Key Points
Human IL-6 improves T-cell engraftment and serum IgG production in humanized mice.
IgG-switched memory B cells in IL-6 knock-in mice displayed a diverse antibody repertoire and high specificity against immunized antigen.
Abstract
Humanized mice are a powerful tool for the study of human hematopoiesis and immune function in vivo. However, the existing models cannot support robust adaptive immune responses, especially the generation of class-switched, antigen-specific antibody responses. Here we describe a new mouse strain, in which human interleukin 6 (IL-6) gene encoding the cytokine that is important for B- and T-cell differentiation was knocked into its respective mouse locus. The provision of human IL-6 not only enhanced thymopoiesis and periphery T-cell engraftment, but also significantly increased class switched memory B cells and serum immunoglobulin G (IgG). In addition, immunization with ovalbumin (OVA) induced OVA-specific B cells only in human IL-6 knock-in mice. These OVA-specific antibodies displayed the highest frequency of somatic mutation, further suggesting that human IL-6 is important for efficient B-cell activation and selection. We conclude that human IL-6 knock-in mice represent a novel and improved model for human adaptive immunity without relying on complex surgery to transplant human fetal thymus and liver. These mice can therefore be used to exploit or evaluate immunization regimes that would be unethical or untenable in humans.
Introduction
The adaptive immune system plays a central role in the pathogenesis of many diseases, such as cancer, autoimmune disorders, and infection. To study how T and B lymphocytes orchestrates the immune responses, scientists have used small vertebrates over the past decades. Because many aspects of mammalian biological systems, particularly their immune systems, are species specific,1 small-animal models that more closely recapitulate human immunity, such as “humanized” mice, are currently required.
To establish a functional human immune system that contains the multiple cell lineages required to provoke cellular and humoral activities, several models such as the severe combined immunodeficiency (SCID) mice engrafted with hematopoietic stem cells and the bone marrow (BM)-liver-thymus (BLT) model have been developed.2-4 However, the generation of class-switched, antigen-specific antibody responses by human B cells is still a major challenge. Although antigen-specific human IgM antibody responses are generated, the achievement of affinity maturation and class-switching from the IgM to the IgG isotype has been particularly difficult.5,6 Several reports demonstrated that antigen-specific human IgG can be detected in humanized mice by enzyme-linked immunosorbent assay (ELISA).7,8 The B-cell response was nevertheless not robust and the scarcity of IgG+ memory B cells makes them extremely difficult to be identified and isolated.
One explanation for the suboptimal B-cell responses may be a lack of adequate T-cell help.9,10 Inappropriate selection on mouse major histocompatibility complex molecules may contribute to the weak T-cell responses and insufficient interactions between B and T cells. In agreement with this notion, the expression of human HLA class II molecule in immune-deficient mice has marginally improved both T- and B-cell function.11 In the BLT model, although T-cell engraftment and human major histocompatibility complex-restricted T-cell function were enhanced due to thymocyte selection by implanted human thymic tissue, IgM remained the predominant antibody response,12,13 suggesting that other factors may be more important for B-cell maturation and antibody class switching.
Interleukin 6 (IL-6) was initially identified as a B-cell differentiation factor both in vivo and in vitro. It is capable of inducing the final maturation of B cells into immunoglobulin-secreting plasma cells.14 In fact, IL-6 has been shown to stimulate the secretion of antibodies to such a degree that serum IgG1 levels can rise 120- to 400-fold.15 Because murine and human IL-6 show 65% sequence homology at the DNA level and 42% homology at the protein level,16 and murine IL-6 is not active in human cells, we reasoned that physiological expression of human IL-6 in the mouse may result in improved B-cell differentiation and human antibody production. Therefore, we generated immunodeficient Rag2−/−Il2rg−/− mice, in which the gene encoding human IL-6 was knocked into its orthologous mouse locus. We found human IL-6 not only improves thymopoiesis and peripheral T-cell engraftment, but also significantly increases the level of total IgG and antigen-specific IgG. Consistent with enhanced antibody production, higher frequencies of memory B cells and IgG+ B cells, and lower frequencies of transitional and immature B cells were also detected. Furthermore, immunization with OVA induced OVA-specific B cells only in human IL-6 knock-in mice. These OVA-specific antibodies displayed the highest frequency of somatic mutation, further suggesting that human IL-6 is important for efficient antigen-specific B-cell activation and selection.
Materials and methods
Analysis of IL-6 expression
Total tissue RNA was purified and reverse-transcribed. The following primers were used for polymerase chain reaction (PCR) amplification: mIL-6 forward, AGTTGCCTTCTTGGGACTGA; mIL-6 reverse, CCTCCGACTTGTGAAGTGGT; hIL-6 forward, ATGCAATAACCACCCCTGAC; hIL-6 reverse, TAAAGCTGCGCAGAA-TGAGA; mouse glyceraldehyde-3-phosphate dehydrogenase (mGAPDH) forward, TGCACCACCAACTGCTTAGC; and mGAPDH reverse, GGAAGGCCATGCCAGTGA. Plasma concentrations of mouse and human IL-6 protein were detected with species-specific ELISA kits from R&D Systems according to the manufacturer’s instructions. Mice received 1 dose of intraperitoneal (IP) injections of 20 μg ultrapure lipopolysaccharide (LPS) Escherichia coli 0111:B4 (InvivoGen). Plasma was harvested 2 hours after the injection.
Transplantation of human hematopoietic progenitor cells into mice
Fetal liver samples were obtained from the Human Fetal Tissue Repository at Albert Einstein College of Medicine, Bronx, NY, and from Advanced Biosciences Resources, Alameda, CA. Tissues were cut in small fragments, treated for 45 minutes at 37°C with Collagenase D (100 ng/mL; Roche) and a cell suspension was prepared. Human CD34+ cells were purified by density gradient centrifugation, followed by positive immunomagnetic selection using anti-human CD34+ microbeads (Miltenyi Biotec). Newborn pups were sublethally irradiated with 180 cGy and 100 000 CD34+ cells were injected into the liver. The mice were bled 8 to 10 weeks post-engraftment to check for the reconstitution of the human immune system. More than 20 fetal liver samples were used and the same donor sample was transplanted for both human IL-6–positive and negative recipients to exclude variations that related to the donor. All experiments were performed in compliance with Yale University Human Investigation Committee protocol and Yale Institutional Animal Care and Use Committee protocols.
Flow cytometry
Cell suspensions were prepared from thymus, spleen, BM, lymph node (LN) (including mesenteric, axillary, and cervical LN), and the blood of mice 10- to 18-weeks posttransplantation. Samples were stained with fluorochrome-labeled monoclonal antibodies (mAbs) against mouse and human cell-surface antigens, and analyzed on an LSRII flow cytometer (BD Biosciences). Data were analyzed using FloJo software.
Immunization
A total of 100 μg of chicken OVA (Sigma-Aldrich) in 100 μL of phosphate-buffered saline (PBS) was mixed with an equal volume of Complete Freund’s Adjuvant (Difco); and 14-week-old humanized mice were immunized by IP injection. Two weeks later, mice were boosted with 100 μg of OVA in 100 μL of PBS mixed with an equal volume of Incomplete Freund’s Adjuvant (Difco). Seven to 10 days later, mice were bled to analyze the levels of antigen-specific immunoglobulins and boosted again with OVA + Incomplete Freund’s Adjuvant. Seven to 10 days after the last booster, the mice were euthanized and tissue samples were collected.
Analysis of total human immunoglobulin and antigen-specific IgG levels
Levels of human IgM and IgG were determined by ELISA. Multisorp plates (NUNC) were coated overnight at 4°C with rabbit polyclonal anti-human IgM or IgG (Southern Biotech). After washing and blocking the wells, diluted samples were applied for 2 hours at room temperature. Human serum with known concentrations of IgM and IgG (Bethyl Laboratories) was used as a standard. Secondary biotinylated rabbit polyclonal anti-human IgM or IgG and streptavidin-horseradish peroxidase were then sequentially added. ELISAs were developed using tetramethylbenzidine, and the reaction was stopped using stop solution. Plates were analyzed using a Bio-Rad plate reader.
Levels of OVA-specific antibodies were determined similarly to total immunoglobulin levels with a single change. Plates were coated with 50 μg/mL OVA in PBS overnight at 4°C instead of anti-human IgM/IgG antibodies.
Single-cell real-time (RT)-PCR and immunoglobulin gene sequencing
Single CD10−CD27+IgG+ B cells from spleens of immunized mice or from peripheral blood in healthy donors were sorted directly into 96-well PCR plates. Plates were sealed and immediately frozen on dry ice before storage at −80°C. IgH and Igλ and Igκ gene transcripts were amplified independently by nested PCR with primer mix (see supplemental Table 2, available on the Blood Web site). The RT-PCR protocol was carried out manually as previously published.17 Sequences were analyzed by IgBLAST comparison with GenBank to identify germ line V(D)J gene segments with highest identity.
Vector cloning and recombinant antibody production
Vector cloning and recombinant antibody production was carried out as previously published.17 Colonies were screened by PCR using 5′Absense as forward primer and 3′IgG internal, 3′Cκ, or 3′Cλ as reverse primer, respectively (supplemental Table 2). A total of 293 cells were transiently transfected with equal amounts of IgH and corresponding IgL chain expression vector DNA. Cell culture supernatants were harvested at day 11.
Polyreactivity and OVA ELISA
Antibody concentrations in supernatants were adjusted to 1 μg/mL and two consecutive 1:5 dilutions in PBS were prepared. ELISA plates were coated with 50 μL per well of individual antigens at concentrations of 5 μg/mL (insulin) or 10 μg/mL (double-stranded DNA, LPS), or 100 μg/mL (OVA) in PBS. Human recombinant insulin solution (Sigma), LPS from E coli serotype 055:B5 (Sigma), and double-stranded DNA from calf thymus (Sigma) were stored at +4°C. Positive control for polyreactivity is the recombinant human mAb ED3818 and for OVA is the mouse anti-OVA mAb TOSG1C6 (BioLegend). Negative controls for OVA reactivity were recombinant human mAbs originally cloned from B cells of 2 healthy human donors (supplemental Table 3). Antibody sequences cloned from humanized mice can be found in supplemental Tables 4 and 5. Bound antibodies were detected using 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid as substrate (BioRad). Antibodies were considered polyreactive if they bound to at least 2 different types of antigens (DNA, insulin, or LPS).
Statistical analysis
Statistical analyses were performed using the GraphPad Prism version 4.00 for Mac (GraphPad Software, San Diego, CA). Statistical significance was evaluated by one-way analysis of variance (ANOVA) using a Bonferroni test, two-way ANOVA using a Tukey’s multiple comparisons test, and unpaired two-tailed Student t test.
Results
Human IL-6 is expressed physiologically in the IL-6 knock-in mice
To replace the gene encoding mouse IL-6 by its human homolog, we applied VelociGene technology19 and designed a vector to replace the sequence encompassing the ORF of mouse IL-6 with its human counterpart, but to maintain the promoter and other regulatory elements (such as 5′ untranslated region) of mouse origin (supplemental Figure 1). This targeting construct was electroporated into F1 BALB/c × 129 Rag2+/−Il2rgY/− embryonic stem cells, and correctly targeted embryonic stem cell clones were identified by RT-PCR. After obtaining the chimeras, their progeny were then intercrossed to generate Rag2−/−Il2rg−/− mice with IL-6 wild-type (IL-6m/m), heterozygous knock-in (IL-6h/m), or homozygous knock-in (IL-6h/h) animals.
Since we have previously reported that mice bearing a transgene of human signal regulatory protein α (SIRPα) in Rag2−/−Il2rg−/− background showed improved engraftment of human hematopoietic cells and antigen-specific humoral immune responses,20 we then further crossed homozygous human IL-6 knock-in mice (Rag2−/−Il2rg−/−IL6h/h) with SIRPa knock-in mice (Rag2−/−Il2rg−/−SIRPαh/m, referred to as RG SKI), a newer SIRPa generation to ensure that the SIRPα gene is expressed at an approximately physiological level. The final Rag2−/−Il2rg−/−SIRPαh/mIL-6h/h mice are referred to RG SKI IL-6.
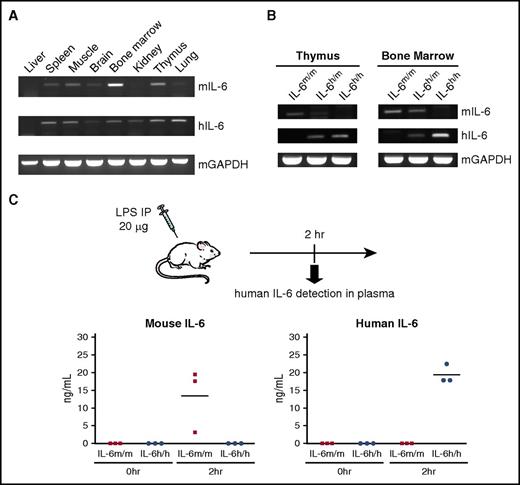
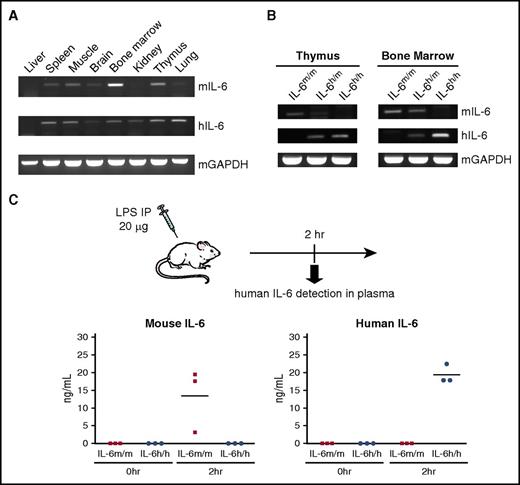
To determine whether human IL-6 is faithfully expressed, organs from a non-engrafted heterozygous knock-in (IL-6h/m) mouse were harvested and analyzed for murine and human IL-6 messenger RNA (mRNA) expression using primers that are species specific. We observed a similar pattern of expression for both mouse and human IL-6 mRNA in all analyzed organs by RT-PCR (Figure 1A). When we compared IL-6 expression in two tissues (thymus and BM) that showed relatively higher levels of basal mRNA in the steady state, we only detected the expression of mouse IL-6 in samples from IL-6m/m and IL-6h/m mice, whereas human IL-6 was expressed in IL-6h/m and IL-6h/h mice (Figure 1B). Because IP injection of LPS induces a marked increase in circulating IL-6 levels, we next measured the concentrations of IL-6 protein in the plasma of IL-6m/m and IL-6h/h mice 2 hours after LPS treatment and found the protein level of human IL-6 was comparable with mouse IL-6 (Figure 1C).
Validation of human IL-6 expression in knock-in mice. (A) RT-PCR analysis of human IL-6 (hIL-6) and mouse IL-6 (mIL-6) expression in different tissues of a Rag2−/−Il2rg−/−IL6h/m mouse. mGAPDH served as an endogenous control. (B) RT-PCR analysis of mIL-6 and hIL-6 expression in thymus (left) and BM (right) of Rag2−/−Il2rg−/−IL-6m/m, IL-6m/h, and IL-6h/h mice. (C) ELISA measurement of mouse (left) and human (right) IL-6 protein in plasma of non-engrafted IL-6m/m or IL-6h/h mice after LPS challenge (in ng/mL, n = 3). Each dot represents 1 mouse. Horizontal bars indicate mean values.
Validation of human IL-6 expression in knock-in mice. (A) RT-PCR analysis of human IL-6 (hIL-6) and mouse IL-6 (mIL-6) expression in different tissues of a Rag2−/−Il2rg−/−IL6h/m mouse. mGAPDH served as an endogenous control. (B) RT-PCR analysis of mIL-6 and hIL-6 expression in thymus (left) and BM (right) of Rag2−/−Il2rg−/−IL-6m/m, IL-6m/h, and IL-6h/h mice. (C) ELISA measurement of mouse (left) and human (right) IL-6 protein in plasma of non-engrafted IL-6m/m or IL-6h/h mice after LPS challenge (in ng/mL, n = 3). Each dot represents 1 mouse. Horizontal bars indicate mean values.
Human IL-6 enhances thymopoiesis and peripheral T-cell engraftment
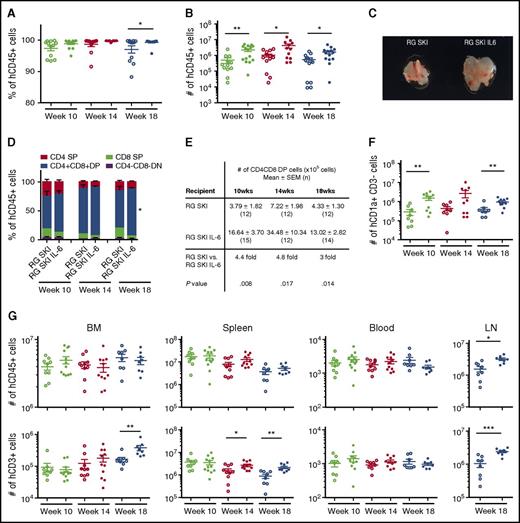
Newborn RG SKI and RG SKI IL-6 mice were irradiated and engrafted with human CD34+ cells purified from the fetal liver. The engraftment levels were analyzed 10, 14, or 18 weeks after transplantation. We observed a significant increase in the absolute numbers of human hematopoietic cells (hCD45+) in the thymus of RG SKI IL-6 mice compared with RG SKI recipients at all 3 time points (Figure 2A-B). A larger thymus size was also noticed in IL-6 knock-in mice, which was most obvious at week 14 (Figure 2C). Although the numbers of human thymocytes declined in both mouse strains at week 18, RG SKI IL-6 mice still maintained significant numbers of thymocytes, at a level which is comparable with the peak level of thymic engraftment in RG SKI mice and approximately fivefold higher than the peak level of thymic engraftment level in nonobese diabetic-scid Il2rg−/− or NOD Rag1−/−Il2rg−/− mice.21,22 A more detailed analysis of thymic subsets revealed that the percentages and numbers of CD4+CD8+ DP thymocytes were significantly lower in RG SKI mice (Figure 2D-E). These results are consistent with the previously described observation that IL-6 promotes thymocyte proliferation23,24 or a contribution to the survival/self-renewal of hematopoietic stem cells and early progenitors.25 To further dissect the role of human IL-6 in T-cell development, we stained human CD34+CD1a− common lymphoid progenitors (CLPs) and T-cell commitment progenitors CD1a+CD3− in the thymus. Although the percentage of CLPs showed no difference between two strains (supplemental Figure 2A), the absolute numbers of CLPs were higher in RG SKI IL-6 mice at early time points (supplemental Figure 2B). Higher frequencies and numbers of T-cell commitment progenitors were also detected in RG SKI IL-6 mice (supplemental Figure 2C; Figure 2F).
Human CD45+hematopoietic cell and T-lymphocyte development in RG SKI (open dots) and RG SKI IL-6 mice (closed dots). The frequency (A) and number (B) of human hematopoietic (hCD45+) cells in the thymus (n = 12-15 per group) were compared at 10, 14, and 18 weeks (C). Representative image of thymus size at week 14. (D) Comparison of frequencies of immature DN (CD4−CD8−), immature DP (CD4+CD8+), and mature SP (CD4+CD8− and CD4−CD8+) subpopulations in RG SKI and RG SKI IL-6 mice. (E) Comparison of the absolute numbers of DP thymocytes at different time points. (F) Comparison of absolute numbers of human T-cell commitment progenitor cells in the thymus. (G) Numbers of human CD45+ and CD3+ cells in BM, spleen, blood (per μL), and LN (left to right) were compared after 10 to 18 weeks (n = 8-12 per group). Because LNs start to appear in ∼15-week-old humanized mice, LN data were only obtained at week 18. Each dot represents 1 mouse. Horizontal bars indicate mean ± SEM. Two independent experiments were performed and the results were pooled. Data were analyzed by one-way ANOVA and unpaired Student t test. Individual P values for posttest are displayed. *P < .05; **P < .01; ***P < .001. DN, double-negative; DP, double-positive; SEM, standard error of the mean; SP, single-positive.
Human CD45+hematopoietic cell and T-lymphocyte development in RG SKI (open dots) and RG SKI IL-6 mice (closed dots). The frequency (A) and number (B) of human hematopoietic (hCD45+) cells in the thymus (n = 12-15 per group) were compared at 10, 14, and 18 weeks (C). Representative image of thymus size at week 14. (D) Comparison of frequencies of immature DN (CD4−CD8−), immature DP (CD4+CD8+), and mature SP (CD4+CD8− and CD4−CD8+) subpopulations in RG SKI and RG SKI IL-6 mice. (E) Comparison of the absolute numbers of DP thymocytes at different time points. (F) Comparison of absolute numbers of human T-cell commitment progenitor cells in the thymus. (G) Numbers of human CD45+ and CD3+ cells in BM, spleen, blood (per μL), and LN (left to right) were compared after 10 to 18 weeks (n = 8-12 per group). Because LNs start to appear in ∼15-week-old humanized mice, LN data were only obtained at week 18. Each dot represents 1 mouse. Horizontal bars indicate mean ± SEM. Two independent experiments were performed and the results were pooled. Data were analyzed by one-way ANOVA and unpaired Student t test. Individual P values for posttest are displayed. *P < .05; **P < .01; ***P < .001. DN, double-negative; DP, double-positive; SEM, standard error of the mean; SP, single-positive.
As expected, improvement of human T-cell engraftment was observed in peripheral tissues such as BM, spleen, and LNs, which was most profound at week 18 (Figure 2G; supplemental Figure 3A). However, the numbers of human B cells and myeloid cells were very similar between the two mouse strains (supplemental Figure 3B).
IL-6 did not improve B-cell maturation and differentiation at steady state
The original identification of IL-6 as a major stimulus for B-cell growth and differentiation prompted us to analyze the B-cell population in these mice. We found that most of the CD19+ B cells in mouse BM were CD10+, suggesting that they were immature. In contrast to the BM, there were more mature CD19+ B cells in the spleen and blood of RG SKI and RG SKI IL-6 mice, although the proportions were very similar between these two mouse strains (supplemental Figure 4A-B). Of note, B-cell maturation increased in these mice with a longer duration of reconstitution and the percentages of mature B cells in peripheral blood at 18 weeks were very similar to the percentage of those in human peripheral blood mononuclear cells.26
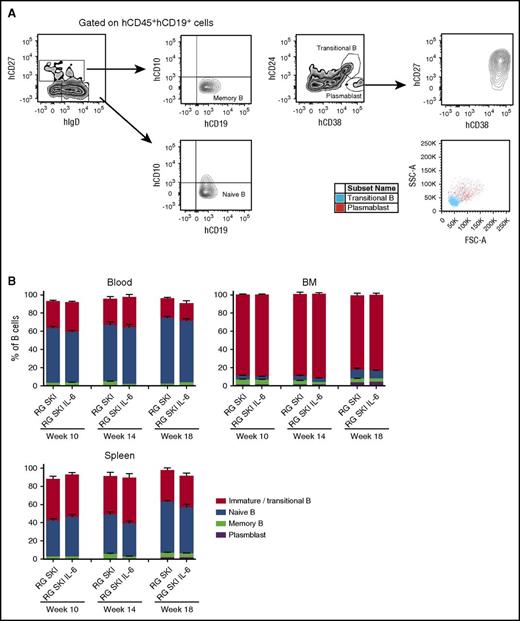
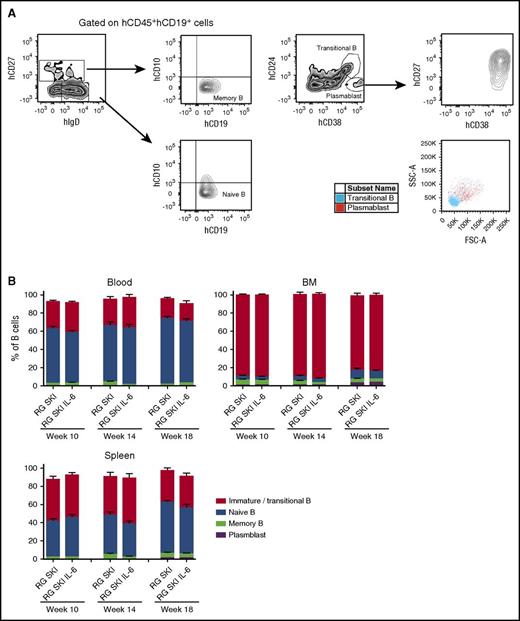
The various stages of B-cell development were also analyzed in blood, BM, and spleen for naïve B cells (CD19+CD10−IgD+CD27−), memory B cells (CD19+CD10−IgD+/−CD27+), plasmablasts (CD19+CD24−CD38+CD27+), and immature/transitional B cells (CD19+CD24+CD38+) (Figure 3A). We saw no significant differences in the B-cell subset populations between the two mouse strains at steady state (Figure 3B). Although the average percentage of transitional B-cell population in the spleen is lower in our mouse model (34.93 ± 2.33%, mean ± SEM, n = 25) compared with NOD-scid γc−/− (62.0 ± 13.8%),10 it is still higher when compared with the percentage in the human spleen (4.3 ± 1.9%).27
Characteristics of human CD19+cells recovered from blood, BM, and spleen of RG SKI and RG SKI IL-6 mice. (A) Gates used for identification of human naïve, memory, transitional/immature, or plasmablast B cells by flow cytometry based on the expression of hCD27, hCD10 hIgD, hCD24, and hCD38. Plasmablasts also have large cell size measured by forward scatter (FSC)/side scatter (SSC). (B) Relative abundance of each B-cell subset compared between two mouse strains at different time points. Two independent experiments were performed and the results were pooled. Data represent the mean ± SEM (n = 12-15 per group) and were analyzed by two-way ANOVA, Tukey’s multiple comparisons test.
Characteristics of human CD19+cells recovered from blood, BM, and spleen of RG SKI and RG SKI IL-6 mice. (A) Gates used for identification of human naïve, memory, transitional/immature, or plasmablast B cells by flow cytometry based on the expression of hCD27, hCD10 hIgD, hCD24, and hCD38. Plasmablasts also have large cell size measured by forward scatter (FSC)/side scatter (SSC). (B) Relative abundance of each B-cell subset compared between two mouse strains at different time points. Two independent experiments were performed and the results were pooled. Data represent the mean ± SEM (n = 12-15 per group) and were analyzed by two-way ANOVA, Tukey’s multiple comparisons test.
Human IL-6 strongly increases antigen-specific immune responses after immunization
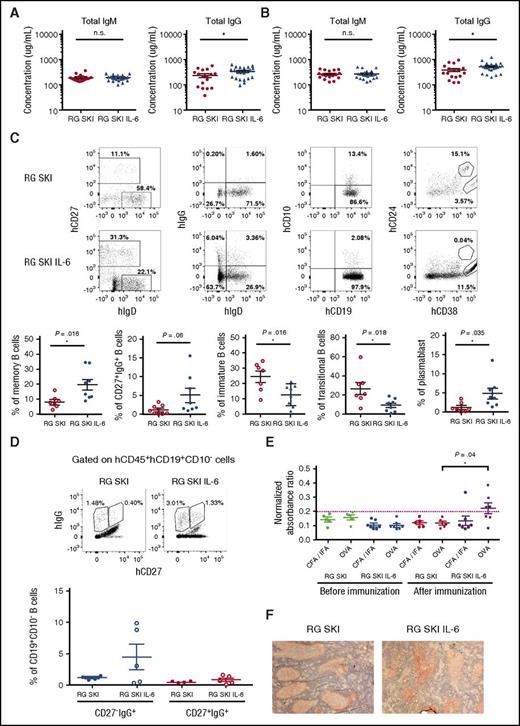
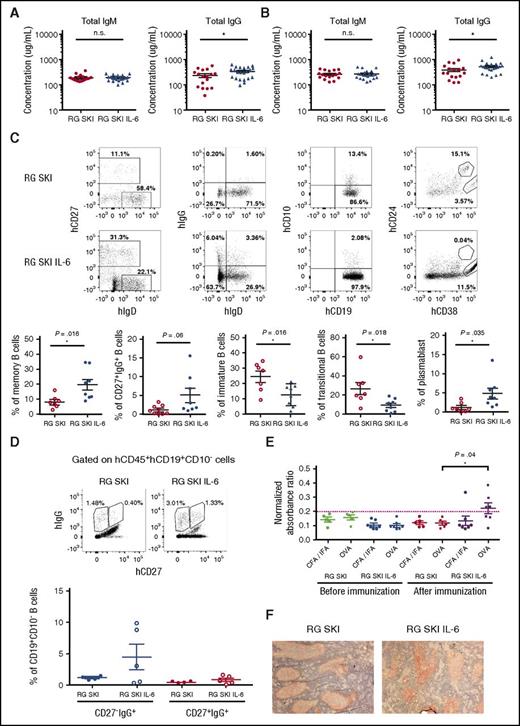
To test whether human IL-6 quantitatively and qualitatively improves the humoral immune responses in vivo, we first analyzed the total levels of human immunoglobulins in mice 12 to 14 weeks post-engraftment. Compared with RG SKI, RG SKI IL-6 mice had similar levels of human IgM (187.9 ± 13.9 μg/mL vs 193.9 ± 10 μg/mL), but a higher level of human IgG (220.4 ± 34.8 μg/mL vs 319.3 ± 31.8 μg/mL, P < .05) in the serum (Figure 4A). Although immunization with a protein antigen (OVA) increased total IgM and IgG levels in both mouse strains, a significantly higher level of IgG was observed in the RG SKI IL-6 mice (351.6 ± 52.2 μg/mL vs 517.0 ± 51.6 μg/mL, P < .05) (Figure 4B). Consistent with antibody production after immunization, higher frequencies of CD19+CD10−CD27+ memory B cells, CD19+CD10−CD27+IgG+ B cells, and CD19+CD24−CD38+CD27+ plasmablasts, as well as lower frequencies of CD19+CD24+CD38+ transitional and CD19+CD10+ immature B cells were detected in the spleen of human IL-6 knock-in mice (Figure 4C). In addition to conventional CD27+IgG+ B cells, a novel subset of CD27−IgG+ class-switched B cells have recently been described as the product of primary germinal center (GC) reactions.28,29 We observed a higher frequency of these CD27−IgG+ over CD27+IgG+ B cells in RG SKI IL-6 mice (Figure 4D).
Improved total IgG and antigen-specific IgG responses in RG SKI IL-6 mice. (A) Concentration of total hIgM and hIgG in sera. (B) Humanized mice were immunized with OVA as described in “Materials and methods.” Concentration of total hIgM and hIgG in sera were shown after immunization. The data represent the mean ± SEM (n = 17-22 per group). (C) Spleen cells were collected and analyzed after immunization. Gates used for identification of human memory, IgG+, immature, transitional, or plasmablast B cells by flow cytometry based on the expression of hCD27, hIgG, hCD10, hCD24, and hCD38 (top). The relative abundance of each B-cell subset is compared between two mouse strains (bottom). (D) Gates used for identification of CD27−IgG+ and CD27+IgG+ B cells (top). The relative abundance of each cell subset is compared between two mouse strains (bottom). (E) Representative ELISA results from humanized mice with serum sample dilutions of 1:100. Data were presented as normalized absorbance ratio by dividing the OD value of antigen-specific IgG with the OD value of total IgG in each mouse to exclude the variation between the individual animals. The dotted line represents the cutoff value (cut off value = mean OD values of the negative control + 2 × SD). Negative control is the mean OD value of OVA-specific IgG of RG SKI and RG SKI IL-6 mice before the immunization. (F) Histologic examination of spleen tissues from engrafted mice. Sections were stained with hematoxylin and hCD20 (stained in red). All the data were analyzed by unpaired Student t test. *P < .05; **P < .01; ***P < .001. OD, optical density; SD, standard deviation.
Improved total IgG and antigen-specific IgG responses in RG SKI IL-6 mice. (A) Concentration of total hIgM and hIgG in sera. (B) Humanized mice were immunized with OVA as described in “Materials and methods.” Concentration of total hIgM and hIgG in sera were shown after immunization. The data represent the mean ± SEM (n = 17-22 per group). (C) Spleen cells were collected and analyzed after immunization. Gates used for identification of human memory, IgG+, immature, transitional, or plasmablast B cells by flow cytometry based on the expression of hCD27, hIgG, hCD10, hCD24, and hCD38 (top). The relative abundance of each B-cell subset is compared between two mouse strains (bottom). (D) Gates used for identification of CD27−IgG+ and CD27+IgG+ B cells (top). The relative abundance of each cell subset is compared between two mouse strains (bottom). (E) Representative ELISA results from humanized mice with serum sample dilutions of 1:100. Data were presented as normalized absorbance ratio by dividing the OD value of antigen-specific IgG with the OD value of total IgG in each mouse to exclude the variation between the individual animals. The dotted line represents the cutoff value (cut off value = mean OD values of the negative control + 2 × SD). Negative control is the mean OD value of OVA-specific IgG of RG SKI and RG SKI IL-6 mice before the immunization. (F) Histologic examination of spleen tissues from engrafted mice. Sections were stained with hematoxylin and hCD20 (stained in red). All the data were analyzed by unpaired Student t test. *P < .05; **P < .01; ***P < .001. OD, optical density; SD, standard deviation.
Because human IL-6 increased the total IgG level, we reasoned that this cytokine might also improve antigen-specific IgG responses. To test this hypothesis, we measured the OVA-specific antibody production by ELISA. Although the level of OVA-specific IgM antibodies showed no difference between two mouse strains (data not shown), OVA-specific IgG antibodies were significantly increased in the majority of human IL-6 knock-in mice (Figure 4E). Because the development of class-switched plasma and memory B cells depends on the GC reaction, we performed immunohistochemistry on spleen sections. Although human B cells can form follicle-like structures after immunization (Figure 4F), we did not detect GC formation with positive BCL-6 staining.
IgG+ B cells generated in humanized mice used a diverse antibody repertoire and displayed somatic hypermutation
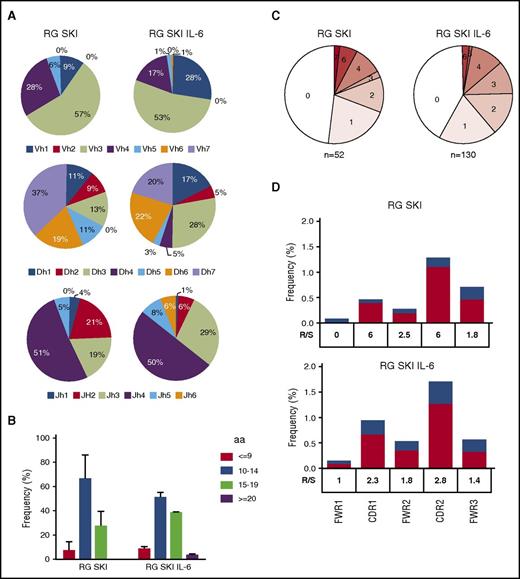
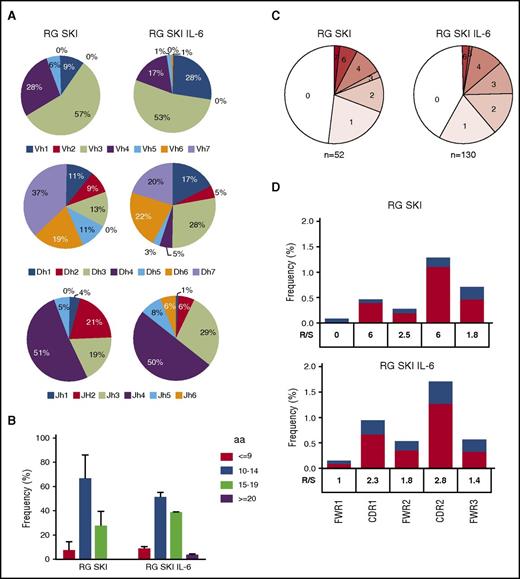
To characterize the antibody gene repertoire of IgG+ memory B cells and the rate of somatic hypermutation, we single-cell sorted classical IgG+CD27+CD19+CD10− B cells from the spleens of immunized RG SKI and RG SKI IL-6 mice, and sequenced their IgH chains. A total of 96 cells from RG SKI and 288 cells from RG SKI IL-6 mice were analyzed and an identifiable PCR product was obtained from 52 and 130 cells, respectively (supplemental Table 1). Human B cells generated in RG SKI and RG SKI IL-6 mice use a diverse antibody repertoire. The repertoire in IgG+ B cells was similar between the two mouse strains and also similar to that of humans (Figure 5A).30 For example, the most frequent immunoglobulin heavy chain variable (IGHV) subgroups used in B cells from our gene sequence data, IGHV3 and IGHV4 are also the most frequently used IGHV genes in B cells isolated from human blood.31,32 However, the length of complementarity-determining region 3 (CDR3) showed no difference between RG SKI and RG SKI IL-6 mice (Figure 5B), and was shorter than that of humans. The average length is 11.1 for RG SKI and 11.7 for RG SKI IL-6 mice, whereas the median length of IGH-CDR3 is 13.7 amino acids in human memory B-cell subsets.29 When comparing IgH sequence data to unmutated germ line forms, we noticed a low level of hypermutation (Figure 5C) with a maximum number of 7. Because a high ratio of replacement vs silent (R/S) mutations in IGHV-CDRs is regarded as the molecular sign of affinity maturation, we compared the R/S ratio in CDRs vs framework regions (FWRs). A higher R/S ratio in CDRs was observed in both mouse strains, suggesting that antigen-driven selection takes place in the humanized mice after immunization (Figure 5D).
IGHV gene features from IgG+memory B-cell antibodies. IgG+ memory B cells were sorted from 3 spleens each of RG SKI mice and RG SKI IL6 mice. (A) Pie charts depict VH family usage in RG SKI and RG SKI IL-6 mice. (B) Bar graphs show frequencies of IGVH-CDR3 length with ≤9, 10 to 14, 15 to 19, and ≥20 amino acids (aa). (C) Pie charts showing proportion of IgH sequences with 0, 1, 2, 3, 4, 5, 6, and 7 somatic mutations; 0 and 7 represents the minimum and maximum number of total mutations. (D) The ratio of R/S mutations in IGHV-FWRs and CDRs were calculated in RG SKI (top) and RG SKI IL6 (bottom) as mutated nucleotides per total base pairs analyzed. Replacement (R; black bar) and silent (S; white bar). The R/S ratio for each region is indicated. DH, heavy chain diversity segment; JH, heavy chain joining segment; VH, heavy chain variable segment.
IGHV gene features from IgG+memory B-cell antibodies. IgG+ memory B cells were sorted from 3 spleens each of RG SKI mice and RG SKI IL6 mice. (A) Pie charts depict VH family usage in RG SKI and RG SKI IL-6 mice. (B) Bar graphs show frequencies of IGVH-CDR3 length with ≤9, 10 to 14, 15 to 19, and ≥20 amino acids (aa). (C) Pie charts showing proportion of IgH sequences with 0, 1, 2, 3, 4, 5, 6, and 7 somatic mutations; 0 and 7 represents the minimum and maximum number of total mutations. (D) The ratio of R/S mutations in IGHV-FWRs and CDRs were calculated in RG SKI (top) and RG SKI IL6 (bottom) as mutated nucleotides per total base pairs analyzed. Replacement (R; black bar) and silent (S; white bar). The R/S ratio for each region is indicated. DH, heavy chain diversity segment; JH, heavy chain joining segment; VH, heavy chain variable segment.
Human IL-6 is important for the development of antigen-specific IgG+ B cells
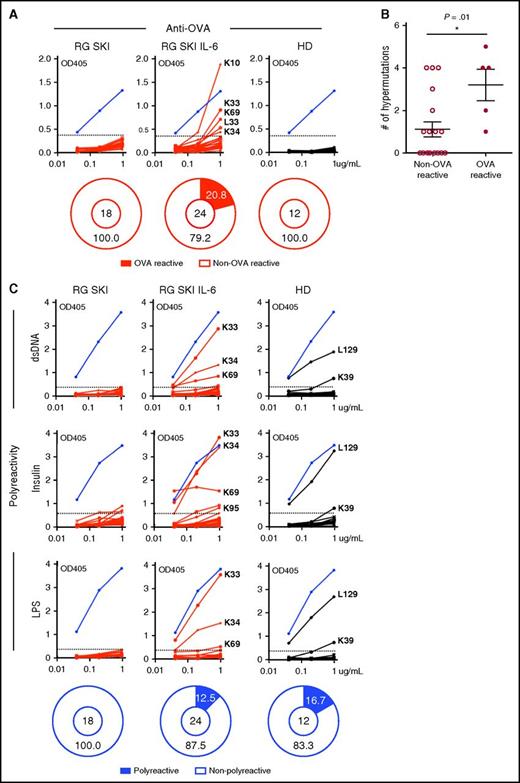
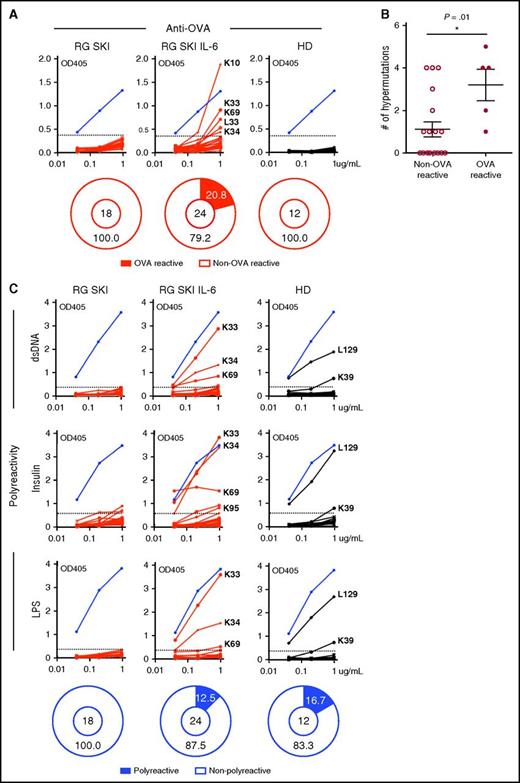
To characterize antigen-specific responses, we cloned paired heavy and light chain genes amplified from single B cells and expressed recombinant antibodies in the HEK293 cell line. None of the antibodies cloned from IgG-switched B cells isolated from immunized RG SKI mice, or from healthy donors, bound to OVA. In contrast, 5 out of 24 antibodies cloned from RG SKI IL-6 mice displayed OVA reactivity and their binding affinity was comparable to a commercially available mouse anti-OVA antibody (Figure 6A), suggesting that human IL-6 plays an essential role in the development of antigen-specific IgG+ B cells. More IGHV mutations were observed in OVA reactive clones than in non-OVA reactive clones (Figure 6B), consistent with the implication that somatic hypermutations are involved in the generation of antigen-specific antibodies. IgG-switched B cells from healthy donors have been reported to often express polyreactive antibodies, a feature reflecting binding to multiple antigens with low affinity that appeared to be associated with broad antibacterial activity.33,34 We found that 12.5% of clones isolated from IgG-switched B cells in RG SKI IL-6 mice were polyreactive and resembled representative clones isolated from healthy donors (Figure 6C), whereas none of the recombinant antibodies cloned from RG SKI mice showed polyreactivity (Figure 6C). Hence, IL-6 knock-in mice represent a more physiologic model for human immune IgG responses.
Human IL-6 promotes the generation of antigen-specific IgG-switched B cells in humanized mice. A total of 18, 24, and 12 antibodies cloned from IgG+ B cells in OVA-immunized RG SKI mice, RG SKI IL-6 mice, and control HD were tested by ELISA for OVA reactivity (A) and polyreactivity (C). Blue lines represent a mouse anti-OVA specific antibody (TOSG1C6) in (A) or polyreactive ED38 clone in (C). Except for the purified TOSG1C6 clone, all other tested antibodies are collected from cell culture supernatants. Solid black lines represent antibodies cloned from HD and solid red lines represent recombinant antibodies cloned from either immunized RG SKI or RG SKI IL-6 mice. Reactive antibodies are indicated by IgL designation followed by clone number. Horizontal dotted lines define the cutoff OD405nm for positive reactivity. The cutoff OD value was determined as twice the value of the average OD when all clones from RG SKI mice were pooled. The frequency of reactive (black area) and nonreactive (white area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. (B) Increased IGHV mutation numbers in OVA-reactive vs non-OVA reactive clones from immunized RG SKI IL-6 mice. *P < .01. HD, healthy donors; OD, optical density.
Human IL-6 promotes the generation of antigen-specific IgG-switched B cells in humanized mice. A total of 18, 24, and 12 antibodies cloned from IgG+ B cells in OVA-immunized RG SKI mice, RG SKI IL-6 mice, and control HD were tested by ELISA for OVA reactivity (A) and polyreactivity (C). Blue lines represent a mouse anti-OVA specific antibody (TOSG1C6) in (A) or polyreactive ED38 clone in (C). Except for the purified TOSG1C6 clone, all other tested antibodies are collected from cell culture supernatants. Solid black lines represent antibodies cloned from HD and solid red lines represent recombinant antibodies cloned from either immunized RG SKI or RG SKI IL-6 mice. Reactive antibodies are indicated by IgL designation followed by clone number. Horizontal dotted lines define the cutoff OD405nm for positive reactivity. The cutoff OD value was determined as twice the value of the average OD when all clones from RG SKI mice were pooled. The frequency of reactive (black area) and nonreactive (white area) clones is summarized in pie charts, with the total number of clones tested indicated in the center. (B) Increased IGHV mutation numbers in OVA-reactive vs non-OVA reactive clones from immunized RG SKI IL-6 mice. *P < .01. HD, healthy donors; OD, optical density.
Discussion
In the present study, we have established a new mouse model with improved T- and B-cell engraftment and differentiation. The involvement of IL-6 in thymopoiesis is consistent with previous murine studies. For example, in adult mice, IL-6 deficiency leads to a 20% to 40% reduction of thymocytes and peripheral T cells.23 Administration of IL-6 induces the differentiation of CD4−CD8− thymocytes into CD4+CD8+ and CD4+CD8− cells.35 Furthermore, in agreement with the previous finding that B-cell maturity in humanized mice increased with longer time post-reconstitution, surpassing 60% after 24 weeks,9 we noticed in our model that >75% of human B cells in blood and spleen were mature at week 18. More importantly, and in contrast to other models, we also found significantly increased total IgG and antigen-specific IgG production. This increase is associated with enhanced differentiation of IgG+ memory B cells and plasmablasts. Therefore, our model provides an alternative and improved strategy for adaptive immunity without relying on surgery to transplant human fetal tissues as reported in the BLT model,13,36 which are not readily available due to ethical, legal, or practical reasons. In addition, given the timeline of B-cell maturation, the usage of BLT mice in a humoral immunity study is recommended when mice are 5 to 6 months old.37 However, the occurrence of xenogeneic graft-versus-host disease and its associated morbidity and mortality,4,37,38 can make older BLT mice impractical for experimental design and the ease of repetition of studies. In contrast, our RG SKI IL-6 mice do not show signs of graft-versus-host disease and demonstrate a high survival rate (>90%) after 6 months.
Most of the published literature analyzes the frequency and ratio of R/S mutations in the CDRs and FWRs of antibody genes to identify the signature of antigenic selection. The basic assumption of this statistical method is that antigenic selection creates a bias for R mutations in the CDRs and for S mutations in the FWRs.39 We analyzed memory B cells using single-cell sorting and sequenced the B-cell receptors of antigen-induced IgG+ memory B cells. Our results agree with the expected R/S ratios in both CDRs and FWRs. Furthermore, we also observed a typical pattern with a high frequency of mutations in CDR loops, which usually contact the antigen and a low frequency of mutations in FWRs, which provide the scaffold for CDRs, and are both resistant to and less tolerant of mutations. By cloning paired heavy and light chain genes amplified from single B cells and characterizing expressed recombinant antibodies, we found that only antibody clones isolated from RG SKI IL-6 mice displayed OVA reactivity and polyreactivity. Therefore, IL-6 knock-in mice represent a more physiologic model for investigating human immune IgG responses, thus enabling the exploration of immunization regimes that would be unethical or untenable in humans. Our study also provides a “proof of concept” for the idea that, despite the absence of detectable GCs, human IgG mAbs can be generated against a target antigen of interest in humanized mice. Although their human effector functions need to be further explored, these mAbs showed antigen specificity, have naturally processed glycoproteins to maintain glycoform fidelity, and should have very limited immunogenicity because of their native human structure.
Although RG SKI IL-6 mice represent a marked improvement in adaptive immunity over existing humanized mouse models, they also have limitations. For example, a higher than normal percentage of B cells are blocked at the transitional stage of B-cell development. Splenic GCs are disorganized, likely preventing the development of a more robust antigen-specific antibody response. In addition, an increased production of CD27−IgG+ B cells over CD27+IgG+ B cells was observed in RG SKI IL-6 mice, suggesting that IL-6 improves the generation of the first wave of class-switched memory B cells after primary immune response.28,40 However, the emergence of a second wave of memory B cell is limited likely due to the lack of GC formation. The present results raise the possibility that activated B cells progress to GC B cells and may start to form small foci in the spleen post-immunization, but these foci cannot develop in size vigorously without help from follicular dendritic cells. As a result, the average level of somatic hypermutation in IgG+ B cells isolated from humanized mice is 10-fold lower than the average frequency of mutations in human IgGs.29 Hence, approaches to improve effective T-B interaction and follicular dendritic cell development might be useful. Future refinements, such as knocking in additional human genes into the mouse host, should lead to a better mouse model that may ultimately fully recapitulate functional human adaptive immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Regeneron Pharmaceuticals’ Velocigene team for generating human IL-6 knock-in mice; J. Alderman for managerial support; C. Weibel, E. Henchey, and P. Ranney for technical assistance; and C. Lieber for manuscript submission.
This study was supported by the Bill and Melinda Gates Foundation, the National Institutes of Health, National Cancer Institute (R01 CA156689) (R.A.F.), and the American Diabetes Association Research Foundation Mentorship Fellowship (H.Y.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Authorship
Contribution: H.Y. designed and performed experiments, and analyzed the data; C.B., J.-N.S., S.Z., and E.E.E. performed experiments and edited the manuscript; T.S. contributed to the research; D.F., C.G., A.J.M., and G.D.Y. generated the IL-6 knock-in mice and edited the manuscript; E.M. and M.G.M. provided valuable input for this study; R.A.F. conceived the project and supervised its participants and interpreted its results; and H.Y., E.M., M.G.M., and R.A.F. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.B. is Università del Piemonte Orientale, Novara Area, Italy; J.-N.S. is Adimab, Lebanon, NH; T.S. is Helmholtz Centre for Infection Research, Braunschweig, Germany.
Correspondence: Richard A. Flavell, Department of Immunobiology, Yale School of Medicine, 300 Cedar St, TAC S-569, POB 208011, New Haven, CT 06520-8011; e-mail: richard.flavell@yale.edu.