In this issue of Blood, Badros et al bring the checkpoint inhibitor wave to multiple myeloma (MM), presenting clinical evidence supporting a role for T-cell–mediated immunity in controlling myeloma plasma cells.1
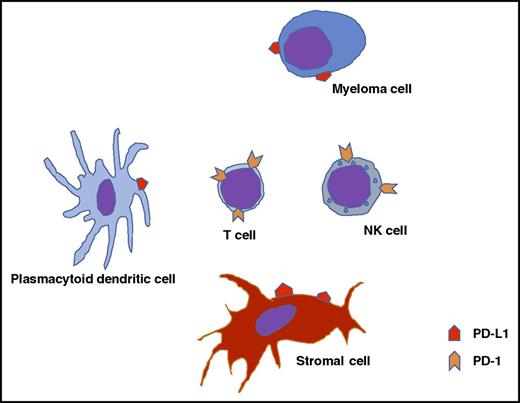
Potential interactions between myeloma cells, the microenvironment, and immune effector cells.
Potential interactions between myeloma cells, the microenvironment, and immune effector cells.
In a phase 2 trial, 48 patients with relapsed MM who had a median of 3 prior therapies were treated with the programmed death 1 (PD-1) inhibitor pembrolizumab combined with pomalidomide and dexamethasone. Despite extensive prior therapy (73% refractory to both a proteasome inhibitor and an immune modulatory drug [IMiD] and 70% with prior autologous transplant), overall response was 60% including deep responses (≥ very good partial response [VGPR]) in 27%. The responses were durable, with a response duration of nearly 15 months. These results are concordant with those of the combination of pembrolizumab and lenalidomide in relapsed MM, further confirming the efficacy of this combinatorial approach.
Harnessing the immune system to control the disease is not a new concept in MM, though it has had limited success in MM, unlike some other cancers.2 Multiple aspects of the immune system are compromised particularly in relapsed disease, and have been blamed for the failures. These include reduced expression of tumor antigens and HLA molecules by the malignant plasma cell (PC), enhanced expression of inhibitory ligands such as PD-1 ligand (PD-L1) by PCs, and recruitment of regulatory T cells and myeloid-derived suppressor cells, all of which can contribute to immune suppression. The PD-1/PD-L1 pathway is a negative costimulatory pathway that leads to a T-cell exhaustion phenotype that prevents adequate cellular response to foreign antigens and appears to be relevant in MM (see figure). The recent success with checkpoint inhibitors in solid tumors and lymphomas has spurred interest in exploring this pathway as a therapeutic strategy in MM.3 Increased expression of PD-1 on T cells has been described in the setting of MM with associated “exhausted” T-cell phenotype.4,5 In vitro and in vivo work show enhanced expression of PD-L1 on MM cells, suggesting a potential role for PD-1/PD-L1 inhibitors in treating MM. Expression of PD-L1 on MM may be driven by various factors, with increased expression seen in the context of hyperdiploidy in the MM cells, among MM cells with a higher proliferation rate and in the setting of stimulation with interferon-γ and interleukin-6. PD-L1 expression on MM cells appears to be increased in the setting of relapsed/refractory disease and associated with the presence of high tumor burden in the marrow and increased serum lactate dehydrogenase levels. Natural killer (NK) cells from MM patients have been shown to express PD-1, and engagement by PD-L1 on primary MM cells can downregulate the NK-cell vs MM effect.6 The plasmacytoid dendritic cells in MM also express PD-L1 and may contribute to the T-cell exhaustion phenotype observed.7 Finally, the high mutational load in the MM cell may make these cells particularly sensitive to immune-based approaches, as has been observed in other solid tumors.8
Despite strong laboratory evidence supporting immune-checkpoint blockade as a therapeutic strategy in MM, the initial trial of the PD-1 inhibitor nivolumab was disappointing. In a phase 1 study of 27 patients with relapsed refractory MM, no objective responses were noted with single-agent nivolumab.9 Given this background, the efficacy observed with the addition of an IMiD to pembrolizumab, while surprising, has been consistent across two phase 2 studies, including the one by Badros et al in this issue. In a phase 1/2 study with pembrolizumab with lenalidomide and dexamethasone, Mateos and colleagues studied 50 patients (17 dose escalation, 33 at maximum tolerated dose level) with relapsed disease who had at least 2 prior lines of therapy.10 Overall response rate was 50% including 4 VGPRs (2 in lenalidomide-refractory) and 9 partial responses (3 in lenalidomide-refractory), with a median duration of response of 9.7 months. Based on these results, there are 2 ongoing large phase 3 trials of pembrolizumab in combination with lenalidomide (for newly diagnosed MM) or pomalidomide (for relapsed MM). These studies will provide conclusive evidence regarding the role of pembrolizumab (and other PD-1 inhibitors) in the treatment of MM. Overall, the combination was well tolerated with manageable toxicities. Specifically, autoimmune adverse events from pembrolizumab were grades 1 and 2 and included pneumonitis, hypothyroidism, adrenal insufficiency, hepatitis, and vitiligo.
Clearly, much remains to be learned regarding the role of PD-1/PD-L1 in the treatment of MM and the specific mechanisms at play. The efficacy seen in combination with IMiDs, especially in the context of no activity with the single-agent PD-1 inhibitor, suggests a beneficial, and likely an immunomodulatory, effect of this class of drugs. It is also possible that the antimyeloma drugs lead to MM cytotoxicity and the consequent release of tumor antigens is playing a role in the observed efficacy. This question is being addressed through combinations of PD-1/PD-L1 inhibitors with other MM drugs such as proteasome inhibitors as well as in combination with radiation to plasmacytomas. This therapeutic approach may be particularly beneficial in the setting of minimal residual disease, where a more robust immune system is likely to be more amenable to modulation by the checkpoint inhibitors. In addition to various combinations and disease settings, several other checkpoint inhibitors are being introduced though clinical trials, including those targeted toward LAG-3 and CTLA4. The ability to combine pembrolizumab with new monoclonal antibodies such as daratumumab is being explored in clinical trials as well.
Finally, as the treatment choices for MM increase and the biological as well as financial toxicity from these drugs become problematic, the ability to select the most effective agent(s) for a given patient becomes more relevant. In the setting of checkpoint inhibitors, available data suggest that expression of the receptors and ligands can serve as biomarkers for response to treatment. The authors of the current study examined PD-L1 expression on myeloma cells and noticed a trend toward a higher rate of VGPR or better in PD-L1–positive patients compared with negative and weakly positive patients (54% vs 20%). PD-1 expression on the lymphocytes correlated weakly with the extended progression-free survival in a small smaller subset of patients. This is an important area of investigation and can significantly enhance the cost–toxicity benefit ratio for this therapeutic approach. This is particularly relevant given the potential for toxicity with immune therapies, as has been highlighted by the termination of the pembrolizumab trials in combination with lenalidomide or pomalidomide secondary to the higher death rate seen in the investigational arms. This underscores the importance of clinical experience in using this class of drugs and the ability to identify toxicities early and institute appropriate management. Development of tools that helps identify patients likely to respond as well as those who are likely to develop specific toxicities will lead to safer and effective use of these modalities.
Conflict-of-interest disclosure: S.K.K. has participated in advisory boards for Takeda, Celgene, Amgen, Janssen, AbbVie, Merck, and Skyline Dx and receives clinical trial funding from Takeda, Janssen, AbbVie, Merck, Celgene, Sanofi, and Roche.