Key Points
Tissue-type plasminogen activator inhibits the activity of the innate immune system in macrophages in vitro and in vivo in mice.
Suppression of macrophage proinflammatory responses by tPA requires the NMDA receptor.
Abstract
Tissue-type plasminogen activator (tPA) is the major intravascular activator of fibrinolysis and a ligand for receptors involved in cell signaling. In cultured macrophages, tPA inhibits the response to lipopolysaccharide (LPS) by a pathway that apparently requires low-density lipoprotein receptor-related protein-1 (LRP1). Herein, we show that the mechanism by which tPA neutralizes LPS involves rapid reversal of IκBα phosphorylation. tPA independently induced transient IκBα phosphorylation and extracellular signal-regulated kinase 1/2 (ERK1/2) activation in macrophages; however, these events did not trigger inflammatory mediator expression. The tPA signaling response was distinguished from the signature of signaling events elicited by proinflammatory LRP1 ligands, such as receptor-associated protein (RAP), which included sustained IκBα phosphorylation and activation of all 3 MAP kinases (ERK1/2, c-Jun kinase, and p38 MAP kinase). Enzymatically active and inactive tPA demonstrated similar immune modulatory activity. Intravascular administration of enzymatically inactive tPA in mice blocked the toxicity of LPS. In mice not treated with exogenous tPA, the plasma concentration of endogenous tPA increased 3-fold in response to LPS, to 116 ± 15 pM, but remained below the approximate threshold for eliciting anti-inflammatory cell signaling in macrophages (∼2.0 nM). This threshold is readily achieved in patients when tPA is administered therapeutically for stroke. In addition to LRP1, we demonstrate that the N-methyl-D-aspartic acid receptor (NMDA-R) is expressed by macrophages and essential for anti-inflammatory cell signaling and regulation of cytokine expression by tPA. The NMDA-R and Toll-like receptor-4 were not required for proinflammatory RAP signaling. By mediating the tPA response in macrophages, the NMDA-R provides a pathway by which the fibrinolysis system may regulate innate immunity.
Introduction
Thrombosis is a leading cause of death in stroke, myocardial infarction, deep vein thrombosis/pulmonary embolism, trauma, and sepsis and is a complication of cancer.1-4 In these conditions, bidirectional crosstalk between pathways that control hemostasis and inflammation is important but remains incompletely understood.5-10 We recently identified enzymatically inactive (EI) tissue-type plasminogen activator (tPA) as a protein that suppresses expression of inflammatory mediators by macrophages in vitro.11 Because EI-tPA was inactive when the gene encoding low-density lipoprotein receptor-related protein-1 (LRP1) was deleted, we proposed that LRP1 is essential for EI-tPA activity.11 LRP1 functions in endocytosis and cell signaling in response to diverse ligands, including proteinases and intracellular proteins released in tissue injury.12-15
tPA is the principal intravascular activator of fibrinolysis and is a US Food and Drug Administration–approved drug for acute ischemic stroke.16 The efficiency of plasminogen activation by tPA is greatly increased by fibrin, providing a mechanism to localize fibrinolysis and spare fibrinogen.17 The structure of tPA includes a fibronectin type I (FN) domain, an epidermal growth factor–like (EGF-like) domain, 2 kringle domains, and the serine proteinase module.18 tPA binding to fibrin involves the FN and second kringle domains.19 tPA is rapidly inactivated by the serpin, plasminogen activator inhibitor-1 (PAI-1), which is overwhelmed when tPA is administered therapeutically because the concentration of PAI-1 in plasma is low.8
In addition to fibrin, tPA associates with extracellular matrix proteins and cell surface receptors, including LRP1,20 annexin-A2-S100A10 complex,21,22 Plg-RKT,23 the N-methyl-D-aspartic acid (NMDA) receptor (NMDA-R),24,25 and mannose receptors.26 Collectively, these receptors regulate the kinetics of plasminogen activation, control circulating levels of tPA, and trigger cell-signaling events. tPA receptors may be similar in function to other receptors that have evolved to inform cells of the presence of active proteinases in the cellular microenvironment.13,27,28
We and others have shown that LRP1 gene deletion in macrophages is proinflammatory.11,13,29-32 In wild-type (WT) macrophages, LRP1 ligands control the effects of LRP1 on cell physiology in a ligand-specific manner.11 The ability to initiate ligand-specific cell signaling may empower LRP1 to survey the macrophage microenvironment and initiate context-appropriate cellular responses. In neuron-like cells, ligand-specific LRP1 signaling has been attributed to assembly of distinct LRP1 coreceptor complexes.25
Herein, we show that enzymatically active tPA (EA-tPA) and EI-tPA demonstrate similar anti-inflammatory cell-signaling activity in macrophages. We also show that EI-tPA blocks the activity of lipopolysaccharide (LPS) in vivo in mice. The ability of tPA to antagonize LPS was explained by rapid reversal of IκBα phosphorylation. Although tPA independently activated cell signaling in macrophages, the tPA response was distinct from that elicited by the proinflammatory LRP1 ligand, receptor-associated protein (RAP), which included sustained IκBα phosphorylation and activation of all 3 MAP kinases: extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun kinase (JNK), and p38 MAP kinase. Finally, we show that the NMDA-R is expressed by macrophages and essential for anti-inflammatory tPA signaling and suppression of cytokine expression. These results define a novel pathway by which the fibrinolysis system may regulate innate immunity and inhibit inflammation.
Materials and methods
Proteins and reagents
Human EI-tPA (h-EI-tPA), h-EA-tPA, and mouse EI-tPA (m-EI-tPA) were from Molecular Innovations. E. coli LPS serotype 055:B5 was from Sigma-Aldrich. All in vivo studies were performed using the same batch of LPS. Bovine lactoferrin (LF) was from Sigma-Aldrich. α2-Macroglobulin (α2M) was purified from human plasma and activated for binding to LRP1.33,34 The hemopexin domain of MMP9 (MMP9-PEX) and RAP were expressed as glutathione S-transferase (GST) fusion proteins, purified as described before,35,36 and subjected to chromatography on Detoxi-Gel endotoxin-removing columns (Pierce). Endotoxin-free, monomeric RAP was provided by Travis Stiles (Novoron Biosciences). Dizocilpine (MK801) was from Millipore. Toll-like receptor-4 specific antibody (TLR4-Ab), which inhibits binding of LPS to TLR4,37 was from BioLegend.
Mice
Mice in which LRP1 is conditionally deleted in myeloid cells (mLRP1−/− mice) in the C57BL/6 background are previously described.11 LRP1 is undetectable in bone marrow derived macrophages (BMDMs) from mLRP1−/− mice.31 Mice that carry 2 copies of the floxed LRP1 gene but are LysM-Cre-negative are referred to as mLRP1+/+ mice. mLRP1+/+ mice express an unchanged level of LRP1 and are phenotypically identical to WT C57BL/6 mice.11,31 Animal experiments were designed to minimize pain and suffering and approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
BMDM cultures
Bone marrow cells were harvested from 16-week-old WT, mLRP1−/− and mLRP1+/+ male mice, as previously described.11,38 Cells were plated in 100-mm dishes and cultured in Dulbecco’s modified Eagle medium/F-12 medium containing 10% fetal bovine serum and 20% L929 cell-conditioned medium for 10 days. Nonadherent cells were then eliminated. Adherent cells included >95% BMDMs as was determined by F4/80 and CD11b immunoreactivity.11
BMDMs were transferred to serum-free medium (SFM) for 30 min and then treated with various proteins, LPS (0.1 µg/mL), or vehicle (20 mM sodium phosphate, 150 mM NaCl, pH 7.4). When indicated, BMDMs were pretreated with MK801 (1 µM) for 30 min or with TLR4-Ab (5 µg/mL) for 3 h.
Analysis of cell signaling
Cell extracts were prepared in radio immunoprecipitation assay buffer containing Protease Inhibitor Mixture and Phosphatase Inhibitor Cocktail (Pierce). Protein concentrations were determined by DC Protein Assay (Bio-Rad). Equivalent amounts of cellular protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene difluoride membranes. Membranes were blocked and probed with primary antibodies (Cell Signaling Technology), followed by horseradish peroxidase conjugated secondary antibodies. Immunoblots were developed using SuperSignal West Pico PLUS substrate (Thermo Scientific) and Blue Devil autoradiography film (Genesee Scientific). Densitometry was performed using Image Studio Lite 5.2.
Reverse transcription polymerase chain reaction (RT-PCR)
RNA was isolated from BMDMs using the NucleoSpin RNA kit (Macherey-Nagel) and reverse-transcribed using the iScript complementary DNA (cDNA) synthesis kit (Bio-Rad). Quantitative PCR (qPCR) was performed using TaqMan gene expression products.11 The messenger RNAs (mRNAs) analyzed included tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6), and CCL3. The relative change in mRNA expression was calculated using the 2ΔΔCt method and GAPDH mRNA as an internal normalizer. To identify cDNAs corresponding to the NR1/GluN1 NMDA-R subunit (GRIN1), the NR2A/GluN2a subunit (GRIN2A), the NR2B/GluN2b subunit (GRIN2B), the NR2C/GluN2c subunit (GRIN2C), and the NR2D/GluN2d subunit (GRIN2D), we PCR-amplified (40 cycles) 500 ng of template cDNA by using primers from Origene and Phusion high-fidelity DNA polymerase. PCR products were subjected to 1.5% agarose gel electrophoresis and visualized using SYBR Safe DNA gel stain (Thermo Fisher Scientific). Control PCR reactions were performed without reverse transcriptase to verify the absence of genomic DNA.
Phosphoprotein proteome profiling
WT BMDMs were transferred to SFM for 30 min and treated with h-EI-tPA (12 nM), RAP (150 nM), or vehicle for 1 h. Cell extracts were prepared using lysis buffer provided in the Proteome Profiler Human Phospho-Kinase Array kit (R&D Systems). An equivalent amount of cellular protein (300 µg) was incubated with the 2 nitrocellulose membranes. Phosphorylated proteins were detected using biotinylated detection antibodies. Although this array was initially developed to identify phosphoproteins in human proteins, we demonstrated excellent cross-reactivity with rodent proteins by comparing human and rat Schwann cells.39
Immunofluorescence (IF) microscopy
BMDMs were fixed with 4% paraformaldehyde and permeabilized in 0.25% Triton X-100. Nonspecific binding sites were blocked with 1% bovine serum albumin. Incubations with primary NR1-specific antibody (Abcam) were performed for 12 h. NR1 antibody was authenticated by comparing its reactivity with Schwann cells in which NR1/GRIN1 was silenced.40 Alexa-Fluor-488-conjugated secondary antibody was added for 1 h. In control studies, primary antibody was omitted. Preparations were mounted on slides with Pro-long Gold and 4′,6-diamidino-2-phenylindole (DAPI). Images were captured using a Leica DMIRE2 fluorescence microscope.
LPS challenge in vivo
The lethal dose of LPS in 50% of test animals (LD50) is reported to be 1-25 µg/g body weight in responsive mouse strains.41 We independently calibrated our LPS stock and determined an LD50 of ∼6 µg/g body weight in 16- to 20-week-old male C57BL/6 mice. To study the activity of h-EI-tPA, we administered LPS at 1.5 × the LD50 by intraperitoneal (IP) injection to 16- to 20-week-old male C57BL/6 mice. One hour later, mice were treated by IV injection with 50 µg h-EI-tPA (n = 6) or vehicle (n = 6). Mice were observed for changes in neurologic status, respiratory rate, and hemostasis abnormalities. Those entering a moribund or poorly responsive state were killed under anesthesia. Survival was plotted in Kaplan-Meier curves, and these curves were subjected to statistical analysis using the log-rank test (GraphPad Prism).
In separate studies, 12-week-old mLRP1−/− and mLRP1+/+ male mice were treated with LPS at 0.5 × the LD50 or vehicle by IP injection. Blood samples (50 µL) were collected from the retro-orbital venous plexus 3 h later, as has been previously described.11 Plasma was prepared, and the concentration of mouse tPA was determined by enzyme-linked immunosorbent assay (ELISA) (Innovative Research).
Statistics
Statistical analysis was performed using GraphPad Prism 5.0. Results are presented as the mean ± standard error of the mean (SEM). RT-qPCR data were analyzed by 1-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test (*P < .05, **P < .01, ***P < .001).
Results
tPA inhibits proinflammatory LPS signaling in macrophages
Treatment of WT BMDMs with 0.1 µg/mL LPS for 1 h caused IκBα phosphorylation and a substantial decrease in total IκBα, as was anticipated (Figure 1A). These events are known to derepress the activity of NFκB. LPS also activated ERK1/2, as was anticipated.42 When BMDMs were treated with LPS and h-EI-tPA (12 nM) for 1 h, IκBα phosphorylation and the decrease in total IκB were no longer observed. ERK1/2 activation also was blocked. Other known LRP1 ligands, including activated α2M (10 nM) and MMP9-PEX (10 nM), inhibited LPS-induced IκBα phosphorylation and ERK1/2 activation, suggesting an essential role for LRP1.
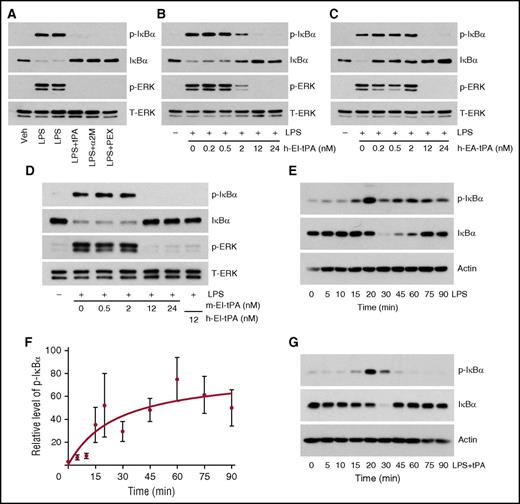
tPA inhibits LPS-initiated cell-signaling in macrophages. (A) BMDMs from WT C57BL/6J mice were treated with LPS (0.1 µg/mL) alone for 1 h or simultaneously with h-EI-tPA (tPA, 12 nM), activated α2M (10 nM), or MMP9-PEX (PEX, 10 nM). (B) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of h-EI-tPA for 1 h. (C) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of h-EA-tPA for 1 h. (D) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of m-EI-tPA for 1 h or with 12 nM h-EI-tPA as a control. Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, phospho-ERK1/2, and total ERK1/2. (E) BMDMs were treated for the indicated times with LPS (0.1 µg/mL). Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (F) Densitometry analysis of 4 independent experiments in which we examined IκBα phosphorylation in BMDMs treated with LPS for the indicated times (mean ± SEM). The analysis was performed using Image Studio Lite 5.2. (G) BMDMs were treated for the indicated times with LPS (0.1 µg/mL) and h-EI-tPA (12 nM). Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. p-ERK, phospho-ERK1/2; t-ERK, total ERK1/2; Veh, vehicle.
tPA inhibits LPS-initiated cell-signaling in macrophages. (A) BMDMs from WT C57BL/6J mice were treated with LPS (0.1 µg/mL) alone for 1 h or simultaneously with h-EI-tPA (tPA, 12 nM), activated α2M (10 nM), or MMP9-PEX (PEX, 10 nM). (B) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of h-EI-tPA for 1 h. (C) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of h-EA-tPA for 1 h. (D) BMDMs were treated with LPS (0.1 µg/mL) and with increasing concentrations (0.2-24 nM) of m-EI-tPA for 1 h or with 12 nM h-EI-tPA as a control. Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, phospho-ERK1/2, and total ERK1/2. (E) BMDMs were treated for the indicated times with LPS (0.1 µg/mL). Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (F) Densitometry analysis of 4 independent experiments in which we examined IκBα phosphorylation in BMDMs treated with LPS for the indicated times (mean ± SEM). The analysis was performed using Image Studio Lite 5.2. (G) BMDMs were treated for the indicated times with LPS (0.1 µg/mL) and h-EI-tPA (12 nM). Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. p-ERK, phospho-ERK1/2; t-ERK, total ERK1/2; Veh, vehicle.
Next, we determined the h-EI-tPA concentration required to antagonize LPS. h-EI-tPA at concentrations of 12 nM or higher completely blocked IκBα phosphorylation and ERK1/2 activation in response to LPS; 2 nM h-EI-tPA was partially effective (Figure 1B), defining an apparent activity range that exceeds the normal plasma concentration of tPA.43 Enzymatically active tPA (h-EA-tPA) replicated the activity of h-EI-tPA, inhibiting LPS-initiated cell signaling when present at concentrations of 12 nM or higher (Figure 1C). Lower concentrations of h-EA-tPA (0.2-2.0 nM) failed to inhibit IκBα phosphorylation; however, loss of IκBα was partially prevented, suggesting that the kinetics of the LPS response may have been modified.
Because BMDMs were isolated from mice, we compared the potency of mouse EI-tPA in regulating LPS. m-EI-tPA behaved similarly to h-EI-tPA, inhibiting LPS when present at concentrations of 12 nM or higher (Figure 1D).
To better understand the effects of tPA on LPS signaling, we studied IκBα phosphorylation and degradation as a function of time in BMDMs treated with LPS alone. IκBα was phosphorylated within 15 min and remained phosphorylated throughout the 90-min time course (Figure 1E). Total IκBα decreased by 30 min but reversed by 75-90 min. Recovery of IκBα in LPS-treated cells has been reported previously.44 The results of 4 independent experiments are summarized in Figure 1F, confirming that in LPS-treated cells, in the absence of tPA, IκBα phosphorylation is sustained.
In BMDMs treated with LPS and h-EI-tPA (12 nM), IκBα phosphorylation was still observed at 20 min; however, by 45-90 min, IκBα phosphorylation was no longer evident (Figure 1G). Total IκB decreased transiently and then recovered. These results demonstrate that h-EI-tPA does not prevent the initial phosphorylation of IκBα in response to LPS but instead rapidly reverses it.
tPA signals independently in macrophages
BMDMs were treated with h-EI-tPA (12 nM) in the absence of LPS. h-EI-tPA independently induced IκBα phosphorylation; however, the response was delayed (45-60 min) and highly transient (Figure 2A). By 75 min, phospho-IκBα was no longer evident. The transient nature of IκBα phosphorylation was reminiscent of the response observed in cells treated with h-EI-tPA + LPS and distinguished from the sustained response observed with LPS alone. Once again, in cells treated with h-EI-tPA, a transient decrease in total IκBα was noted.
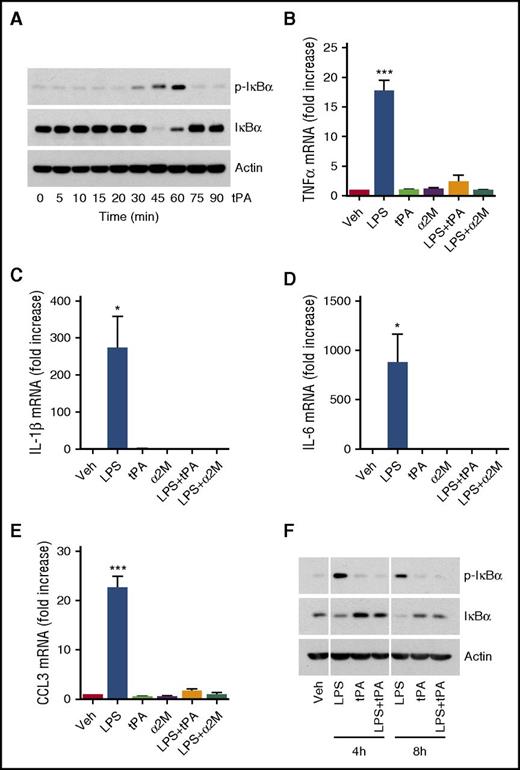
tPA independently activates cell signaling in macrophages. (A) BMDMs from WT C57BL/6J mice were treated with h-EI-tPA (12 nM), in the absence of LPS, for the indicated times. Immunoblot analysis was performed to detect phospho-IκBα, total IκBα, and β-actin. (B-E) BMDMs were treated with LPS (0.1 μg/mL), h-El-tPA (12 nM), activated α2M (10 nM), LPS plus h-EI-tPA, LPS plus activated α2M, or vehicle for 8 h. RT-qPCR was performed to compare mRNA levels for TNFα, IL-1β, IL-6 and CCL3 (mean ± SEM; n = 8; 1-way ANOVA with Tukey’s post hoc analysis). (F) BMDMs were treated for 4 or 8 h with LPS alone (0.1 μg/mL), h-EI-tPA alone (12 nM), or LPS plus h-EI-tPA. Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. *P < .05, ***P < .001.
tPA independently activates cell signaling in macrophages. (A) BMDMs from WT C57BL/6J mice were treated with h-EI-tPA (12 nM), in the absence of LPS, for the indicated times. Immunoblot analysis was performed to detect phospho-IκBα, total IκBα, and β-actin. (B-E) BMDMs were treated with LPS (0.1 μg/mL), h-El-tPA (12 nM), activated α2M (10 nM), LPS plus h-EI-tPA, LPS plus activated α2M, or vehicle for 8 h. RT-qPCR was performed to compare mRNA levels for TNFα, IL-1β, IL-6 and CCL3 (mean ± SEM; n = 8; 1-way ANOVA with Tukey’s post hoc analysis). (F) BMDMs were treated for 4 or 8 h with LPS alone (0.1 μg/mL), h-EI-tPA alone (12 nM), or LPS plus h-EI-tPA. Cell extracts were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. *P < .05, ***P < .001.
The ability of h-EI-tPA to induce IκBα phosphorylation was unanticipated because we previously demonstrated that h-EI-tPA suppresses proinflammatory cytokine expression in BMDMs.11 Given this new result, we re-examined the effects of h-EI-tPA on cytokine expression in BMDMs by using the same h-EI-tPA preparation studied in the cell-signaling experiments. h-EI-tPA (12 nM) blocked the increase in expression of TNFα, IL-1β, IL-6, and CCL3 caused by LPS (Figure 2B-E). Equivalent results were obtained with the alternative anti-inflammatory LRP1 ligand, activated α2M.
We hypothesized that sustained IκBα phosphorylation allows for cyclical decreases in total IκBα, which drive expression of proinflammatory cytokines. To test this hypothesis, we treated BMDMs with LPS, h-EI-tPA, or LPS + h-EI-tPA for 4 or 8 h. At these later time points, IκBα phosphorylation was observed only in cells treated with LPS in the absence of h-EI-tPA and was associated with decreased levels of total IκBα (Figure 2F).
To evaluate cell signaling in h-EI-tPA-treated BMDMs in an unbiased manner, we applied an array approach using the phosphokinase Proteome Profiler, which profiles 43 protein phosphorylation events. BMDMs were treated with 12 nM h-EI-tPA in the absence of LPS for 1 h. The arrays demonstrated activation of ERK1/2 (Figure 3A). Densitometry analysis showed that phospho-ERK1/2 was increased 8-fold (Figure 3B). No other significant increases in phosphorylation were observed. Importantly, the IKK-NFκB pathway is not represented in the array. Decreases in protein phosphorylation were noted in h-EI-tPA-treated cells, including phospho-Akt T308, which is associated with Akt activation. PRAS40 is an Akt substrate and regulator of mTORC1 activity.45 Chk2 is a protein kinase involved in the response to DNA damage and replication stress.46
Profiling protein phosphorylation in tPA-treated BMDMs. (A) BMDMs were isolated from WT C57BL/6J mice and treated with h-EI-tPA (12 nM) or vehicle for 1 h. Protein phosphorylation was analyzed by array. (B) Changes in protein phosphorylation associated with h-EI-tPA treatment in comparison with cells treated with vehicle. Each of the analyzed phoshoproteins is demarcated with a box in panel A. (C) BMDMs were treated with increasing concentrations of h-EI-tPA (0.1-25 nM) for 1 h. Cell extracts were subjected to immunoblot analysis to detect phospho-ERK1/2 and total ERK1/2.
Profiling protein phosphorylation in tPA-treated BMDMs. (A) BMDMs were isolated from WT C57BL/6J mice and treated with h-EI-tPA (12 nM) or vehicle for 1 h. Protein phosphorylation was analyzed by array. (B) Changes in protein phosphorylation associated with h-EI-tPA treatment in comparison with cells treated with vehicle. Each of the analyzed phoshoproteins is demarcated with a box in panel A. (C) BMDMs were treated with increasing concentrations of h-EI-tPA (0.1-25 nM) for 1 h. Cell extracts were subjected to immunoblot analysis to detect phospho-ERK1/2 and total ERK1/2.
The increase in phospho-ERK1/2 in h-EI-tPA-treated BMDMs was unanticipated because when BMDMs were treated with h-EI-tPA + LPS, h-EI-tPA blocked the increase in phospho-ERK1/2 caused by LPS (see Figure 1). To validate that h-EI-tPA activates ERK1/2 in BMDMs, when added independently of LPS, we treated cells with h-EI-tPA for 1 h. h-EI-tPA, at concentrations of 1.0 nM or greater, activated ERK1/2 (Figure 3C).
Activation of cell signaling by proinflammatory LRP1 ligands
The LRP1 ligands, RAP and LF, mimic LRP1 gene deletion, promoting inflammatory cytokine expression in BMDMs.11 However, because RAP is expressed in bacteria, there is risk of endotoxin contamination, and LF is reported to interact directly with LPS and TLR4.47,48 We therefore performed new studies to test the mechanism by which RAP and LF promote cytokine expression.
WT BMDMs were treated with RAP (150 nM) or LF (150 nM) for 1 h. Both proteins induced IκBα phosphorylation and decreased the total level of IκBα (Figure 4A). The responses were similar in magnitude to that elicited by LPS. TLR4-Ab completely blocked IκBα phosphorylation in response to LPS but had no effect on the response to RAP or LF, arguing against LPS contamination and TLR4 as contributing to the activities observed with RAP and LF. As a second test for LPS contamination, RAP and LPS were boiled for 5 min. Boiling LPS had no effect on its activity, as was anticipated, but completely eliminated the response to RAP.
Comparison of proinflammatory LRP1 signaling in BMDMs. (A) BMDMs from WT C57BL/6J mice were pretreated with TLR4-neutralzing antibody (+TLR4-Ab, 5 µg/mL) or with vehicle (−TLR4-Ab) for 3 h and then with LPS (0.1 µg/mL), RAP (150 nM), LF (150 nM), or vehicle for 1 h. Separately, WT BMDMs that were not exposed to TLR4-Ab were treated with LPS (0.1 µg/mL) or RAP (150 nM), which had been boiled for 5 min. Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (B) BMDMs were isolated from littermate mLRP1+/+ and mLRP1−/− mice. The cells from mLRP1−/− mice were treated with h-El-tPA (12 nM), RAP (150 nM), or vehicle for 8 h. Cells from mLRP1+/+ mice were treated with vehicle. RT-qPCR was performed to determine TNFα mRNA (mean ± SEM; n = 8; 1-way ANOVA with Tukey’s post hoc analysis). (C-E) WT BMDMs were treated with monomeric RAP (mRAP, 150 nM) or vehicle for 8 h. RT-qPCR was performed to determine mRNA levels for TNFα, CCL3, and IL-6. (F) WT BMDMs were treated for the indicated times with RAP (150 nM). Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα and β-actin. **P < .01, ***P < .001.
Comparison of proinflammatory LRP1 signaling in BMDMs. (A) BMDMs from WT C57BL/6J mice were pretreated with TLR4-neutralzing antibody (+TLR4-Ab, 5 µg/mL) or with vehicle (−TLR4-Ab) for 3 h and then with LPS (0.1 µg/mL), RAP (150 nM), LF (150 nM), or vehicle for 1 h. Separately, WT BMDMs that were not exposed to TLR4-Ab were treated with LPS (0.1 µg/mL) or RAP (150 nM), which had been boiled for 5 min. Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (B) BMDMs were isolated from littermate mLRP1+/+ and mLRP1−/− mice. The cells from mLRP1−/− mice were treated with h-El-tPA (12 nM), RAP (150 nM), or vehicle for 8 h. Cells from mLRP1+/+ mice were treated with vehicle. RT-qPCR was performed to determine TNFα mRNA (mean ± SEM; n = 8; 1-way ANOVA with Tukey’s post hoc analysis). (C-E) WT BMDMs were treated with monomeric RAP (mRAP, 150 nM) or vehicle for 8 h. RT-qPCR was performed to determine mRNA levels for TNFα, CCL3, and IL-6. (F) WT BMDMs were treated for the indicated times with RAP (150 nM). Equal amounts of cellular protein (20 µg) were subjected to immunoblot analysis to detect phospho-IκBα, total IκBα and β-actin. **P < .01, ***P < .001.
When LRP1 is deleted in macrophages, using tamoxifen-activated Cre, RAP does not stimulate cytokine expression, suggesting an essential role for LRP1.11 As a second model system, we isolated BMDMs from mLRP1−/− and mLRP1+/+ mice. TNFα mRNA was significantly increased in LRP1-deficient BMDMs from mLRP1−/− mice, in comparison with LRP1-expressing BMDMs from mLRP1+/+ mice (Figure 4B). However, RAP failed to significantly stimulate TNFα expression in BMDMs from mLRP1−/− mice, and h-EI-tPA failed to attenuate TNFα expression. We conclude that RAP and h-EI-tPA are inactive in regulating cytokine expression in LRP1-deficient BMDMs, prepared using 2 distinct model systems.
Because RAP is standardly expressed as a GST-fusion protein, which forms dimers,49 for the first time, we studied the effects of monomeric RAP (mRAP) on cytokine expression by WT BMDMs. Treatment of BMDMs with 150 nM mRAP significantly increased expression of TNFα (Figure 4C), CCL3 (Figure 4D), and IL-6 (Figure 4E). RAP activity is thus not dependent on dimerization.
Next, WT BMDMs were treated with 150 nM RAP, and IκBα phosphorylation was studied as a function of time. Figure 4F shows that IκBα phosphorylation was sustained through the end of the time course, mimicking the response observed with LPS. Total IκBα decreased transiently in RAP-treated cells. The ability of RAP to cause sustained IκBα phosphorylation and increase cytokine expression supports our model linking these 2 events in BMDMs.
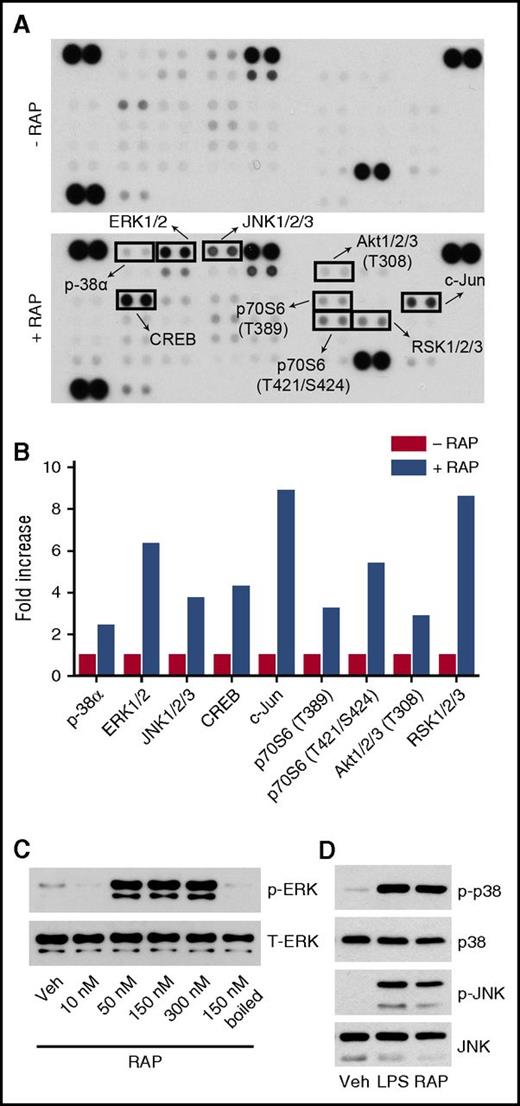
In phosphoprotein array studies, RAP activated all 3 major MAP kinases in WT BMDMs, including ERK1/2, JNK, and p38 MAP kinase (Figure 5A-B), distinguishing RAP from h-EI-tPA and mimicking the reported response to LPS.50 Phospho-c-Jun was observed in RAP-treated cells, an anticipated consequence of JNK activation. Rsk phosphorylation was observed, most likely downstream of ERK1/2, together with Akt and its downstream substrate, p70 S6 kinase. Phospho-cAMP response element binding protein (CREB) was increased in RAP-treated cells. CREB is a transcription factor phosphorylated downstream of diverse cell-signaling enzymes.51
Profiling protein phosphorylation in RAP-treated BMDMs. (A) BMDMs from WT C57BL/6J mice were treated with RAP (150 nM) or vehicle for 1 h. Protein phosphorylation was analyzed by array. (B) Changes in protein phosphorylation associated with RAP treatment in comparison with cells treated with vehicle. Each of the analyzed phoshoproteins is demarcated with a box in panel A. (C) BMDMs were stimulated for 1 h with increasing concentrations of RAP (10-300 nM) and with RAP that had been boiled for 5 min (150 nM). Cell extracts were subjected to immunoblot analysis to detect phospho-ERK1/2 and total ERK1/2. (D) BMDMs were treated with LPS (0.1 µg/mL), RAP (150 nM), or vehicle for 1 h. Cell extracts were subjected to immunoblot analysis to detect phospho-p38 MAP kinase, total p-38 MAP kinase, phospho-JNK, and total JNK.
Profiling protein phosphorylation in RAP-treated BMDMs. (A) BMDMs from WT C57BL/6J mice were treated with RAP (150 nM) or vehicle for 1 h. Protein phosphorylation was analyzed by array. (B) Changes in protein phosphorylation associated with RAP treatment in comparison with cells treated with vehicle. Each of the analyzed phoshoproteins is demarcated with a box in panel A. (C) BMDMs were stimulated for 1 h with increasing concentrations of RAP (10-300 nM) and with RAP that had been boiled for 5 min (150 nM). Cell extracts were subjected to immunoblot analysis to detect phospho-ERK1/2 and total ERK1/2. (D) BMDMs were treated with LPS (0.1 µg/mL), RAP (150 nM), or vehicle for 1 h. Cell extracts were subjected to immunoblot analysis to detect phospho-p38 MAP kinase, total p-38 MAP kinase, phospho-JNK, and total JNK.
Studies were performed to validate that RAP activates ERK1/2 in BMDMs (Figure 5C). The concentration of RAP required for ERK1/2 activation was 50 nM or greater. ERK1/2 activation therefore represents a common property of proinflammatory and anti-inflammatory LRP1 ligands. Boiling RAP completely blocked ERK1/2 activation. We also confirmed that RAP activates JNK and p38 MAP kinase (Figure 5D).
EI-tPA blocks the toxicity of LPS in mice
Male C57BL/6 mice were treated by IV injection with 50 µg EI-h-tPA (n = 6) or vehicle (n = 6) 1 h after injecting LPS at 1.5 × the LD50. The EI-h-tPA dose was approximately twice the recommended dose of EA-h-tPA (activase) for stroke patients.16,52 Assuming a plasma volume of 0.85 mL,53 upon distribution, we estimated the peak concentration of EI-h-tPA to be 850 nM. Five of 6 mice treated with LPS alone succumbed (Figure 6A). All 6 mice treated with LPS + EI-h-tPA survived, showing little or no evidence of toxicity during the observation period. The difference in response was significant (P < .01), as determined by log-rank test.
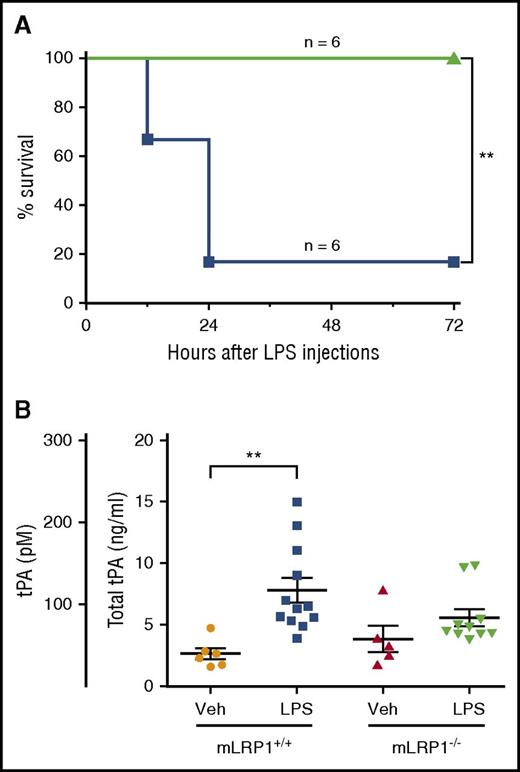
EI-tPA reduces the toxicity of LPS in wild-type mice. (A) Kaplan-Meier survival curves are shown for wild-type C57BL/6J mice treated by IV injection with 50 µg h-EI-tPA (n = 6; green line) or vehicle (n = 6; blue line), 1 h after IP injection of LPS at 1.5 times the LD50. Significance was determined by log-rank test. (B) ELISA was performed to detect endogenous tPA in mouse plasma harvested from mLRP1+/+ and mLRP1−/− mice, 3 h after IP injection of LPS at 0.5 times the LD50 or vehicle. Each data point represents a duplicate analysis of a plasma sample from a different mouse (mean ± SEM; 1-way ANOVA with Tukey’s post hoc analysis). **P < .01.
EI-tPA reduces the toxicity of LPS in wild-type mice. (A) Kaplan-Meier survival curves are shown for wild-type C57BL/6J mice treated by IV injection with 50 µg h-EI-tPA (n = 6; green line) or vehicle (n = 6; blue line), 1 h after IP injection of LPS at 1.5 times the LD50. Significance was determined by log-rank test. (B) ELISA was performed to detect endogenous tPA in mouse plasma harvested from mLRP1+/+ and mLRP1−/− mice, 3 h after IP injection of LPS at 0.5 times the LD50 or vehicle. Each data point represents a duplicate analysis of a plasma sample from a different mouse (mean ± SEM; 1-way ANOVA with Tukey’s post hoc analysis). **P < .01.
Next, we measured endogenous tPA in mouse plasma under basal conditions and after treatment with LPS at 0.5 × the LD50 for 3 h. We compared mLRP1+/+ mice, which are phenotypically WT, and mLRP1−/− mice, which are hypersensitive to LPS.11 The selected LPS dose increased circulating cytokine levels in mLRP1+/+ mice and, to a greater extent, in mLRP1−/− mice.11 In naïve mLRP1+/+ mice, the mean plasma concentration of tPA was 40 ± 7 pM (Figure 6B). After LPS treatment, the concentration of tPA in plasma significantly increased to 116 ± 15 pM (P < .01) but remained below the tPA concentration required to trigger cell signaling in BMDMs in vitro. In mLRP1−/− mice, the plasma concentration of tPA, under basal conditions and after LPS treatment, was not significantly changed in comparison with that of mLRP1+/+ mice.
NMDA-R is essential for tPA activity in macrophages
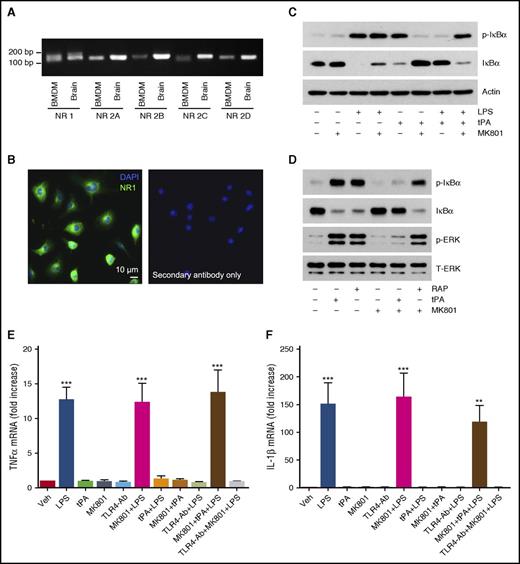
In neurons and Schwann cells, the NMDA-R functions as an independent cell-signaling receptor for tPA and as a coreceptor with LRP1 to form a high-affinity tPA receptor complex.25,40,54,55 The NMDA-R is a heterotetramer of 2 NR1/GluN1 subunits and 2 NR2/ GluN2 subunits.56 NMDA-R expression by macrophages has been previously reported.57,58 By RT-PCR, we identified mRNAs encoding NR1, NR2A, and NR2D in BMDMs (Figure 7A). mRNAs encoding NR2B and NR2C were present at trace levels. NR1 protein was uniformly expressed by BMDMs, as was determined by IF microscopy (Figure 7B). No signal was observed when the primary antibody was omitted.
The NMDA-R functions as an anti-inflammatory tPA signaling receptor in macrophages. (A) RT-PCR analysis comparing NMDA-R subunit mRNA expression in BMDMs and, as a control, extracts of mouse brain. (B) IF microscopy to detect the NMDA-R NR1 subunit in BMDMs. Nuclei are counterstained with DAPI. Primary antibody was omitted in control studies. (C) BMDMs were pretreated for 30 min with MK801 (1.0 µM), as is indicated, and then with LPS (0.1 µg/mL), h-EI-tPA (12 nM), or both for 1 h, as is indicated. Samples were analyzed by immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (D) BMDMs were pretreated with MK801 (1.0 µM) or vehicle and then with h-EI-tPA (12 nM), RAP (150 nM), or vehicle for 1 h, as is indicated. Immunoblot analysis was performed to detect phospho-IκBα, total IκBα, phospho-ERK1/2, and total ERK1/2. (E-F) BMDMs were preincubated with TLR4-Ab (5 µg/mL) for 3 h or with MK801 (1.0 µM) for 30 min. The cells were then treated with LPS (0.1 µg/mL), h-EI-tPA (12 nM), or both, or with vehicle. RT-qPCR was performed to determine mRNA levels for TNFα (E) and IL-1β (F) (mean ± SEM; n = 4). **P < .01, ***P < .001.
The NMDA-R functions as an anti-inflammatory tPA signaling receptor in macrophages. (A) RT-PCR analysis comparing NMDA-R subunit mRNA expression in BMDMs and, as a control, extracts of mouse brain. (B) IF microscopy to detect the NMDA-R NR1 subunit in BMDMs. Nuclei are counterstained with DAPI. Primary antibody was omitted in control studies. (C) BMDMs were pretreated for 30 min with MK801 (1.0 µM), as is indicated, and then with LPS (0.1 µg/mL), h-EI-tPA (12 nM), or both for 1 h, as is indicated. Samples were analyzed by immunoblot analysis to detect phospho-IκBα, total IκBα, and β-actin. (D) BMDMs were pretreated with MK801 (1.0 µM) or vehicle and then with h-EI-tPA (12 nM), RAP (150 nM), or vehicle for 1 h, as is indicated. Immunoblot analysis was performed to detect phospho-IκBα, total IκBα, phospho-ERK1/2, and total ERK1/2. (E-F) BMDMs were preincubated with TLR4-Ab (5 µg/mL) for 3 h or with MK801 (1.0 µM) for 30 min. The cells were then treated with LPS (0.1 µg/mL), h-EI-tPA (12 nM), or both, or with vehicle. RT-qPCR was performed to determine mRNA levels for TNFα (E) and IL-1β (F) (mean ± SEM; n = 4). **P < .01, ***P < .001.
We antagonized the NMDA-R with MK801, which is known to block tPA signaling in PC12 and Schwann cells.25,40 MK801 had no effect on LPS-induced IκBα phosphorylation but blocked the ability of h-EI-tPA to independently induce IκBα phosphorylation and reverse IκBα phosphorylation in response to LPS (Figure 7C). MK801 also blocked ERK1/2 activation by h-EI-tPA (Figure 7D). In BMDMs treated with RAP, MK801 had no effect on IκBα phosphorylation or ERK1/2 activation. Thus, MK801 inhibits cell signaling in BMDMs in response to h-EI-tPA but not RAP.
When BMDMs were treated with LPS and MK801 for 8 h, MK801 failed to inhibit LPS-induced expression of TNFα (Figure 7E) and IL-1β (Figure 7F). In the absence of MK801, either h-EI-tPA or TLR4-Ab effectively neutralized LPS. When BMDMs were treated with LPS and h-EI-tPA, in the presence of MK801, the ability of h-EI-tPA to antagonize LPS was neutralized. MK801 did not interfere with TLR4-Ab. These results indicate that the NMDA-R is essential for the anti-inflammatory activity of h-EI-tPA.
Discussion
In response to injury, platelets and the coagulation system serve as early responders and are highly integrated with innate immunity to demarcate localized infections and prevent dissemination of microorganisms.5,10,59 Coagulation proteinases, such as thrombin and factor VIIa–tissue factor complex, activate protease-activated receptors, increasing the permeability of endothelial cell junctions and inducing expression of cytokines and chemokines by inflammatory cells.27,60 Fibrin clots are infiltrated by inflammatory cells, including monocytes, which may be activated by products released by platelets and associated with the fibrin.61
Anticoagulation systems, such as the Protein C System, suppress inflammation.62 Our results suggest that the fibrinolysis system may suppress inflammation as well. The pivotal interaction involves tPA binding to macrophages and does not require tPA enzyme activity. Instead, tPA exosites are probably involved, such as the FN and EGF-like domains, which have been implicated in LRP1 binding.63 The concentration of tPA required for activation of anti-inflammatory cell signaling in BMDMs exceeded that typically present in plasma; however, it is still possible that endogenously produced tPA regulates macrophage activity at sites of tissue injury or within fibrin clots. In patients receiving tPA/activase for stroke, the plasma tPA concentration is much higher, and pathways that control inflammation may be regulated.
LPS morbidity was prevented in mice treated with h-EI-tPA. Assuming an initial h-EI-tPA plasma concentration of 850 nM and a clearance t1/2 of 4 min,64 the plasma concentration of h-EI-tPA probably exceeded 10 nM for at least 25 min. Because activation of fibrinolysis is not possible with h-EI-tPA, higher and more frequent doses of this derivative may be feasible to counteract various innate immune system responses and pathological inflammation.
We previously deleted LRP1 in cultured BMDMs by using tamoxifen-activated Cre and demonstrated that h-EI-tPA and RAP fail to regulate cytokine expression when LRP1 is absent.11 We have now confirmed this observation using a second model system of LRP1 gene deletion, suggesting an essential role for LRP1 in mediating tPA anti-inflammatory cell signaling. This argument is supported by the fact that the LRP1 ligands, activated α2M and MMP9-PEX, replicate the activity of h-EI-tPA. α2M and MMP9-PEX have more limited repertoires of alternative receptors.13,35 We now report that the NMDA-R also is an essential receptor for the effects of tPA on macrophage cell signaling and for inhibition of cytokine expression. The function of the NMDA-R as a macrophage tPA receptor is unreported. Cooperation between the NMDA-R and LRP1 in mediating the response to tPA in macrophages would suggest conservation of the mechanism by which tPA signals across diverse cell types.25,40,54,55
These results do not preclude a role for other tPA receptors in macrophage signaling. Our studies implicating LRP1 focused on the tPA response in the presence of LPS. One intriguing possibility is that the signaling response elicited by tPA in the absence of LPS involves different receptors. Arguing against that possibility is the fact that MK801 blocked the activity of h-EI-tPA when added independently or together with LPS. Thus, the NMDA-R appears to be necessary under both sets of conditions.
MK801 had no effect on RAP signaling in BMDMs. TLR4-Ab also was ineffective. Boiling RAP eliminated its activity without affecting LPS, authenticating RAP as a reagent. The effects of RAP on cytokine expression mimicked those observed in LRP1-deficient BMDMs. We conclude that the NMDA-R is essential, most likely in conjunction with LRP1, for the response of macrophages to h-EI-tPA but not RAP. In PC12 cells, different LRP1 ligands recruit distinct coreceptors to elicit distinct and sometimes opposing responses.25,65,66 Ligand specificity in LRP1 signaling may allow LRP1 to survey the cellular microenvironment and trigger context-appropriate responses.
When a single time point was examined, h-EI-tPA induced IκBα phosphorylation and ERK1/2 activation, similarly to LPS. This paradoxical finding was resolved with detailed time course experiments. Our results suggest that neutralization of LPS by tPA does not require complete inhibition of IκBα phosphorylation. Instead, the essential event appears to be rapid and permanent reversal of IκBα phosphorylation. The independent effects of tPA on cell signaling did not stimulate cytokine expression. By contrast, RAP induced sustained IκBα phosphorylation, simultaneous activation of p38 MAP kinase and JNK, and increased cytokine expression. The difference in cell-signaling signatures, elicited by anti-inflammatory and proinflammatory LRP1 ligands, may help reconcile our work11 with studies published by others showing activation of NFκB and ERK1/2 in macrophages by tPA and α2M.67-69
Recently, Sugimoto et al.70 demonstrated that plasmin/plasminogen may promote resolution of inflammation by binding to macrophage annexin A1. This pathway is entirely distinct from that described here but potentially complementary, supporting the conclusion that further consideration of the role of fibrinolysis in regulating innate immunity and inflammation is justified.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (Grant R01 HL136395); the NIH National Institute of Neurological Disorders and Stroke (Grant R01 NS097590); and by the Ministry of Education, University and Research (MIUR) (Grant FIRB 2013 RBFR13RBK9).
Authorship
Contribution: E.M., P.A., C.B., M.A.B., and A.S.G. conducted the experiments; E.M. and S.L.G. wrote the paper; and all authors participated in the experiment design and results interpretation, and edited and approved the final draft of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven L. Gonias, Department of Pathology, University of California, San Diego, La Jolla, CA 92093; e-mail: sgonias@ucsd.edu.
References
Author notes
E.M. and P.A. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal