Key Points
This is the first trial to investigate PD-1 inhibitor, pembrolizumab, and an IMiD (pomalidomide) in MM with promising clinical efficacy.
PD-L1 expression on myeloma cells and PD-1 on marrow infiltrating T lymphocytes are potential biomarkers for efficacy of PD-1 blockade.
Abstract
Programmed death 1 (PD-1) receptor and its ligand (PD-L1) facilitate immune evasion in multiple myeloma (MM). We hypothesized that pembrolizumab, PD-1-antibody, can enhance antimyeloma cellular immunity generated by pomalidomide, leading to improved clinical responses. In this single-center, phase 2 study, 48 patients with relapsed/refractory MM (RRMM) received 28-day cycles of pembrolizumab, 200 mg IV every 2 weeks, pomalidomide 4 mg daily for 21 days, and dexamethasone 40 mg weekly. Patients had a median of 3 (range: 2-5) lines of therapy, median age 64 (range: 35-83) years, and had received both an immune modulatory drug (IMiD) and proteasome inhibitor: (35 [73%] of 48) were refractory to both; (31 [70%]) had received an autologous transplant, and (30 [62%]) had high-risk cytogenetics. Adverse events grade 3 to 4 occurred in (19 [40%] of 48 patients), including hematologic toxicities (19 [40%]), hyperglycemia (12 [25%]), and pneumonia (7 [15%]). Autoimmune events included pneumonitis (6 [13%]) and hypothyroidism (5 [10%]), mostly ≤ grade 2. Objective responses occurred in (29 [60%] of 48) patients, including stringent complete response/complete response (4 [8%]), very good partial response (9 [19%]), and partial response (16 [33%]); median duration of response was 14.7 months. At median follow-up of 15.6 months, progression-free survival (PFS) was 17.4 months and overall survival was not reached. Analyses of pretreatment marrow samples revealed a trend for increased expression of PD-L1 in responding patients and longer PFS with increased T-lymphocyte infiltrates, irrespective of PD-1 expression. Pembrolizumab, pomalidomide, and low-dose dexamethasone have acceptable safety and durable responses in RRMM patients. This trial was registered at www.clincialtrials.gov as #NCT02289222.
Introduction
Overall survival (OS) has improved for multiple myeloma (MM) patients; however, most patients eventually relapse. Patients refractory to immune modulatory drugs (IMiDs) and proteasome inhibitors have a poor outcome.1
Programmed death 1 (PD-1) and its coinhibitory ligands PD-L1 and PD-L2 represent an emerging therapeutic target in cancer; blockade of this pathway has led to impressive durable remissions in subsets of patients with solid tumors and hematologic malignancies.2,3 PD-L1 is overexpressed on myeloma cells.4 Expression is heterogeneous and is higher in the relapsed/refractory (RR) setting and in patients with residual disease after therapy and those with hyperdiploid karyotype. High levels of expression correlate with increased risk of progression from monoclonal gammopathy of unknown significance to MM.5-7 PD-L1 expression has been shown to induce drug resistance in myeloma cell lines through the PI3K/Akt signaling pathway.8 PD-L1 expression on antigen-presenting dendritic cells (DCs) and myeloid-derived suppressor cells provide a tumor-promoting, immune-suppressive microenvironment in MM.9,10 PD-1 is expressed on antigen-activated as well as exhausted T cells, natural killer (NK) cells, and DCs, and its expression in combination with other inhibitory signals leads to dysfunction of cytotoxic T lymphocytes.2 A recent study of T cells in MM showed low-level expression of PD-1 on T-cell clones, suggesting that these cells are not exhausted and that there would be a suboptimal response to checkpoint blockade alone.11 This observation may explain the lack of objective responses among 27 patients with MM treated with single-agent anti–PD-1 antibody nivolumab.12 These data suggest that other immune-stimulatory strategies are needed in combination with PD-1/PD-L1 blockade to restore effector T-cell function in MM.
Immune modulatory drugs (IMiDs), such as lenalidomide and pomalidomide, in addition to their direct proapoptotic effect on myeloma cells, enhance the immunologic bone marrow (BM) microenvironment by stimulating effector cytotoxic T lymphocytes and NK cells, inhibiting regulatory T cells, and altering a wide range of cytokines, including interferon-γ (IFN-γ) and interleukin-2 (IL-2).13-15 In vitro exposure to IMiDs resulted in decreased PD-1 expression on T cells and enhanced T-cell proliferative responses to allogeneic DCs.16 In vivo pomalidomide therapy led to increased production of multiple cytokines, including IFN-γ, tumor necrosis factor-α (TNF-α), IL-2, and IL-4 by CD4+ and CD8+ T cells and TNF-α and IFN-γ by NK cells. Pomalidomide also induces polyfunctional T-cell activation (particularly CD8+ T cells) with increased proportion of coinhibitory immune checkpoint B- and T-lymphocyte attenuator T cells and T-cell immunoglobulin and mucin domain 3 NK cells, but no change in PD-1+ T cells.17,18
Based on the above data, blocking inhibitory signals with PD-1 inhibitor in the setting of the immune stimulatory environment generated with IMiDs is an appealing approach to enhancing antimyeloma cellular immunity. Here we report the safety, efficacy, and exploratory biomarker results from a phase 2 study combining pembrolizumab with pomalidomide and low-dose dexamethasone in RRMM patients.
Methods
Study design and participants
This phase 2, single-center study combined pomalidomide and low-dose dexamethasone, with pembrolizumab, a humanized monoclonal immunoglobulin G4 (IgG4) antibody against PD-1. Patients were enrolled from January 2015 until May 2016. The data cutoff for this analysis was 1 November 2016.
Eligible patients had RRMM after at least 2 lines of prior therapy that included proteosome inhibitor (bortezomib or carfilzomib) and an IMiD (thalidomide or lenalidomide); prior exposure to pomalidomide was allowed if they had a response and were off therapy for 6 months. Patients should have a measurable disease as defined by serum paraprotein ≥5 g/L, urine Bence Jones protein ≥200 mg/24 hours, and/or involved free light chain level ≥100 mg/L. Refractory status was defined as progressing on or within 60 days of therapy. Patients who progressed on lenalidomide maintenance (10 mg) must have received higher doses (25 mg) with dexamethasone to be considered refractory to lenalidomide. Patients were at least 18 years old with an Eastern Cooperative Oncology Group performance status of 0 to 2 (0 to 5 scale with less disability with lower scores). Patients had adequate organ and BM functions. Female patients of childbearing potential had a negative pregnancy test and agreed to use adequate contraception during study treatment and for 120 days afterward. Patients were excluded if they had a diagnosis of acquired immunodeficiency, untreated hepatitis B or C, or active autoimmune disease, including noninfectious pneumonitis, coexisting second malignancy, plasma cell leukemia, or MM affecting the central nervous system.
All study patients were enrolled at the University of Maryland; the Institutional Review Board approved the protocol. The study was conducted in accordance with the Declaration of Helsinki and followed Good Clinical Practice guidelines. All of the patients provided written informed consent before study procedures and treatment.
Procedures
Pembrolizumab was administered IV at a dose of 200 mg every 2 weeks (the first 6 patients received 200 mg every 28 days). Pomalidomide was given orally at 4 mg daily for 21 days, and dexamethasone was given at 40 mg orally weekly Patients older than age 70 years received 20 mg. Cycles were repeated every 28 days for 2 years; afterward, responding patients continued on monthly pembrolizumab with pomalidomide and dexamethasone. All patients received antiviral and antibiotic prophylaxis with acyclovir and a quinolone. All patients received aspirin for deep venous thrombosis prophylaxis.
All patients underwent MM evaluations (M-protein in blood and urine and serum free light chains) at screening and monthly thereafter. Radiologic evaluations were performed at screening and as clinically indicated. Pretreatment BM specimens were obtained from all patients. Cytogenetics and fluorescence in situ hybridization (FISH) analyses were performed for risk assessment. Standard risk was defined as normal, hyperdiploid, and/or t(11:14). High risk was defined as deletion of 17p, t(14:16), t(14:20), t(4:14), and/or 1q+ in CD-138 selected myeloma samples. The normal cutoff value to define FISH positivity for each probe was 10% for fusions and 20% for numerical abnormalities, as previously reported.19 BMs were obtained yearly for those remaining on the study and for confirmation of complete responses (CRs).
Immunohistochemistry was performed on formalin-fixed paraffin-embedded sections of pretreatment BM biopsies. The decalcification was performed with Immunocal (formic acid–based bone decalcifier; Stat Laboratory), standardized to 90 minutes at 37°C. For PD-L1 (analysis done by E.H.), the antibody used was a rabbit monoclonal antibody anti–PD-L1 (clone 28-8) Abcam (ab 205921) and Envision Flex rabbit detection system with Envision Flex rabbit linker (DAKO). Antigen retrieval was performed using a pressure cooker using Target Retrieval Solution pH 6.0 (DAKO). Immunohistochemistry was performed on automated immunohistochemistry (IHC)/in situ hybridization platform Bond-III (Leica Microsystems). Positive controls were fixed and decalcified tonsils. To limit the PD-L1 scores to myeloma cells, sections immunostained for PD-L1 were double stained for MUM1 using mouse monoclonal antibody anti-MUM1 (clone MUM1p) (DAKO; catalog number M7259). Continuous plasma member staining was interpreted as positive. A proportion score of PD-L1 expression in <1% of the cells was considered negative; a score of 1% to 49% was scored as weakly positive, and a score of >50% was positive. For T cells (analysis done by A.L. and A.D.), immunostaining was performed using an antihuman CD3 monoclonal antibody (DAKO; clone F7.2.38) and antihuman PD-1 monoclonal antibody (Abcam; clone NAT105). CD3 and PD-1 IHC staining were graded as negative (<5% for CD3, <1% for PD-1) or positive (≥5% for CD3, ≥1% for PD-1). BM lymphocytes were classified into 3 groups: CD-3+/PD-1+ (activated T cells), CD3+/PD-1− (nonactivated T cells), and CD-3−/PD-1− (no detectable T cells).
Statistical analysis
The study size was designed to provide an adequate 82% power to detect a 25% improvement in the objective response rate (ORR) between the study population and historical control regimen of pomalidomide and low-dose dexamethasone (ORR of 30%). The research objectives of this study were to assess safety and clinical efficacy, including ORR, progression-free survival (PFS), OS, and duration of response (DOR). Responses and progressions were assessed using the International Myeloma Working Group criteria.20 Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Patients who received at least 1 cycle of treatment were included in the safety analysis. Overall response rate was defined as a 50% reduction in serum M protein or 90% reduction in Bence Jones protein (ie, partial response [PR] or better) and was based on intent-to-treat analysis. Efficacy assessment (PFS and OS) included patients who completed at least 2 cycles of therapy to allow for confirmation of response. DOR, assessed for responding patients who achieved ≥ PR, was defined as the time interval between the date of first response and the date of disease progression or death, whichever occurs first. For patients alive without disease progression, the end date for response duration was the last disease assessment. PFS was defined as time from the date of the first dose of pembrolizumab to the date of disease progression or death, whichever occurred earlier. Patients alive without a progression reported were censored at date of last contact. OS was defined as time from start of therapy to date of death (from any cause), censored at date of last contact. Survival and PFS functions were estimated using the Kaplan-Meier method. The Cox regression model was used to assess the following plausible risk factors for OS and PFS: age, isotype, number of cycles of therapy, and cytogenetic profile. The main exploratory biomarker analysis was to examine potential correlation between expression of PD-1 on T cells and PD-L1 on myeloma cells with clinical outcome using the following parameters: response rate focusing on responses ≥ very good partial response (VGPR) and PFS. SAS software (v.9.4; SAS Institute, Inc, Cary, NC) was used for statistical analyses.
Results
A total of 48 patients with RRMM enrolled in the study. Patient demographics and disease characteristics at baseline are shown in Table 1. Median age was 64 years (range: 35 to 83 years). High-risk cytogenetic findings were present in 30 (62%) patients. Median time from MM diagnosis to study entry was 4 years (range: 1.2-26 years). Patients received a median of 3 lines of prior therapy (range: 2-5), and 31 patients (72%) had prior autologous stem cell transplant. All patients (48 [100%]) received bortezomib (for induction and/or relapse) and 24 (50%) received carfilzomib (for relapsed disease). Overall (38 patients [79%]) were refractory to proteasome inhibitors; 100% had received lenalidomide (43 [90%]) and were refractory. Thirty-five (73%) patients were “double” refractory to both an IMiD and a proteasome inhibitor.
Treatment-related adverse events of any grade occurred in 35 (73%) patients, whereas grade 3 and higher events were observed in 20 (42%) patients (Table 2). One patient died of neutropenia and sepsis during cycle 2. The most common nonhematologic adverse events were fatigue, hyperglycemia, dizziness, and constipation. Respiratory symptoms of all grades occurred in a third of patients, including dyspnea (15 [31%]), upper respiratory tract infections (16 [33%]), pneumonia (10 [21%]), and influenza A (4 [8%]).
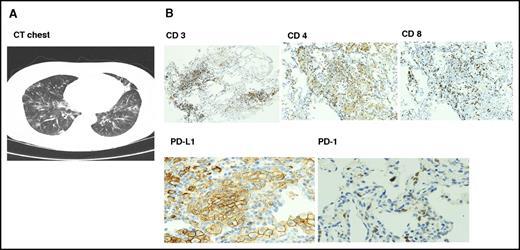
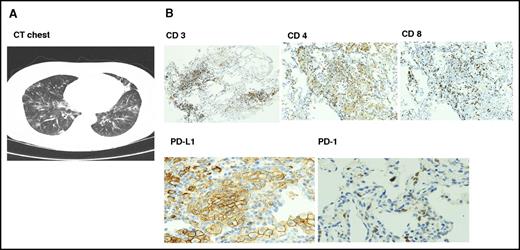
Six patients had documented autoimmune interstitial pneumonitis, mostly grades 1 to 2; only 1 patient had grade 3 toxicity. Time to onset of pneumonitis was variable from 2 to 15 months. Patients presented with fever, cough, and dyspnea, and computed tomographic scans showed nonspecific ground glass opacity. Transbronchial biopsies performed in 4 patients showed intense T-cell infiltration with reversal of CD4/CD8 ratio and upregulation of PD-L1 on type II pneumocytes (Figure 1). All 6 patients responded to drug interruption, and methylprednisolone was given to 3 patients. One patient (was in PR) withdrew consent; 3 patients were restarted on the same dose of pembrolizumab after resolution of symptoms, and 2 patients had recurrent episodes and stopped therapy. Both responded to methylprednisolone with resolution of symptoms. Other immune-mediated events included hypothyroidism (n = 5) and adrenal insufficiency (n = 2), both treated with hormone replacement therapy with levothyroxine and hydrocortisone. There were 2 cases of hepatitis and 1 case of vitiligo. To emphasize, most immune-mediated events were grade 1 to 2 and resolved with interruption of therapy.
Radiologic and pathologic findings in autoimmune pneumonitis. (A) Computed tomographic scan findings were nonspecific with multifocal ground glass opacities. (B) Transbronchial biopsy showed immune peroxidase staining of lymphocytic infiltrate with reversal of CD4/8 ratio and upregulation of PD-L1 on type I pneumocytes with absent PD-1 expression. Original magnification ×100 (CD 3, 4, and 8) and ×200 (PD-L1 and PD-1).
Radiologic and pathologic findings in autoimmune pneumonitis. (A) Computed tomographic scan findings were nonspecific with multifocal ground glass opacities. (B) Transbronchial biopsy showed immune peroxidase staining of lymphocytic infiltrate with reversal of CD4/8 ratio and upregulation of PD-L1 on type I pneumocytes with absent PD-1 expression. Original magnification ×100 (CD 3, 4, and 8) and ×200 (PD-L1 and PD-1).
Overall, 22 patients (49%) required dose reductions. Pembrolizumab dose was reduced to 200 mg monthly for autoimmune side effects (n = 2). Pomalidomide dose was reduced in 13 patients for fatigue (n = 5), neutropenia (n = 3), rash (n = 2), palpitation (n = 1), muscle spam (n = 1), and peripheral neuropathy (n = 1). Dexamethasone dose was reduced in 7 patients for uncontrolled hyperglycemia (n = 3), weight gain (n = 2), cellulitis (n = 1), and insomnia (n = 1). Five patients discontinued the study drugs because of regimen toxicity (11%), including pneumonitis (n = 3, 1 withdrew consent and 2 for recurrent episodes), dyspnea (n = 1), and fatigue (n = 1).
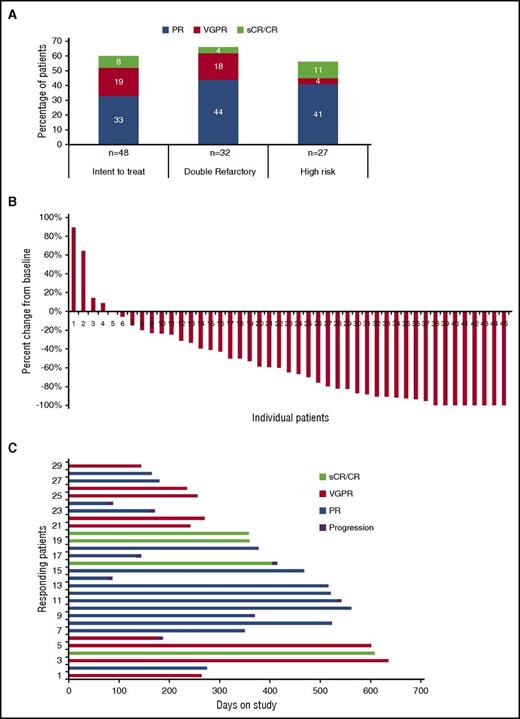
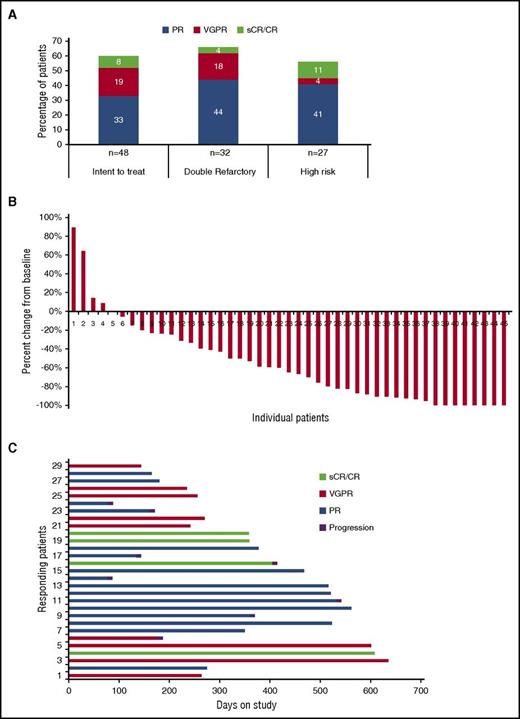
Responses are summarized in Figure 2. All 48 patients were included in the intent-to-treat response assessment. The ORR was 60%, with 3 of 48 (6%) achieving a stringent complete response (sCR), 1 (2%) CR, 9 (19%) VGPR, and 16 (33%) PR. Additional response evaluations showed minimal response in 3 (6%), stable disease in 11 (23%), and progressive disease in 2 (4%) patients. Three patients were not evaluable for response. Among 29 patients achieving objective responses, the median DOR was 14.7 months (95% confidence interval [CI] 7.9-17.5). The ORR rate was 68% and 56% for double refractory and high-risk cytogenetic patients, respectively. Responses of VGPR or better were 28% for the study population and 24% and 15% for double-refractory and high-risk cytogenetic patients, respectively.
Response data. (A) Summary of overall response data for all patients and by refractory status (double refractory to IMiDs and proteosome inhibitors) and by high-risk cytogenetics. (B) Relative change in paraprotein level from baseline for all evaluable patients (n = 45). (C) Duration of remission for patients achieving objective responses.
Response data. (A) Summary of overall response data for all patients and by refractory status (double refractory to IMiDs and proteosome inhibitors) and by high-risk cytogenetics. (B) Relative change in paraprotein level from baseline for all evaluable patients (n = 45). (C) Duration of remission for patients achieving objective responses.
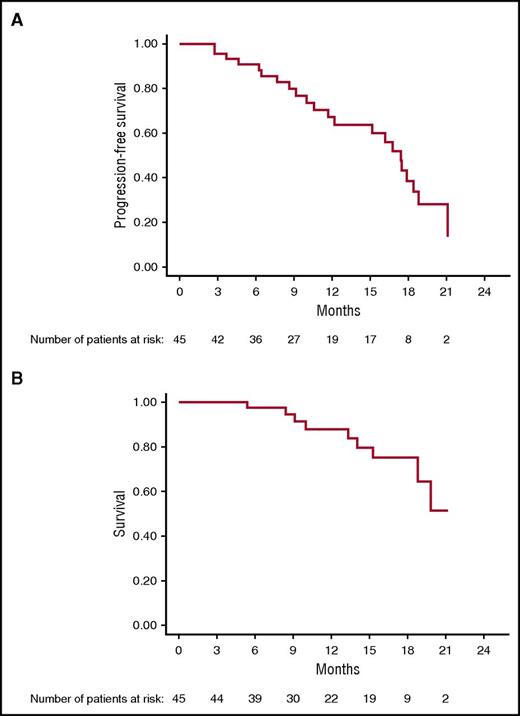
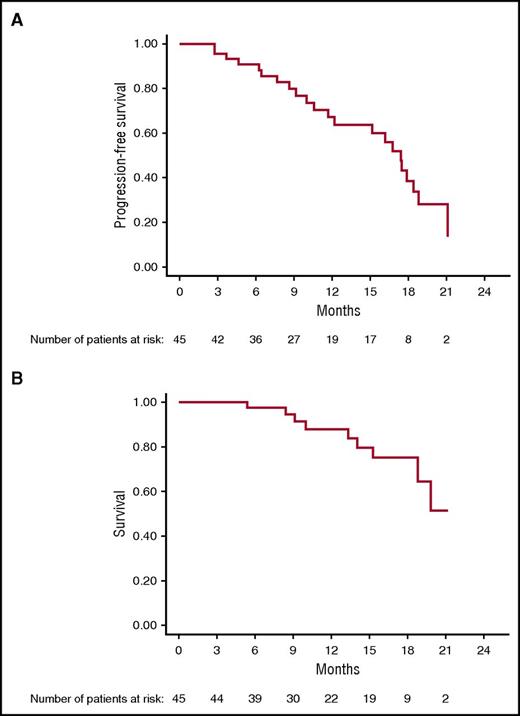
After a median follow-up of 15.6 months (95% CI 9.2-17.5), Kaplan-Meier analysis yielded an estimated median PFS of 17.4 months (95% CI 11.7-18.8); median OS has not been reached (95% CI, 18.9-NR [not reached]) (Figure 3). Median PFS for cytogenetically defined high-risk patients was 15.1 months (95% CI 9.1-17.9) and 19 months (95% CI 16-NR) for low-risk patients (P = .04).
On Cox regression analysis, the number of lines of prior therapy correlated with shorter PFS, relative risk: 0.8 (CI: 0.8-0.9, P = .003). High-risk cytogenetic, age, stage, isotype, and refractory status had no impact on PFS. There was no association between clinical efficacy (ORR, DOR, PFS, and OS) and autoimmune adverse events. As of 1 November 2016, 22 patients (49%) have progressed; 9 (20%) have died, and 23 continue to receive treatment.
Several patients who achieved sCR (n = 2) and VGPR (n = 4) have never achieved such deep responses with prior therapies that included proteasome inhibitors, IMiDs, and transplantation; all were double refractory at study entry. An additional 4 patients with soft tissue extramedullary disease responded: sCR (n = 1) and VGPR (n = 2); the fourth patient had stable disease for 4 cycles, and after receiving radiation to a painful soft tissue tumor, achieved a VGPR, supporting a possible abscopal effect, as previously described with PD-1 inhibitors.12,21
Correlative studies
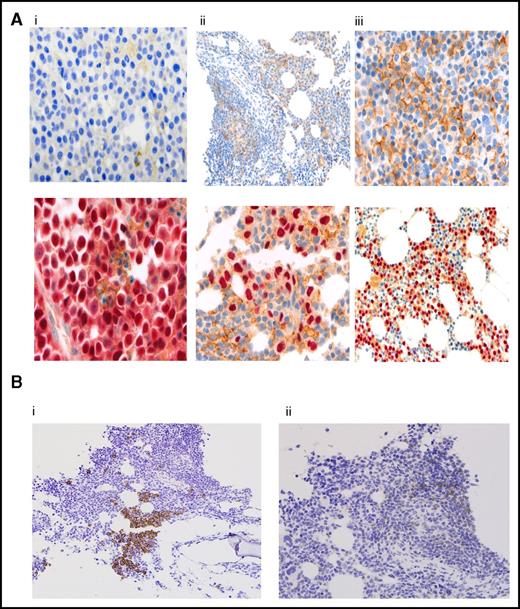
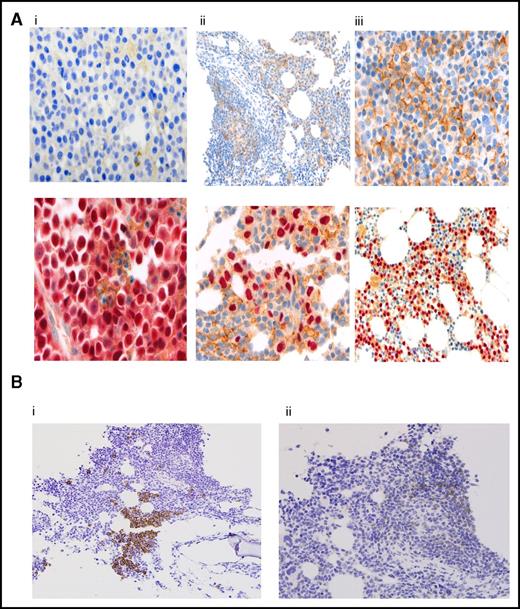
Figure 4A shows expression patterns for PD-L1, as detected by immunohistochemical staining, from pretreatment BM biopsies in 29 patients; it was negative in (10 [34%]) patients, weakly positive in (6 [21%]) patients, and positive in (13 [45%]) patients; the results correlated with ORR at 60%, 50%, and 77%, respectively. Responses ≥ VGPR or better were more frequent in PD-L1-positive patients compared with negative and weakly positive patients (54% vs 20%, P = .06); but was of marginally statistical significance. There was no correlation between PD-L1 staining and PFS, Table 3.
Correlative studies. (A) PD-L1 expression by immunohistochemistry in myeloma cells. Results are reported as percentage of myeloma cells showing membranous staining for PD-L1 (proportion score). Shown are BM samples with a proportion score of <1% (i), 1% to 49% (ii), and >50% (iii). The lower panels show corresponding MUM1 stain. PD-L1 stain is shown by the presence of brown chromogen, and the red color is MUM1 stain for myeloma cells. (B) CD3 and PD-1 expression by immunohistochemistry on T lymphocytes. CD3 staining (i) and PD-1 staining (ii). BM infiltrating T lymphocytes were graded as negative (<5% CD3, <1% PD-1) or positive (≥5% CD3, ≥1% PD-1). Original magnifications ×100 (Aii, upper panel; Aiii, lower panel; and Bi-ii), ×200 (Ai-ii, upper panels), and ×400 (Ai-ii, lower panels).
Correlative studies. (A) PD-L1 expression by immunohistochemistry in myeloma cells. Results are reported as percentage of myeloma cells showing membranous staining for PD-L1 (proportion score). Shown are BM samples with a proportion score of <1% (i), 1% to 49% (ii), and >50% (iii). The lower panels show corresponding MUM1 stain. PD-L1 stain is shown by the presence of brown chromogen, and the red color is MUM1 stain for myeloma cells. (B) CD3 and PD-1 expression by immunohistochemistry on T lymphocytes. CD3 staining (i) and PD-1 staining (ii). BM infiltrating T lymphocytes were graded as negative (<5% CD3, <1% PD-1) or positive (≥5% CD3, ≥1% PD-1). Original magnifications ×100 (Aii, upper panel; Aiii, lower panel; and Bi-ii), ×200 (Ai-ii, upper panels), and ×400 (Ai-ii, lower panels).
PD-1 expression on lymphocytes was analyzed in 38 patients: 6 patients (16%) were CD-3+/PD-1+, 10 (26%) were CD3+/PD-1−, and 22 (58%) had no detectable T-cell infiltrate (CD-3−/PD-1−) (Figure 4B). There was no significant correlation between PD-1 expression on marrow infiltrating lymphocytes and ORR, but there was a trend, marginally significant, for longer PFS irrespective of PD-1 expression at 16.5 months (95% CI, 10.6-NR) vs 6.3 months (95% CI, 2.6-NR), respectively (P = .05) (Table 3).
Discussion
The PD-1–blocking antibody pembrolizumab in combination with pomalidomide is a highly active combination for patients with RRMM with a favorable safety profile. The clinical activity is much higher than that seen in other pomalidomide and dexamethasone studies. In the NIMBUS trial, 302 heavily treated patients (5 lines of prior therapy) were randomized to pomalidomide and low-dose dexamethasone with an ORR of 32% and a median PFS of 4.0 months (95% CI 3.6-4.7).22 In the STRATUS study, among 682 patients with a median number of 5 prior regimens and mostly double refractory (80.2%) who received pomalidomide and low-dose dexamethasone, after a median follow-up of 16.8 months, the ORR was 32.6%, and median PFS was 4.6 months.23 In 50 high-risk RRMM patients defined by adverse cytogenetics (deletion 17p and translocation [4;14]) who had a median of 3 lines of prior therapy, pomalidomide and dexamethasone, resulted in an ORR of 22% and PFS of 2.8 months.24 These results compare favorably to our high-risk cytogenetically defined patients (n = 27); we reported an ORR of 56% and a median PFS of 15.1 months. The addition of cyclophosphamide to pomalidomide and dexamethasone improved ORR to 60% (only 1 patient achieved CR and 3 achieved VGPR), and the median PFS was 10 months in patients with a median of 4 lines of prior therapy.25 In another pomalidomide, cyclophosphamide-prednisone study, patients who had 3 lines of prior therapy had an ORR of 51% and median PFS of 10.4 months.26 Similarly, the addition of carfilzomib to pomalidomide resulted in an ORR of 50% (no CRs; 5 of 32 achieved VGPR) and median PFS of 7.2 months.27 With the understanding of the limitation of comparing different studies, the clinical data from the current study support a synergistic activity between pomalidomide and pembrolizumab that led to high ORRs (60%) that were remarkably durable (median PFS 17 months). The lack of single-agent activity for the PD-1 inhibitor, nivolumab, in MM patients suggests that myeloma is refractory to anti–PD-1 therapy.12 Patients in the current study had a median of 3 lines of prior therapy, which alone cannot account for the improved outcome because 90% were refractory to lenalidomide, 80% were refractory to proteasome inhibitors (bortezomib and or carfilzomib), and 73% were double refractory. In addition, there are emerging data that the addition of pembrolizumab can restore sensitivity in pomalidomide refractory patients. In a small retrospective study, there was 30% PR (3 of 9 patients) in pomalidomide-refractory patients.28 In the keynote-23 study (NCT02036502) presented in abstract form, the combination of lenalidomide with pembrolizumab and low-dose dexamethasone showed impressive results in RRMM patients; with a median follow-up of 9·7 months, 20 of 40 (50%) evaluable patients responded to treatment, including sCR (1 [3%]), VGPR (5 [13%]), and PR (14 [35%]).29
The regimen had an acceptable safety profile in patients with RRMM, and the toxicities noted in the current study were identical to what has been reported with pomalidomide and dexamethasone in phase 2 and 3 trials except for the autoimmune effects. Nearly all patients treated with pomalidomide and low-dose dexamethasone experienced at least 1 adverse reaction (99%).30 The most common adverse reactions in the NIMBUS trial included neutropenia (51%), fatigue (47%), upper respiratory tract infection (31%), dyspnea (25%), pneumonia (19%), grade 3 or 4 adverse reactions including neutropenia (48%), thrombocytopenia (22%), and pneumonia (16%).22 In our study, there was a 42% incidence of grade 3 neutropenia associated with increased risk of infections. Pneumonia grade 3 was noted in 15%, which is similar to what is reported in the NIMBUS trial (16%) and also from the STRATUS trial (11%).22,23 Influenza A (n = 4 [8%]) occurred in patients who received the influenza vaccine while on study; PD-1 inhibition may affect humoral response to vaccines by affecting the quantity and quality of long-lived plasma cells.31
Pneumonitis occurred at a similar rate to that seen with PD-1 inhibitors in solid tumors. In a recent study of 915 patients who received anti–PD-1 antibodies as a monotherapy, pneumonitis developed in 5% of patients, whereas those receiving combination immunotherapy had a 10% incidence of pneumonitis.32 In our study, only 1 patient had grade 3 pneumonitis. All 6 patients responded to drug interruption alone (n = 3) or after a short (2 week) course of steroids (n = 3); 2 patients had recurrent episodes and stopped therapy. None of the cases resulted in infection, compromised lung function, or death. The incidence of pneumonitis in the current study is similar to what is reported for PD-1 antibody therapy, and surprisingly, not much higher despite the combination with pomalidomide, which has been associated with pneumonitis.33 Other autoimmune effects were grade 1 and 2 and were easily managed with hormone therapy and supportive care.
The biomarker analysis suggests that ORR to pomalidomide and pembrolizumab seems to correlate with PD-L1 expression on myeloma cells. This study is the first to establish a new method for IHC staining of PD-L1 in BM biopsies. The testing was conclusive in 29 patients. Similarly, analysis of marrow infiltrating lymphocytes for PD-1 was successfully performed in 38 patients suggests that T-cell infiltration, independent of PD-1 expression, correlates with PFS. The data are preliminary and need confirmation in a larger cohort with additional studies assessing other features of the marrow microenvironment (DCs, NK cells, etc) to fully understand the mechanism of action.6,9 So far, none of the biomarkers examined (PD-L1 on myeloma cells and PD-1 on T cells) are adequately standardized to guide clinical decisions on use of PD-1 inhibitors in MM; nevertheless, it is encouraging to observe clinical responses and biomarker data associations that are similar to what is observed in solid tumor experience.34-37
In conclusion, combination immunotherapy with antibodies targeting the PD-1/PD-L1 pathway and IMiDs provides a novel therapeutic approach for patients with MM refractory to current available therapies. The combination of pembrolizumab, pomalidomide, and low-dose dexamethasone shows promising durable clinical antimyeloma activity; the results are encouraging and support an ongoing phase 3 trial in patients with RRMM (NCT02576977). These promising results emphasize the therapeutic potential of immune cells in MM.
Note added in proof
On 12 June 2017, one of the authors (A.B.) received a notification that Merck, in agreement with the Food and Drug Administration (FDA), is suspending enrollment for randomized trials of pembrolizumab with pomalidomide and lenalidomide in relapsed and newly diagnosed multiple myeloma, respectively, based on an External Data Monitoring Committee recommendation due to an imbalance of deaths in the pembrolizumab arms. Patients enrolled in these 2 trials continued therapy per protocol. On 6 July 2017, the FDA notified the same author that the clinical trial presented here had been placed on full clinical hold and that study treatment should be discontinued for all patients. The letter states, “The data available at the present time indicate that the risks of pembrolizumab plus pomalidomide and dexamethasone outweigh any potential benefit for patients.”
In our study, the combination of pembrolizumab and pomalidomide had significant clinical activity with durable responses in a subset of patients. As of 11 July 2017, 12 (25%) patients continued on the study, many into the third year. However, we were also aware of higher-than-expected rates of infection and autoimmunity. We were able to manage these events with close monitoring by a dedicated research team and strict dose modification measures for side effects as outlined in this paper.
The exact reason for the imbalanced death rate in the pembrolizumab arms remains speculative until Merck releases the data. But the results raise a concern about using immunotherapy that benefits a yet-to-be-defined subset of patients, especially in the newly diagnosed patients, who theoretically have a robust immune system and are at risk for more immunotoxicities. As a consequence, 10 patients who are receiving this therapy without any adverse events were notified of the FDA's decision and study procedures were terminated. Patients will continue pomalidomide and low-dose dexamethasone until progression, and all will be monitored for safety. A careful analysis of the causes of the side effects observed in these trials will be mandatory to define the future (if any) of checkpoint inhibitors combined with IMiDs in myeloma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors especially thank the patients and their families, study coordinators, and support staff; Maria Baer for the critical review of the paper; and the Merck & Co, Inc multiple myeloma team.
This is an investigator-initiated study with funding from Merck & Co, Inc for research support and correlative studies (A.B.). A.L. receives grant support from the Sawiris Family Foundation. Merck also provided pembrolizumab free to participants.
Authorship
Contribution: A.B. and O.G. designed the study; A.B., N.M., A.P.R., and M.K. enrolled patients and followed patients in the study; E.H. and Z.S. performed the IHC analysis for PD-L1; A.L. and A.D. performed IHC analysis for PD-1; T.M., E.L., S.P., and C.D. collected the data; O.G. performed the statistical analysis; A.B. wrote the first draft; and all authors provided critical revisions, gave final approval, and agreed to be accountable for the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ashraf Badros, University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, 22 South Greene St, Baltimore, MD 21201; e-mail: abadros@umm.edu.