Key Points
PVD is an active combination in relapsed lenalidomide-refractory MM patients.
PVD with weekly bortezomib offers a simpler, more convenient, and well-tolerated regimen option.
Abstract
This phase 1/2 trial evaluated the maximum tolerated doses, safety, and efficacy of pomalidomide, bortezomib, and dexamethasone (PVD) combination in patients with relapsed lenalidomide-refractory multiple myeloma (MM). In phase 1, dose level 1 consisted of pomalidomide (4 mg by mouth on days 1 to 21), IV or subcutaneous bortezomib (1.0 mg/m2 on days 1, 8, 15, and 22), and dexamethasone (40 mg by mouth on days 1, 8, 15, and 22) given every 28 days. Bortezomib was increased to 1.3 mg/m2 for dose level 2 and adopted in the phase 2 expansion cohort. We describe the results of 50 patients. Objective response rate was 86% (95% confidence interval [CI], 73-94) among all evaluable patients (stringent complete response, 12%; complete response, 10%; very good partial response, 28%; and partial response, 36%) and 100% among high-risk patients. Within a median follow-up of 42 months, 20% remain progression free, 66% are alive, and 4% remain on treatment. Median progression-free survival was 13.7 months (95% CI, 9.6-17.7). The most common toxicities were neutropenia (96%), leukopenia (84%), thrombocytopenia (82%), anemia (74%), and fatigue (72%); however, the majority of these were grade 1 or 2. The most common grade ≥3 toxicities included neutropenia (70%), leukopenia (36%), and lymphopenia (20%). Deep vein thrombosis occurred in 5 patients. In conclusion, PVD is a highly effective combination in lenalidomide-refractory MM patients. Weekly administration of bortezomib enhanced tolerability and convenience. Toxicities are manageable, mostly consisting of mild cytopenias with no significant neuropathy. This trial was registered at www.clinicaltrials.gov as #NCT01212952.
Introduction
Multiple myeloma (MM) is a heterogeneous disease characterized by the clonal proliferation of plasma cells producing immunoglobulins.1 The clonal plasma cell in MM is primarily confined to the bone marrow and highly dependent on its microenvironment.2 The introduction of novel agents, such as the immunomodulatory drugs (IMiDs) lenalidomide or pomalidomide and the proteasome inhibitor bortezomib, has changed the MM treatment paradigm and markedly improved survival.3 The precise mechanism of action of IMiDs remains unclear; however, disruption of MM–microenvironment interactions plays a major role in the antimyeloma activity of IMiDs.4 Proteasome inhibitors not only exert a direct cytotoxic effect in the MM cells but also target the bone marrow microenvironment and seem to have a synergetic effect with IMiDs.5
The success of the combination regimen lenalidomide, bortezomib, and dexamethasone6 prompted interest in combining pomalidomide with proteasome inhibitors and dexamethasone. The choice of pomalidomide is especially interesting owing to the synergistic antiproliferative activity of pomalidomide and dexamethasone in lenalidomide-resistant MM cells.7 The phase 1 trial MM-005 phase8 of twice-weekly bortezomib with pomalidomide and dexamethasone showed promising results, with an overall response rate (ORR) of 75%, including 30% who achieved complete or very good partial response. That trial used escalating doses of pomalidomide doses in conjunction with twice-weekly bortezomib and dexamethasone given the day of and the day after each dose of bortezomib. A phase 3 trial post-hoc analysis comparing weekly versus twice-weekly bortezomib dosing showed equivalent efficacy but significantly decreased peripheral neuropathy, with the weekly schedule of bortezomib.9
Therefore, building on these promising results, we decided to explore a simpler, more convenient, and potentially better tolerated schedule. This phase 1/2 trial was designed to evaluate the safety and efficacy of the combination of pomalidomide, once-weekly bortezomib, and dexamethasone.
Patients and methods
Study design and objectives
This was a prospective, nonrandomized, multicenter, open-label, investigator-initiated, phase 1 and 2 clinical trial. The primary goal of phase 1 was to determine the maximum tolerated dose (MTD) of bortezomib (2 dose levels [DLs]) in combination with pomalidomide and dexamethasone. The phase 2 expansion cohort at the bortezomib MTD primary goal was to evaluate the confirmed objective response rate (partial response [PR], very good partial response [VGPR], and complete response [CR]) of pomalidomide, bortezomib, and dexamethasone (PVD) in patients with relapsed or refractory myeloma. Secondary goals included assessments of overall survival (OS), progression-free survival (PFS), duration of response, and toxicity. This study was approved by the Mayo Clinic Institutional Review Board in accordance with federal regulations and the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as #NCT01212952.
Eligibility
Patients were eligible to participate in the study if they had previously treated lenalidomide-refractory MM requiring therapy. Refractory disease was defined as relapse on or within 60 days of stopping treatment. Patients were required to have measurable disease defined by one of the following: (1) serum monoclonal protein (M-protein) ≥10 g/L, (2) urine M-protein ≥200 mg/24 hours, (3) serum immunoglobulin free light chain (FLC) >10 mg/dL and an abnormal FLC ratio, (4) measurable soft tissue plasmacytoma not previously radiated, or (5) monoclonal bone marrow plasmacytosis ≥30%. Furthermore, a platelet count ≥75 × 109/L, an absolute neutrophil count ≥1.0 × 109/L, and a creatinine ≤221 μmol/L (2.5 mg/dL) were required. All previous cancer therapy, including chemotherapy and investigational agents, must have been discontinued ≥2 weeks prior to study registration.
Exclusion criteria included uncontrolled infection, another active malignancy, deep vein thrombosis not on therapeutic anticoagulation, Eastern Cooperative Oncology Group performance score ≥3, peripheral neuropathy grade >2, pregnant or nursing women, women of child-bearing potential who were unwilling to use a dual method of contraception, and men who were unwilling to use a condom.
Treatment schedule
Pomalidomide was given orally at a dose of 4 mg daily on days 1 to 21 of a 28-day cycle. Dexamethasone was given orally at a dose of 40 mg daily on days 1, 8, 15, and 22 of each cycle. During the phase 1, the 2 DLs of bortezomib evaluated were IV 1.0 mg/m2 and 1.3 mg/m2 on days 1, 8, 15, and 22 of each cycle. The bortezomib MTD was defined as the DL below the lowest dose that induces dose-limiting toxicities (DLT) in at least one-third of patients (at least 2 of a maximum of 6 patients) or the highest DL tested if all DLs were found to be safe. DLTs included any of the following treatment-related events that occurred during the first cycle: grade 4 decreased in the absolute neutrophil count or platelet count for ≥7 days, grade 4 infection, grade ≥3 febrile neutropenia, or any grade ≥3 nonhematologic adverse event (AE) as per the NCI’s Common Terminology Criteria for Adverse Events, version 4.10 Once the MTD of bortezomib was established (1.3 mg/m2 intravenously), patients were enrolled in the phase 2 expansion cohort. After 8 cycles of the 3-drug combination, bortezomib and dexamethasone were discontinued and patients were allowed to continue single-agent pomalidomide for 21 days per cycle until disease progression or significant toxicity. After the majority of patients were enrolled, an interim protocol amendment allowed the use of a subcutaneous route for bortezomib administration.
Patients received aspirin 325 mg once daily for thromboprophylaxis or were allowed to substitute full-dose anticoagulation with either low-molecular-weight heparin or warfarin at physician discretion. All patient received varicella zoster prophylaxis with acyclovir or an equivalent antiviral. Dose adjustments of PVD were permitted based on toxicity according to a standard prespecified dose-modification algorithm (see supplemental Material, available on the Blood Web site). All concomitant drugs for supportive care were allowed, as indicated, apart from systemic steroids and antineoplastic therapies with antimyeloma effects. Bisphosphonates were administered according to established guidelines. Among patients who had a previous dose reduction, escalation was allowed as long as there was no current grade 3 or 4 toxicity.
Response and toxicity criteria
Responses were assessed according to the International Myeloma Working Group criteria.11 A PR was defined as ≥50% reduction in the level of the serum M-protein and reduction in 24-hour urinary M-protein excretion by ≥90% or to <200 mg or as ≥50% reduction in bone marrow plasma cells, provided the baseline bone marrow involvement was ≥30% and it was the only measurable parameter. In addition to the above criteria, if a plasmacytoma was present at baseline, ≥50% reduction in the size of soft tissue plasmacytomas was also required. CR required complete disappearance of the serum and urine M-protein by immunofixation studies and <5% plasma cells on bone marrow examination. Stringent complete response (sCR) required CR as defined above plus normal FLC ratio and absence of clonal cells in bone marrow by immunohistochemistry or immunofluorescence. VGPR required, in addition to criteria for PR, serum and urine M-protein detectable only by immunofixation or ≥90% reduction in serum M-protein and 24 hour urine M-protein <100 mg. In patients whose only measurable disease was by serum FLC levels, CR required a normal FLC ratio of 0.26 to 1.65 in addition to CR criteria listed above. VGPR in such patients was defined as a >90% decrease in the difference between involved and uninvolved FLC levels. Disease progression required any one of the following criteria: (1) increase in serum M-protein ≥25% above the lowest response level and an absolute increase of more than 5 g/L, (2) increase in urine M-protein ≥25% above the lowest remission value and an absolute increase in excretion ≥200 mg/24 hours, (3) increase in size of soft tissue plasmacytoma by ≥50% or appearance of a new plasmacytoma, (4) definite appearance of new bone lesions or increase in the size of existing bone lesions by ≥50%, or (5) unexplained hypercalcemia >2.875 mmol/L (>11.5 g/dL). All response categories required 2 consecutive assessments made at any time for confirmation before the institution of any new therapy.
The NCI Common Terminology Criteria for Adverse Events, version 4, was used to grade AEs as well as to assign perceived attribution to the study treatment regimen.10 Toxicity was defined as an AE classified as being possibly, probably, or definitely related to study treatment.
Statistical analysis
The phase 1 portion of the study was designed to determine the MTD of this treatment combination. A total of 6 patients treated at the MTD were considered sufficient to identify common toxicities at the MTD. A standard 3 + 3 phase 1 study design was used. The phase 2 study was designed to evaluate the safety and efficacy of the treatment regimen. The primary endpoint for the phase II portion of this trial was confirmed objective response rate (patients who achieved a PR or better response). All patients who met the eligibility criteria, signed a consent form, and received at least 1 dose of therapy were considered evaluable for response and toxicity assessment. A 2-stage Fleming design was used to evaluate the confirmed objective response rate. A total of 42 evaluable patients were required to test the null hypothesis that the true objective response rate is at most 25%, versus the alternative hypothesis that the true objective response rate is at least 45%. At the final analysis, at least 15 responses were required in the first 42 evaluable patients to recommend further testing of this regimen in subsequent studies in this patient population. This study design had 90% power, with a 10% type I error rate.
Continuous and categorical data were summarized with descriptive statistics. The objective response rate was estimated by the number of responses divided by the total number of evaluable patients. A confidence interval for the objective response rate at the MTD DL was calculated according to the approach of Duffy and Santner. PFS was defined as the time from registration to the date of disease progression or death from any cause. OS was defined as the time from registration to death from any cause. The Kaplan-Meier method was used to analyze time-to-event end points, where differences between groups were assessed using log-rank statistics. Statistical analyses were conducted using SAS version 9.4.
Results
Patient characteristics
From March 2012 to July 2014, a total of 50 patients were accrued to the study (DL 1: 3 patients, DL 2: 6 patients, phase 2: 41 patients). The 6 phase 1 patients treated at the MTD were also included in the phase 2 portion, for a total of 47 patients in phase 2. All patients were evaluable. Patient characteristics and previous therapies at study entry are presented in Tables 1 and 2. The median number of prior regimens was 2 (range, 1-5) and all patients were refractory to lenalidomide therapy. Twenty-nine (58%) patients were also exposed to bortezomib prior to entering the study, including 5 (17%) bortezomib-refractory patients. Bortezomib was given intravenously in 39 (78%) patients, while the remaining received it subcutaneously. The median time from diagnosis to enrollment on study was 50 months (range, 15-142). Twenty-one (42%) patients were classified as intermediate or high risk by mSMART criteria (Table 1).
Baseline characteristics at PVD initiation
| . | Total (N = 50) . |
|---|---|
| Age, y | |
| Median (range) | 65.5 (45-83) |
| ≥65 | 29 (58) |
| Sex | |
| Male | 26 (52) |
| ECOG performance score | |
| 0 | 32 (64) |
| 1 | 17 (34) |
| 2 | 1 (2) |
| mSMART risk | |
| Standard | 29 (58) |
| Intermediate | 9 (18) |
| High | 12 (24) |
| FISH results | |
| Normal | 4 (8) |
| Abnormal* | 40 (80) |
| del 17p | 11 (22) |
| del 13 | 8 (16) |
| t(4;14) | 5 (10) |
| t(14;16) | 2 (4) |
| Other | 31 (62) |
| Not done/insufficient cells | 6 (12) |
| Cytogenetics result | |
| Normal | 29 (58) |
| Abnormal | 19 (38) |
| Deletion 13† | 7 (14) |
| Hypodiploidy† | 5 (10) |
| Not done | 2 (4) |
| . | Total (N = 50) . |
|---|---|
| Age, y | |
| Median (range) | 65.5 (45-83) |
| ≥65 | 29 (58) |
| Sex | |
| Male | 26 (52) |
| ECOG performance score | |
| 0 | 32 (64) |
| 1 | 17 (34) |
| 2 | 1 (2) |
| mSMART risk | |
| Standard | 29 (58) |
| Intermediate | 9 (18) |
| High | 12 (24) |
| FISH results | |
| Normal | 4 (8) |
| Abnormal* | 40 (80) |
| del 17p | 11 (22) |
| del 13 | 8 (16) |
| t(4;14) | 5 (10) |
| t(14;16) | 2 (4) |
| Other | 31 (62) |
| Not done/insufficient cells | 6 (12) |
| Cytogenetics result | |
| Normal | 29 (58) |
| Abnormal | 19 (38) |
| Deletion 13† | 7 (14) |
| Hypodiploidy† | 5 (10) |
| Not done | 2 (4) |
Values are reported as n (%) of patients unless otherwise indicated.
ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization.
A few patients were found to harbor ≥1 fluorescence in situ hybridization abnormality.
Most common cytogenetic abnormalities.
Prior treatment characteristics
| . | Total (N = 50) . |
|---|---|
| Number of prior regimens | |
| 1 | 14 (28) |
| 2 | 14 (28) |
| 3 | 12 (24) |
| 4 | 9 (18) |
| 5 | 1 (2) |
| Refractory to immediate prior therapy | |
| Yes | 13 (26) |
| Prior regimens | |
| IMiD | |
| Thalidomide | 10 (20) |
| Lenalidomide | 50 (100) |
| Proteasome inhibitors | |
| Bortezomib | 29 (58) |
| Other proteasome inhibitors | 17 (34) |
| Corticosteroids | 48 (96) |
| Alkylators | 28 (56) |
| Antimetabolites | 2 (4) |
| Vinca alkaloids | 1 (2) |
| Other | 4 (8) |
| Prior transplant | 36 (72) |
| Lenalidomide refractory | 50 (100) |
| . | Total (N = 50) . |
|---|---|
| Number of prior regimens | |
| 1 | 14 (28) |
| 2 | 14 (28) |
| 3 | 12 (24) |
| 4 | 9 (18) |
| 5 | 1 (2) |
| Refractory to immediate prior therapy | |
| Yes | 13 (26) |
| Prior regimens | |
| IMiD | |
| Thalidomide | 10 (20) |
| Lenalidomide | 50 (100) |
| Proteasome inhibitors | |
| Bortezomib | 29 (58) |
| Other proteasome inhibitors | 17 (34) |
| Corticosteroids | 48 (96) |
| Alkylators | 28 (56) |
| Antimetabolites | 2 (4) |
| Vinca alkaloids | 1 (2) |
| Other | 4 (8) |
| Prior transplant | 36 (72) |
| Lenalidomide refractory | 50 (100) |
Values are reported as n (%) of patients.
MTD
Nine patients were enrolled in the phase 1 portion of the study. No DLTs were observed at DL 1 (bortezomib 1 mg/m2). At DL 2 (bortezomib 1.3 mg/m2), 1 out of 6 patients was hospitalized for pancytopenia and infection but did not meet the definition of DLT. Dose level 2 was defined as the MTD and selected for the phase 2 part of this trial.
Efficacy
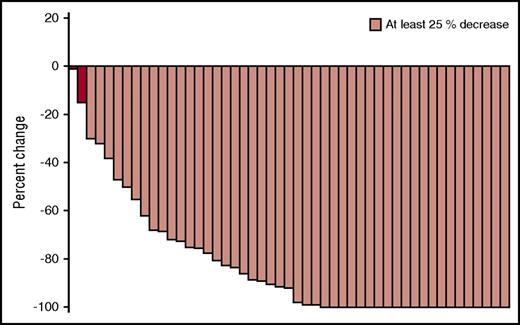
The median follow-up was 42 months (range, 13-59). The objective response rate for all 50 patients was 86% (95% confidence interval [CI], 73-94), including sCR in 6 patients (12%), CR in 5 patients (10%), VGPR in 14 patients (28%), and PR in 18 patients (36%). One patient (2%) achieved a minimal response, and 6 patients (12%) had stable disease (Figure 1). Among the 3 patients treated at DL 1, 2 patients responded and achieved an sCR. Among the 47 patients treated at MTD, 41 responded (ORR, 87%; 95% CI, 75-94), and the predefined criteria to conclude the regimen warrants further study was met. The objective response rate was similar between the intermediate-/high-risk group (90% objective response rate) and the standard risk group (83% objective response rate, P = .68). Among the 11 patients with deletion 17p, the objective response rate was 100% (95% CI, 72-100), including sCR in 1 patient (9%), CR in 2 patients (18%), VGPR in 4 patients (36%), and PR in 4 patients (36%). No significant difference in objective response rate was seen in bortezomib-refractory (80%) and bortezomib-sensitive patients (92%, P = .45). The median duration of response was 14.3 months (95% CI, 8.6-18.1).
Best response to PVD. Waterfall plot for all patients treated with PVD.
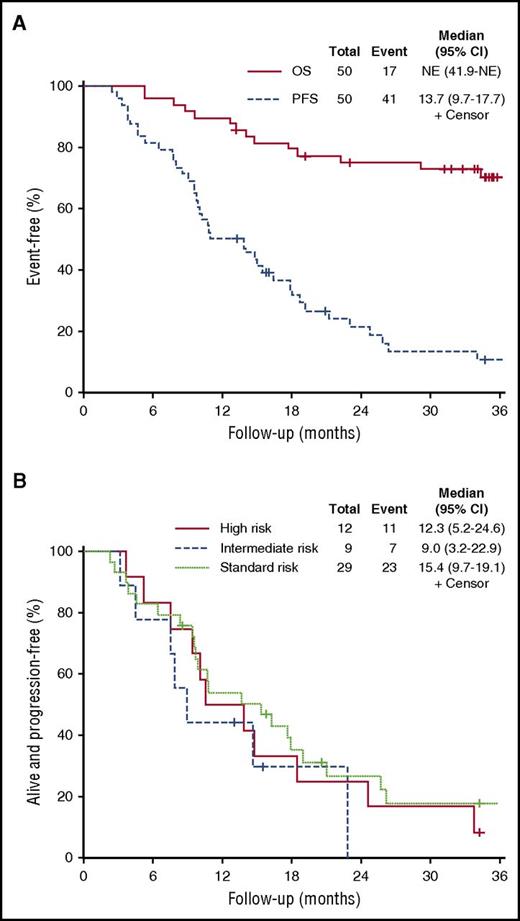
At the time of analysis, 40 patients (80%) had progressed and 17 were deceased (34%). The median PFS was 13.7 months (95% CI, 9.7-17.7). No difference was seen in median PFS among the high-risk group (12.3 months; 95% CI, 5.2-24.6), intermediate-risk group (9 months; 95% CI, 3.2-22.9), and standard-risk group (15.4 months; 95% CI, 9.5-19.1; P = .5). The median OS was not reached (Figure 2).
OS and PFS curves. (A) PFS and OS for all patients treated with PVD. NE, not evaluated. (B) PFS by mSMART risk groups.
OS and PFS curves. (A) PFS and OS for all patients treated with PVD. NE, not evaluated. (B) PFS by mSMART risk groups.
Safety and tolerability
Treatment was well tolerated overall, and AEs consisted primarily of myelosuppression. Grade ≥3 hematologic toxicities occurred in 74% of patients. Grade ≥3 nonhematologic toxicities were seen in 26% of patients and were less frequent than hematologic AEs. Commonly occurring grade ≥3 hematologic toxicities included neutropenia (70%), leukopenia (36%), lymphopenia (20%), anemia (4%), and thrombocytopenia (4%). The most common nonhematologic toxicity was fatigue (reported by 72% of patients), although grade ≥3 fatigue was reported in only 2 patients (4%).
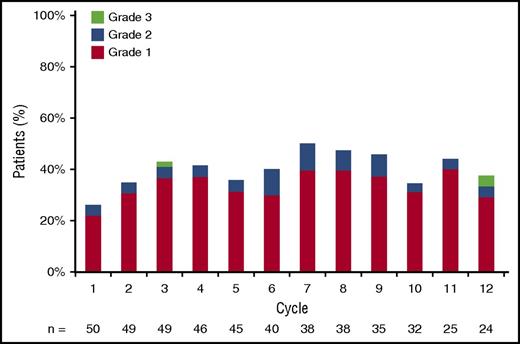
Peripheral sensory neuropathy toxicity of any grade was seen in 68% of patients. However, the majority of peripheral neuropathy was grade 1 (46%), and grade ≥3 peripheral neuropathy was reported in only 2 patients (4%). At baseline, 27 patients (54%) reported peripheral neuropathy, but worsening of symptoms was noted in only 7 patients. In addition, out of the 23 patients who did not report peripheral neuropathy at baseline, 17 developed peripheral neuropathy while on treatment. This is similar to what has been published with other bortezomib regimens and does not follow a cumulative pattern (Figure 3). Peripheral neuropathy rates were stable to slightly lower after discontinuation of bortezomib. Pomalidomide dose was adjusted in 21 patients (42%) at a median of 5 cycles (range, 2-32). The major reason for adjustment was neutropenia. Bortezomib was adjusted in 8 patients (16%). The major reason for adjusting bortezomib was neuropathy. Dexamethasone dose was adjusted in 14 patients (28%). There were 5 episodes of deep vein thrombosis (10%). Adverse events for all treatment cohorts are outlined in Table 3.
Peripheral sensory neuropathy by grade and cycle. Grade ≥1 peripheral sensory neuropathy at least possibly related to the intervention by cycle of treatment.
Peripheral sensory neuropathy by grade and cycle. Grade ≥1 peripheral sensory neuropathy at least possibly related to the intervention by cycle of treatment.
AEs
| . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ≥3 . | Any grade . | |
| Hematologic toxicity | |||||||
| Neutropenia | 8 | 18 | 58 | 12 | — | 70 | 96 |
| Leukopenia | 10 | 38 | 34 | 2 | — | 36 | 84 |
| Thrombocytopenia | 56 | 22 | 4 | — | — | 4 | 82 |
| Anemia | 36 | 34 | 4 | — | — | 4 | 74 |
| Lymphopenia | — | 16 | 10 | 10 | — | 20 | 36 |
| Nonhematologic toxicity | |||||||
| Fatigue | 30 | 38 | 4 | — | — | 4 | 72 |
| Peripheral neuropathy | 46 | 18 | 4 | — | — | 4 | 68 |
| Diarrhea | 36 | 10 | 2 | — | — | 2 | 48 |
| Nausea/vomiting | 40 | 2 | — | — | — | — | 42 |
| Lung infection | — | 6 | 8 | 2 | — | 10 | 16 |
| Thromboembolic event | 10 | — | — | — | — | — | 10 |
| Dyspnea | 2 | 4 | 2 | — | — | 2 | 8 |
| Insomnia | — | 8 | — | — | — | — | 8 |
| Herpes zoster infection | 2 | 2 | 2 | — | — | 2 | 6 |
| Generalized muscle weakness | — | 6 | — | — | — | — | 6 |
| Dizziness | — | 6 | — | — | — | — | 6 |
| Anxiety | — | — | 4 | — | — | 4 | 4 |
| Edema | — | 2 | 2 | — | — | 2 | 4 |
| Constipation | — | 4 | — | — | — | — | 4 |
| . | Grade . | ||||||
|---|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | ≥3 . | Any grade . | |
| Hematologic toxicity | |||||||
| Neutropenia | 8 | 18 | 58 | 12 | — | 70 | 96 |
| Leukopenia | 10 | 38 | 34 | 2 | — | 36 | 84 |
| Thrombocytopenia | 56 | 22 | 4 | — | — | 4 | 82 |
| Anemia | 36 | 34 | 4 | — | — | 4 | 74 |
| Lymphopenia | — | 16 | 10 | 10 | — | 20 | 36 |
| Nonhematologic toxicity | |||||||
| Fatigue | 30 | 38 | 4 | — | — | 4 | 72 |
| Peripheral neuropathy | 46 | 18 | 4 | — | — | 4 | 68 |
| Diarrhea | 36 | 10 | 2 | — | — | 2 | 48 |
| Nausea/vomiting | 40 | 2 | — | — | — | — | 42 |
| Lung infection | — | 6 | 8 | 2 | — | 10 | 16 |
| Thromboembolic event | 10 | — | — | — | — | — | 10 |
| Dyspnea | 2 | 4 | 2 | — | — | 2 | 8 |
| Insomnia | — | 8 | — | — | — | — | 8 |
| Herpes zoster infection | 2 | 2 | 2 | — | — | 2 | 6 |
| Generalized muscle weakness | — | 6 | — | — | — | — | 6 |
| Dizziness | — | 6 | — | — | — | — | 6 |
| Anxiety | — | — | 4 | — | — | 4 | 4 |
| Edema | — | 2 | 2 | — | — | 2 | 4 |
| Constipation | — | 4 | — | — | — | — | 4 |
Values are reported as percentage of patients. All AEs with attribution of possible, probable, or definite occurring in >1 patient are listed.
—, Not applicable.
Discussion
PVD showed remarkable efficacy in patients with relapsed lenalidomide-refractory MM. This phase 1/2 trial was the first to evaluate the combination of pomalidomide and dexamethasone with weekly bortezomib doses. In the phase 1 portion of this trial, the MTD was determined to be 1.3 mg/m2 bortezomib on days 1, 8, 15, and 22. The PVD regimen was highly active, even in high-risk MM patients, with an ORR of 86% and a median PFS of 13.7 months. Treatment was well tolerated, and most of the AEs were related to myelosuppression.
Pomalidomide is an IMiD analog of thalidomide and lenalidomide.12 However, despite similarities in the chemical structure, pomalidomide has a distinct immunomodulatory and antimyeloma activity and has demonstrated clinical and preclinical efficacy in lenalidomide-resistant MM.13-15 Despite the theoretical antagonism between IMiD and proteasome inhibitor mechanisms of action, clinical data support the observation of a synergistic effect with this combination of agents.16,17 The recently described CRBN-independent protein-degradation pathway in IMiD-treated MM cells could explain the synergy observed in the clinical setting.18
In the previous MM-005 phase 1 trial of PVD in relapsed and refractory MM, bortezomib was given intravenously on a twice-weekly schedule.8 The ORR in the MM-005 trial was 75%, with 30% of patients achieving at least a VGPR. In our study, a comparable activity of PVD regimen was demonstrated (86% ORR, with a VGPR or better response in 50% of patients) with weekly bortezomib dosing. A phase 3 randomized trial comparing pomalidomide, twice-weekly bortezomib, and low-dose dexamethasone with twice-weekly bortezomib and low-dose dexamethasone is ongoing (#NCT01734928). As expected, our results shows a more favorable ORR in comparison with that seen in more heavily pretreated patients who received pomalidomide and dexamethasone combination therapy.19
Multiple combinations of IMiD, proteasome inhibitor, and dexamethasone triplet regimens in relapsed and refractory MM have been studied in the recent years. In the phase 3 trial ASPIRE, the combination of lenalidomide, carfilzomib, and dexamethasone showed an ORR of 87%, with a median PFS of 26 months and grade ≥3 AE in 84% of patients.20 In the phase 3 trial TOURMALINE-MM1, the experimental arm with the combination of lenalidomide, ixazomib, and dexamethasone demonstrated an ORR of 78%, a median PFS of 20.6 months, and grade ≥3 AEs in 74% of patients. It is important to note that this trial did not include lenalidomide-refractory MM patients.21 The combination of lenalidomide, bortezomib, and dexamethasone also showed remarkable activity in a phase 2 trial, with an ORR of 64% and median PFS of 9.5 months in the relapsed and refractory setting.16 Finally, in a phase 1 trial of lenalidomide and bortezomib-refractory MM patients, the combination therapy of pomalidomide, carfilzomib, and dexamethasone showed an ORR of 50% and median PFS of 7.2 months, with 94% of patients experiencing a grade ≥3 AE.22 Although caution is warranted when comparing across trials, as the inclusion and exclusion criteria seldom match among different studies, our efficacy and safety results are numerically similar to other combinations of IMiDs, proteasome inhibitors, and dexamethasone in relapsed and refractory MM patients.
The landmark CASTOR23 and POLLUX24 trials showed improved duration of response in patients with relapsed or refractory MM with the addition of daratumumab, an anti-CD38 monoclonal antibody, to either bortezomib-dexamethasone or lenalidomide-dexamethasone. The comparison of those landmark trials with the current study is contentious. The POLLUX trial specifically excluded lenalidomide-refractory patients, and the CASTOR trial did not report the rate of lenalidomide refractoriness. Neither trial assessed a triplet regimen contain an IMiD, proteasome inhibitor, and dexamethasone such as the PVD regimen used in the current study.
Once-weekly bortezomib administration was shown to be as effective and better tolerated than twice-weekly dosing with various combination regimens for the treatment of MM. A small study of 63 patients treated with cyclophosphamide, IV bortezomib, and dexamethasone using either weekly or twice-weekly bortezomib dosing found a similar ORR (93% vs 88%) but fewer dose reductions and lower grade ≥3 toxicities (40% vs 60%), despite a higher cumulative bortezomib dose (6 mg/m2 vs 5.2 mg/m2) with the weekly schedule.25 The phase 3 GIMENA clinical trial of bortezomib, melphalan, and prednisone with or without thalidomide enrolled 139 patients in a twice-weekly bortezomib dosing before the protocol was amend to a weekly bortezomib schedule enrolling an additional 372 patients.9 In a post-hoc analysis comparing the weekly vs twice-weekly bortezomib dosing, both schedules seemed to be equally effective (ORR 85% vs 86%), with similar grade ≥3 hematologic AEs (44% vs 45%). The incidence of grade ≥3 nonhematologic AEs, however, was significantly reduced in the weekly bortezomib schedule (35% vs 51%), including a lower rate of peripheral neuropathy (8% vs 28%, P < .001).26 In this phase 1/2 trial, the most common grade ≥3 hematologic AE was neutropenia, which was seen in 70% of patients. This incidence of grade ≥3 neutropenia is similar to that previously reported with pomalidomide and dexamethasone combination therapy.27 Grade ≥3 peripheral neuropathy was noted in only 4% of patients. A small proportion of patients received subcutaneous bortezomib after a trial amendment; however, the number of patients who received subcutaneous bortezomib was too small for a meaningful subgroup analysis. Even though the use of thromboprophylaxis was mandatory in our study, the rate of venous thromboembolism events was higher than in previously reported clinical trials of pomalidomide and low-dose dexamethasone (10% vs 4%).28
In conclusion, PVD is a highly attractive option in patients with relapsed lenalidomide-refractory MM. PVD is an appealing second-line option in many patients given the well-accepted use of lenalidomide-containing regimens in the frontline setting and lenalidomide maintenance therapy after autologous stem cell transplant. The noteworthy difference in OS and PFS suggests that patients can easily be rescued with subsequent treatment regimens after progressing on PVD. Remarkable activity was also seen in patients with high-risk disease as classified by mSMART criteria. Weekly administration of bortezomib enhanced tolerability and convenience to this regimen. Severe toxicities are manageable, mostly consisting of myelosuppression, and comparable to other IMiD, proteasome inhibitor, and dexamethasone combination regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported in part by the National Institutes of Health, National Cancer Institute (grant CA186781).
Authorship
Contribution: J.R.M., S.K., M.A.G., S.R.H., F.K.B., A.D., J.A.L., P.K, N.L., S.J.R., D.D., R.S.G., Y.L., W.I.G., R.F., P.L.B., V.R., T.S., A.A.C.-K., S.A., A.K.S., C.B.R., P.G.R., S.V.R., M.Q.L. recruited patients and did the study research; B.R.L. and A.E.H. performed the biostatistical analyses; J.P., B.R.L., A.E.H., and M.Q.L. wrote the manuscript and analyzed the data; M.Q.L., J.R.M., V.R., P.G.R. designed the study; and all authors reviewed and approved initial and final versions of the manuscript.
Conflict-of-interest disclosure: S.K. has received honoraria from Skyline Diagnostics. Research support from Abbvie, Celgene, Novartis, Amgen, Takeda, Sanofi, and Janssen has been provided to the Mayo Clinic for conduct of clinical trials on which S.K. serves as a principal investigator. M.A.G. has received research support from Ionis Pharmaceuticals and Prothena and honoraria from Celgene, Millennium Pharmaceuticals, and Novartis. A.D. has received research support from Celgene, Millenium, Pfizer, Jannsen, and Alnylam. P.K. has received research funding from Takeda (Mayo Clinic), Onyx (Mayo Clinic), and Celgene (Mayo Clinic) and consulting fees from Celgene and Sanofi (Mayo Clinic). D.D. has received research funding from Takeda, Karyopharm, and Amgen. Y.L. receives research funding from Janssen. P.L.B. has served as a consultant for Amgen and Jannsen. S.A. has served as a consultant for Novartis Pharmaceuticals, Amgen Pharmaceuticals, Pharmacyclics, Inc., and Takeda and has received research funding from Pharmacyclics, Inc. C.B.R. has received research support from Celgene, Novartis, and Millennium. M.Q.L. receives research funding from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Martha Q. Lacy, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: lacy.martha@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal