability observed in the inhibition of invasion. We then used another agent in the assay that binds to residues conserved in the Rhesus DARC, an anti-FyB antibody and the chemokine IL-828 , and we observed a similar variable impact on the invasion of monkey RBCs (Figure 1D). Given that the P cynomolgi B strain used in this study is not a cloned line, we further excluded possible variations in the sequence of the Duffy binding protein II in parasites used in the different experiments (supplemental Figure 1, available on the Blood Web site). Thus, although these observations show that invasion of human RBCs is clearly dependent on the presence of DARC, this is not the case for M mulatta RBCs. The fact that human normocytes that express DARC were not invaded by P cynomolgi suggested that DARC was not the primary receptor for invasion, a notion supported by the data obtained when monkey RBCs were used. A recent study by Tachibana et al29 on other Macaca species clearly suggests that P cynomolgi targets macaque erythrocytes through a DARC-independent interaction. These observations also suggest that P cynomolgi merozoites have 2 alternative pathways for the invasion of Rhesus RBCs, 1 of them being DARC-dependent and the other not. Comparative genomic/proteomic analyses of the various human and macaque RBC types might provide a means for identifying the primary RBC receptor for P cynomolgi and potentially that of P vivax.
Postinvasion modifications in P cynomolgi- and P vivax-infected reticulocytes
Direct comparison of Giemsa-stained smears from monkeys and humans infected with P cynomolgi or P vivax (Figures 2 and 3A) illustrates the difficulties in distinguishing between the 2 species, even for highly experienced microscopists. One of the characteristics of P vivax-infected RBCs is the dramatic transformation of the rigid reticulocytes into a deformable cell within the space of 6 hours,30,31 a normal maturation process that normally takes at least 48 hours for uninfected reticulocytes.32 This is also observed with P cynomolgi-infected human reticulocytes, as shown by the drop in the shear modulus for these cells (Figure 3B). It was interesting to note that for both human- and macaque-infected RBCs, deformability increased as the parasites matured to schizonts (Figure 3B). Furthermore, the ultra-structural surface modifications on the surface of the RBCs after infection with P vivax were also observed on that of human reticulocytes and Rhesus RBCs infected by P cynomolgi (Figure 2C). These distinctive caveolae that dot the RBC surface (Schüffner dots) at a density of approximately 2/μm2 (Figure 3D) have irregularly shaped openings of 90 to 98 nm in diameter (Figure 3E). Some of the components of these caveolae and the subsurface vesicle complex with which they are associated have been previously identified, namely members of the Plasmodium helical interspersed subtelomeric superfamily,33 but those of the caveolae are yet to be characterized. Given that the caveolae are also formed in monkey normocytes infected with P cynomolgi, it would seem unlikely that they could be associated with proteins scavenged from the clathrin pits (symmetrical openings <100 nM in diameter) primarily found in reticulocytes.32,34
Erythrocytic development of P cynomolgi in monkey and human hosts. Giemsa-stained thin films of P cynomolgi in (A) monkey (M mulatta) normocytes and (B) human reticulocytes matured ex vivo over a period of 42 hours. The parasite was staged into the following categories: tiny ring (0-6 hours after invasion), small ring (6-12 hours after invasion), large rings (12-18 hours after invasion), early trophozoites (18-24 hours after invasion), late trophozoites (24-30 hours after invasion), early schizonts (30-36 hours after invasion), and mature schizonts (36-42 hours after invasion). Scale bar = 10 μm.
Erythrocytic development of P cynomolgi in monkey and human hosts. Giemsa-stained thin films of P cynomolgi in (A) monkey (M mulatta) normocytes and (B) human reticulocytes matured ex vivo over a period of 42 hours. The parasite was staged into the following categories: tiny ring (0-6 hours after invasion), small ring (6-12 hours after invasion), large rings (12-18 hours after invasion), early trophozoites (18-24 hours after invasion), late trophozoites (24-30 hours after invasion), early schizonts (30-36 hours after invasion), and mature schizonts (36-42 hours after invasion). Scale bar = 10 μm.
Comparative morphological and rheological characteristics of P cynomolgi and P vivax infected RBCs. (A) The morphologic similarity of P cynomolgi early trophozoites (18-24 hours after invasion) in M mulatta normocytes and human reticulocytes compared with that of P vivax in human reticulocytes. Giemsa-stained thin film smear is the most commonly used method for species diagnosis in areas where P vivax and P cynomolgi are co-endemic. Scale bar = 10 μm. (B) The effect of P cynomolgi invasion and development on the deformability of monkey and human RBCs. The plot shows the membrane mean shear modulus (SM) (a higher SM indicates a reduced membrane deformability) for different RBC types (monkey and human) and stages of P vivax erythrocytic development (geometric mean ± 95% confidence intervals; n > 15 cells from 3 independent trials). The red lightning icons indicate the tropism of P cynomolgi when invading human or monkey RBCs. Pictures of respective cell types before and during membrane SM measurement by micropipette aspiration are shown under the graph. Mean (geometric) SMs were compared by using ANOVA (Bonferroni correction) and multiple comparison test (Tukey). Uninfected normocytes were significantly more deformable than uninfected reticulocytes (P < .001). However, both ring and trophozoite P vivax stages become progressively more deformable (P < .05) until schizont stage (the very mature schizonts segmenters were especially rigid). Scale bar = 5 μm. (C) Comparative phenotypic characterization of caveolae in P cynomolgi- and P vivax–infected RBCs in monkeys and humans as revealed by atomic force microscopy. (D) The mean density of caveolae per μm2 (n > 10 cells scanned for each condition over 3 separate ex vivo maturation experiments) on early and late trophozoite-infected RBCs. (E) The mean diameter of caveolae of P vivax and P cynomolgi in human reticulocytes. No significant difference in caveola density or diameter was observed.
Comparative morphological and rheological characteristics of P cynomolgi and P vivax infected RBCs. (A) The morphologic similarity of P cynomolgi early trophozoites (18-24 hours after invasion) in M mulatta normocytes and human reticulocytes compared with that of P vivax in human reticulocytes. Giemsa-stained thin film smear is the most commonly used method for species diagnosis in areas where P vivax and P cynomolgi are co-endemic. Scale bar = 10 μm. (B) The effect of P cynomolgi invasion and development on the deformability of monkey and human RBCs. The plot shows the membrane mean shear modulus (SM) (a higher SM indicates a reduced membrane deformability) for different RBC types (monkey and human) and stages of P vivax erythrocytic development (geometric mean ± 95% confidence intervals; n > 15 cells from 3 independent trials). The red lightning icons indicate the tropism of P cynomolgi when invading human or monkey RBCs. Pictures of respective cell types before and during membrane SM measurement by micropipette aspiration are shown under the graph. Mean (geometric) SMs were compared by using ANOVA (Bonferroni correction) and multiple comparison test (Tukey). Uninfected normocytes were significantly more deformable than uninfected reticulocytes (P < .001). However, both ring and trophozoite P vivax stages become progressively more deformable (P < .05) until schizont stage (the very mature schizonts segmenters were especially rigid). Scale bar = 5 μm. (C) Comparative phenotypic characterization of caveolae in P cynomolgi- and P vivax–infected RBCs in monkeys and humans as revealed by atomic force microscopy. (D) The mean density of caveolae per μm2 (n > 10 cells scanned for each condition over 3 separate ex vivo maturation experiments) on early and late trophozoite-infected RBCs. (E) The mean diameter of caveolae of P vivax and P cynomolgi in human reticulocytes. No significant difference in caveola density or diameter was observed.
Discussion
Many biological, immunologic, and ecological factors define the ability of a Plasmodium species to switch and colonize a particular host. Of these, an inability to invade a host’s RBCs would provide an insurmountable barrier. Variations in the tropism of the merozoites would have subtle and pleiotropic effects. Confining the infection to a narrow RBC fraction such as the reticulocyte has been noted for many species and generally leads to low-grade parasitemias. Our demonstration for a restricted RBC tropism and DARC-dependence in humans but not in macaques has no doubt contributed to the paucity of recorded cases of zoonotic P cynomolgi infections. Given the low peak parasitemias, often close to the limits of microscopic detection observed in experimental infections in humans, and the ease with which this species can be misdiagnosed as P vivax, does not allow one to exclude the possibility that the prevalence in humans is consequently merely underestimated. The rapid reduction in the prevalence of P vivax (and P falciparum) in Malaysia but not that of zoonotic P knowlesi tends to argue that P cynomolgi is indeed rarely zoonotic. This matter warrants epidemiologic investigations based on suitably sensitive and specific molecular detection techniques.
The similarity in the tropism to human RBCs and in the evolution of the infected reticulocytes reinforces the usefulness of P cynomolgi as the best model for P vivax. The ability to conduct ex vivo infections of human reticulocytes by P cynomolgi opens up rich possibilities for fundamental investigations into the mechanism of invasion and RBC remodeling, and provides an excellent practical model for preclinical evaluation of vaccine candidates, thus aiding in the development of novel measures to control the globally distributed burdensome P vivax.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yves Colin Aronovicz and Olivier S. Bertrand (UMR_S 1134 INSERM, Université Paris Diderot) for the generous gift of the anti-DARC antibodies and their suggestion to use the anti-FyB antibody and IL-8 in our experiments, and the staff at Department of Veterinary Medicine, Armed Forces Research Institute of Medical Science, Bangkok, Thailand.
This work was supported by a University of Otago start-up grant (B.R.), National University of Singapore Yong Loo Lin School of Medicine Tier 1 (Faculty Research Committee) Grant R-182-000-232-112, a grant from the Wellcome Trust (administered by the Novartis Institute of Tropical Diseases), and by funding from the Singapore Immunology Network and the Horizontal Programme on Infectious Diseases under the Agency for Science, Technology and Research, Singapore (L.R.). Shoklo Malaria Research Unit is part of the Mahidol Oxford University Research Unit, supported by the Wellcome Trust of Great Britain.
Authorship
Contribution: V.K., R.S., A.C.Y.C., B.M., M.I., H.S.-S., B.K.S.Y., J.J.Y.O., and B.R. carried out laboratory work and collected the data; R.Z. and B.R. conducted the biomechanical analyses and imaging; F.N. supervised all collection of human material; V.K., R.S., A.C.Y.C., D.E.K., H.S.-S., K.S.W.T., P.B., G.S., L.R., and B.R. analyzed the data; R.I., R.U., and E.L. managed handling and care of primates; V.K., D.E.K., B.M., F.N., K.S.W.T., P.B., G.S., L.R., and B.R. drafted the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Russell, University of Otago, Microbiology Building, PO Box 56, Dunedin 9054, New Zealand; e-mail: b.russell@otago.ac.nz; and Laurent Rénia, Singapore Immunology Network (SIgN), Agency for Science, Technology, and Research (A*STAR), 8A Biomedical Grove, #03-15, Immunos Building, Biopolis, Singapore 138648; e-mail: renia_laurent@immunol.a-star.edu.sg.