Abstract
Philadelphia-negative classical myeloproliferative neoplasms (MPNs) include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The 2016 revision of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues includes new criteria for the diagnosis of these disorders. Somatic mutations in the 3 driver genes, that is, JAK2, CALR, and MPL, represent major diagnostic criteria in combination with hematologic and morphological abnormalities. PV is characterized by erythrocytosis with suppressed endogenous erythropoietin production, bone marrow panmyelosis, and JAK2 mutation. Thrombocytosis, bone marrow megakaryocytic proliferation, and presence of JAK2, CALR, or MPL mutation are the main diagnostic criteria for ET. PMF is characterized by bone marrow megakaryocytic proliferation, reticulin and/or collagen fibrosis, and presence of JAK2, CALR, or MPL mutation. Prefibrotic myelofibrosis represents an early phase of myelofibrosis, and is characterized by granulocytic/megakaryocytic proliferation and lack of reticulin fibrosis in the bone marrow. The genomic landscape of MPNs is more complex than initially thought and involves several mutant genes beyond the 3 drivers. Comutated, myeloid tumor-suppressor genes contribute to phenotypic variability, phenotypic shifts, and progression to more aggressive disorders. Patients with myeloid neoplasms are at variable risk of vascular complications, including arterial or venous thrombosis and bleeding. Current prognostic models are mainly based on clinical and hematologic parameters, but innovative models that include genetic data are being developed for both clinical and trial settings. In perspective, molecular profiling of MPNs might also allow for accurate evaluation and monitoring of response to innovative drugs that target the mutant clone.
Introduction
The concept of myeloproliferative disorders was developed by Dameshek in a visionary editorial, in which he described these conditions as being characterized by excessive proliferation of hematopoietic precursors in the bone marrow and excessive production of mature blood cells.1 The term “neoplasm” was introduced in 2008 by the authors of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues to underscore the clonal nature of myeloproliferative disorders.2 The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms includes the following myeloproliferative neoplasms (MPNs)3 : chronic myeloid leukemia (CML), BCR-ABL1+; chronic neutrophilic leukemia; polycythemia vera (PV); primary myelofibrosis (PMF); essential thrombocythemia (ET); chronic eosinophilic leukemia, not otherwise specified; and MPN, unclassifiable.
Because of their overlapping features, PV, ET, and PMF have been traditionally grouped into the category of Philadelphia-negative classical MPNs. In 2005, the identification of a unique base substitution in JAK2, the gene encoding Janus kinase 2, in patients with PV, ET, and PMF provided a unifying genetic basis for these disorders.4-7 Our understanding of the genetic basis of classical MPNs further improved with the identification of somatic mutations of MPL in patients with ET or PMF,8 of JAK2 exon 12 mutations in patients with PV or idiopathic erythrocytosis,9 and more recently of somatic mutations of CALR in patients with ET or PMF.10,11 For a comprehensive analysis of the molecular pathophysiology of classical MPNs, the reader is referred to the review article by Vainchenker and Kralovics in this issue.12
MPNs are classified as rare cancers because their incidence is lower than 6 per 100 000 persons per year. A recent study provided the following incidence estimates: PV from 0.4 to 2.8, ET from 0.38 to 1.7, and PMF from 0.1 to 1.0 per 100 000 persons per year.13 These disorders generally occur in middle- or advanced-age adults, with a median age of 65 years for PV, 68 years for ET, and 70 years for PMF.14 The classification of MPNs into 3 nosologic entities has considerable prognostic relevance, as shown by a population-based study from the Swedish Cancer Register,15 and more recently by an international study conducted on patients with molecularly annotated MPNs.16 Basically, although MPN patients overall have reduced life expectancy compared with the general population, the relative survival rate is lower in PMF compared with PV, and in PV compared with ET.15 Of note, survival of patients with WHO-defined ET is similar to that of the sex- and age-standardized European population.17 A study of the Swedish Cancer Register has shown that excess mortality in MPN patients is overall attributable to death from hematologic malignancies or from bacterial infections, and in young patients also from cardiovascular and cerebrovascular disease.18 There has been a clear improvement in survival between 1973 and 2005, mainly because of decreased probabilities of dying as a result of these complications.
In the present article, we will examine diagnosis, risk stratification, and response evaluation in classical MPNs.
The 2016 revision of the WHO classification of classical MPNs
WHO classification of classical MPNs
Abnormalities of peripheral blood cell count and alterations of bone marrow morphology represent major components of the WHO diagnostic criteria for classical MPNs. Thrombocytosis is a major criterion for diagnosis of ET, although this abnormality may be found in all MPNs, and the diagnostic approach to ET primarily involves differential diagnosis of thrombocytosis, as described in detail elsewhere.20 Erythrocytosis is a major diagnostic criterion for PV, and the diagnostic approach to PV primarily involves differential diagnosis of erythrocytosis: however, marginally elevated hemoglobin levels may be found both in JAK2 (V617F)-mutant ET and PMF. Leukocytosis may be found in MPN patients with advanced disease, and overall represents an unfavorable prognostic factor. Patients with PMF may present various combinations of blood “cytosis” and cytopenia, but many of them present with anemia, and therefore the diagnostic approach to PMF typically involves differential diagnosis of anemia.21
Figure 1 illustrates representative abnormalities of bone marrow biopsy in patients with classical MPNs. In ET, bone marrow cellularity is normal or sometimes even slightly reduced, and the most apparent abnormality is megakaryocytic proliferation with increased numbers of enlarged, hyperlobulated megakaryocytes. In PV, bone marrow is hypercellular for age with trilineage proliferation, a pattern defined as panmyelosis. In prefibrotic/early PMF (prePMF), bone marrow biopsy shows megakaryocytic proliferation combined with increased age-adjusted bone marrow cellularity and granulocytic proliferation. In overt PMF, megakaryocytic proliferation and atypia are typically combined with either reticulin and/or collagen fibrosis.
Representative bone marrow biopsies from patients with MPNs. (A) ET: Normocellular marrow, proliferation of giant megakaryocytes with hyperlobulated nuclei, scattered or in loose clusters (hematoxylin and eosin [H&E], original magnification ×40). (B) PV: Hypercellular marrow with erythroid proliferation and scattered pleomorphic megakaryocytes (H&E, original magnification ×20). (C) PMF: Hypercellular marrow with granulocytic proliferation and large megakaryocytes with atypical bulbous nuclei (H&E, original magnification ×40). (D) Overt PMF: Hypercellular marrow, proliferation of atypical megakaryocytes forming dense clusters, and dilated vessels with intraluminal hematopoiesis (H&E, original magnification ×40). (E) Overt PMF (collagen fibrosis): Bands of collagen fibrosis within hematopoietic lacunae (Masson trichrome staining, original magnification ×40). Courtesy of Emanuela Boveri, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy.
Representative bone marrow biopsies from patients with MPNs. (A) ET: Normocellular marrow, proliferation of giant megakaryocytes with hyperlobulated nuclei, scattered or in loose clusters (hematoxylin and eosin [H&E], original magnification ×40). (B) PV: Hypercellular marrow with erythroid proliferation and scattered pleomorphic megakaryocytes (H&E, original magnification ×20). (C) PMF: Hypercellular marrow with granulocytic proliferation and large megakaryocytes with atypical bulbous nuclei (H&E, original magnification ×40). (D) Overt PMF: Hypercellular marrow, proliferation of atypical megakaryocytes forming dense clusters, and dilated vessels with intraluminal hematopoiesis (H&E, original magnification ×40). (E) Overt PMF (collagen fibrosis): Bands of collagen fibrosis within hematopoietic lacunae (Masson trichrome staining, original magnification ×40). Courtesy of Emanuela Boveri, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy.
In the 2016 revision of the WHO classification,3 the diagnostic criteria for ET have been only slightly modified with the inclusion of CALR mutation. In addition, the revised WHO criteria underscore the importance of differentiating “true” ET from prefibrotic PMF as these 2 entities have different clinical outcomes.17 As illustrated in Figure 1, bone marrow biopsy is of fundamental importance for distinguishing between ET and prefibrotic PMF (see also “Prefibrotic/early PMF” later in text).
The diagnostic criteria for PV have been substantially modified in the 2016 revision. The hemoglobin or hematocrit thresholds for diagnosis of PV have been lowered (16.5 g/dL or 49% in men, and 16.0 g/dL or 48% in women, respectively), whereas bone marrow morphology has been upgraded to a major diagnostic criterion. Hemoglobin and hematocrit thresholds have been lowered mainly with the purpose of distinguishing JAK2-mutant ET from the so-called masked PV.22,23 However, rigorous application of the new hemoglobin threshold for men (16.5 g/dL) might increase the number of “potential” PV patients by up to 12-fold.24 In clinical practice, the use of a hemoglobin threshold of 17.0 g/dL (upper normal limit) in men, and consideration of other abnormalities of complete blood cell count (that is, thrombocytosis and/or leukocytosis) in subjects with hemoglobin levels between 16.5 and 17.0 g/dL, might represent a better compromise between the need of diagnosing PV early and the risk of considerably expanding the number of “potential” PV patients. Another debatable point is the possibility, provided by the revised WHO criteria, of diagnosing PV without any evidence of a driver mutation: all patients diagnosed with PV at our institution since 2005 carried JAK2 (V617F) (96%) or a JAK2 exon 12 mutation (4%).
Prefibrotic/early PMF
This condition was included in the 2008 WHO classification of myeloid neoplasms as a prodromal phase of PMF, but no specific diagnostic criteria were defined at that time.25 The 2008 description was primarily focused on hypercellularity with an increased number of neutrophils and atypical megakaryocytes: megakaryocyte atypia included “cloud-like” and “balloon-shaped” nuclei. The revised criteria undoubtedly represent a useful tool for clinicians.
Most patients with prePMF present with thrombocytosis,26 and therefore the diagnostic approach typically implies a differential diagnosis with ET. Bone marrow biopsy represents the most important criterion for distinguishing between these 2 MPNs, as illustrated in Figure 1. In addition, diagnosis of prePMF requires the presence of at least 1 of the following minor criteria (Table 2): (1) anemia not attributed to a comorbid condition; (2) leukocytosis (white blood cell [WBC] count ≥11 × 109/L); (3) palpable splenomegaly; (4) lactate dehydrogenase (LDH) level increased to above upper normal limit of institutional reference range. The available evidence suggests that prePMF is a presentation mode of PMF,26 and that the clinical outcome of PMF patients is worse than that of ET patients17 but better than that of PMF patients26 : these observations signify the importance of distinguishing between these different conditions.
Patients meeting the WHO criteria for MPN but lacking a driver mutation
Patients with a PV phenotype apparently lacking JAK2 (V617F) or an exon 12 mutation are extremely rare in our experience. Facing such a patient, the differential diagnosis process should be reassessed ab initio. A small clone of hematopoietic cells carrying a JAK2 exon 12 mutation might be the underlying molecular basis.27 Atypical, germ line mutations of JAK2 may be responsible for hereditary erythrocytosis and may also cooperate with JAK2 (V617F).28,29
About 10% of patients with ET and 5% to 10% of those with PMF meet the WHO criteria for MPN but have no evidence of a canonical somatic mutation in 1 of the 3 drivers, that is, JAK2, CALR, or MPL: these patients are defined as triple negative. A few triple-negative patients with ET carry activating mutations of MPL outside exon 10 (Table 1), and these noncanonical mutations may be either somatically acquired or inherited.30,31 Therefore, these patients may have a true MPN associated with noncanonical mutations of MPL, or, alternatively, hereditary thrombocytosis (see “Distinguishing hereditary disorders attributable to germ line JAK2 or MPL mutation from MPNs”). A substantial portion of triple-negative patients with ET have evidence of polyclonal hematopoiesis, and most likely do not have a true MPN30,31 : the use of cytoreductive drugs in these patients is questionable.
About 5% to 10% of patients with PMF are triple negative and have poor clinical outcome (see “Risk stratification in PMF”). According to WHO criteria,3 in the absence of any of the 3 driver mutations, clinicians should search for the most frequent accompanying mutations (eg, ASXL1, EZH2, TET2, IDH1/IDH2, SRSF2, SF3B1) to determine the clonal nature of the disease. Indeed, triple-negative PMF is similar to the myelodysplastic syndrome associated with bone marrow fibrosis, a condition characterized by hypercellular bone marrow, multilineage dysplasia, severe cytopenia involving high transfusion requirement, unfavorable cytogenetics, and poor survival.32 Bone marrow fibrosis can be driven by somatic mutation of myeloid tumor-suppressor genes like EZH233 or SRSF2,12 and therefore this abnormality is not infrequent in myeloid neoplasms other than PMF. Patients with triple-negative PMF should be investigated using a gene panel sequencing approach,34 and exclusion of the BCR-ABL1 rearrangement is important in these cases.
Identifying familial cases of MPNs: how to deal with the issue of genetic predisposition
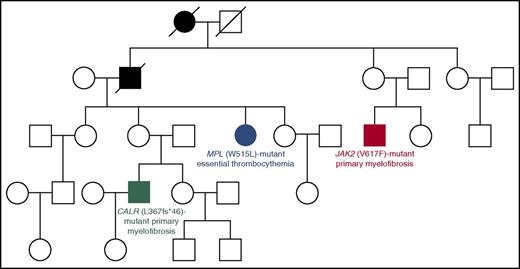
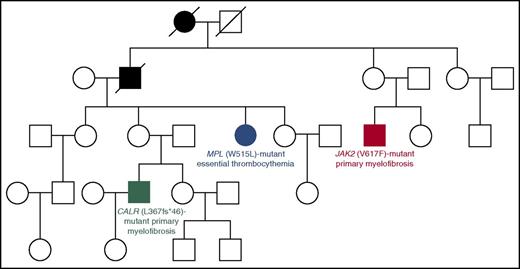
In our experience, about 7% to 8% of patients with an apparently sporadic MPN belong to familial trees with at least 2 cases of MPN: these represent familial MPNs, in which the somatic mutation in the MPN driver gene has likely been acquired as a result of genetic predisposition.35-38 Although this predisposition has been molecularly defined only in few families so far (Figure 2),28,39,40 identifying familial cases of MPNs may allow early diagnosis of these myeloid malignancies in other individuals. In our clinical practice,20 we interview all MPN patients to find out if there is a family history of these disorders. In familial trees with at least 2 cases of MPN, we suggest performing a complete blood cell count in all apparently healthy relatives with the aim of identifying an early asymptomatic MPN phenotype.
Four-generation Australian pedigree with different types of familial MPNs associated with different driver mutations. Full symbols indicate affected individuals. In this pedigree, a germ line mutation (R1569H) in the RBBP6 gene segregated with an MPN phenotype. The RBBP6 protein is a RING finger E3 ubiquitin ligase that contributes to ubiquitinate and degrade p53 in association with MDM2: mutant RBBP6 may cause an elevation in somatic mutagenesis rates through inhibition of p53 function and deregulation of cell cycle. The fact that individuals with the germ line mutation acquired somatic mutations in different genes supports the notion of genetic predisposition. Modified from Harutyunyan et al39 with permission.
Four-generation Australian pedigree with different types of familial MPNs associated with different driver mutations. Full symbols indicate affected individuals. In this pedigree, a germ line mutation (R1569H) in the RBBP6 gene segregated with an MPN phenotype. The RBBP6 protein is a RING finger E3 ubiquitin ligase that contributes to ubiquitinate and degrade p53 in association with MDM2: mutant RBBP6 may cause an elevation in somatic mutagenesis rates through inhibition of p53 function and deregulation of cell cycle. The fact that individuals with the germ line mutation acquired somatic mutations in different genes supports the notion of genetic predisposition. Modified from Harutyunyan et al39 with permission.
Recent studies have identified germ line MPN-predisposition alleles on a population level.41,42 These observations are extremely important to understand how the expansion of mutant clones is influenced by heritable genetic polymorphisms present in the host’s genome,43 but should not be used at present for estimating the risk of developing an MPN in individual subjects.
Distinguishing hereditary disorders attributable to germ line JAK2 or MPL mutation from MPNs
Hereditary thrombocytosis may be associated with germ line mutations in THPO, the gene encoding thrombopoietin, MPL, or JAK2.44 In a Japanese family with hereditary thrombocytosis,45 affected members carried the germ line MPL (S505N) mutation, which has been reported also as an acquired somatic mutation.31 More recently, families with hereditary thrombocytosis attributable to germ line JAK2 mutations have been described, including JAK2 (R564Q), JAK2 (H608N), JAK2 (V617I), JAK2 (R867Q), and JAK2 (S755R/R938Q).46-49
Patients with hereditary thrombocytosis might present as sporadic cases, and in the initial workup they might be diagnosed with triple-negative ET. In order to detect the germ line mutation, it is important to sequence all coding regions of JAK2 or MPL on DNA from both granulocytes and T lymphocytes (or from nonhematopoietic control tissue). Although these are rare disorders and evidence concerning treatment is lacking, individuals with hereditary thrombocytosis should probably not be given a cytoreductive treatment.
Toward a deeper integration of clinical features, morphology, immunophenotype, and genetics for defining and managing MPNs
The WHO classification is based on a multiparameter approach to disease definition, using all available information, that is, clinical features, morphology, immunophenotype, and genetic data. In perspective, we should move toward a deeper integration of these data.
Flow cytometry enumeration of circulating CD34+ cells as a tool for estimating abnormal stem cell trafficking
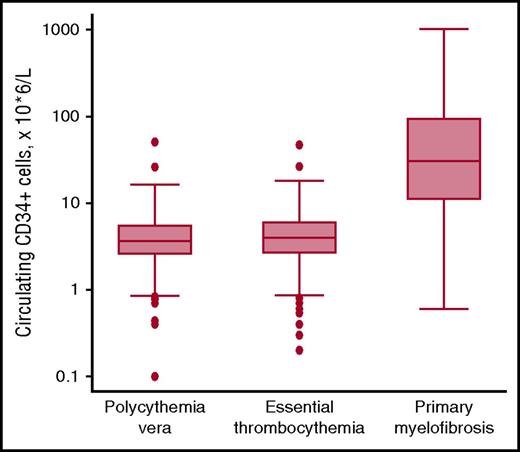
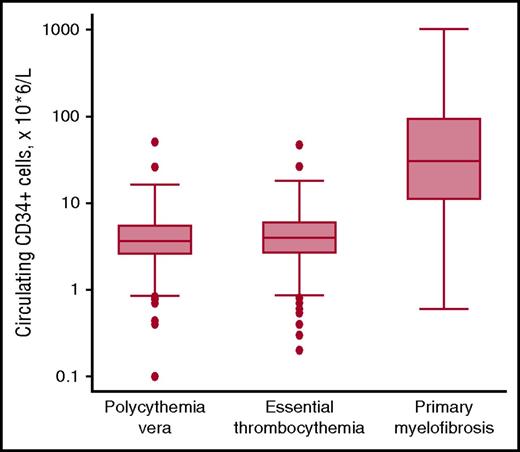
Abnormal stem cell trafficking is not only a typical feature of PMF but is also found in patients with PV or ET who have progressed to myelofibrosis. In 2001, Barosi et al showed that flow cytometry enumeration of circulating CD34+ cells provides a reliable estimation of abnormal stem cell trafficking and allows PMF to be distinguished from other MPNs.50 We confirmed these observations, showing that the absolute number of circulating CD34+ cells is normal in most patients with PV or ET, and elevated in most patients with PMF or secondary myelofibrosis51 : representative data are illustrated in Figure 3. A recent study has shown that circulating CD34+ cell count is normal or only marginally elevated in prefibrotic PMF, whereas it increases along the continuum of bone marrow fibrosis in overt PMF.26 Patients with PMF and counts >300 × 106/L are at high risk of leukemic transformation50 : these markedly elevated counts likely include not only hematopoietic stem cells but circulating CD34+ leukemic blasts as well.
Circulating CD34+ cells in patients with MPNs. Flow cytometry enumeration of circulating CD34+ cells in patients with PV (n = 239), ET (n = 391), or PMF (n = 106). Data are shown in a box plot depicting the upper and lower adjacent values (highest and lowest horizontal line, respectively), upper and lower quartile with median value (box), and outside values (dots). Values found in PMF patients are significantly higher than those found in PV or ET patients (P < .0001): the existence of overlaps is consistent with the notion that abnormal stem cell trafficking may be found also in some patients with PV or ET, especially those with advance disease. Overall, the available evidence indicates that flow cytometry enumeration of circulating CD34+ cells represents a simple, useful tool for estimating abnormal stem cell trafficking in patients with MPNs.
Circulating CD34+ cells in patients with MPNs. Flow cytometry enumeration of circulating CD34+ cells in patients with PV (n = 239), ET (n = 391), or PMF (n = 106). Data are shown in a box plot depicting the upper and lower adjacent values (highest and lowest horizontal line, respectively), upper and lower quartile with median value (box), and outside values (dots). Values found in PMF patients are significantly higher than those found in PV or ET patients (P < .0001): the existence of overlaps is consistent with the notion that abnormal stem cell trafficking may be found also in some patients with PV or ET, especially those with advance disease. Overall, the available evidence indicates that flow cytometry enumeration of circulating CD34+ cells represents a simple, useful tool for estimating abnormal stem cell trafficking in patients with MPNs.
We use flow cytometry enumeration of circulating CD34+ cells in patients with PMF to assess the extent of abnormal stem cell trafficking, and in patients with advanced PV or ET to early detect myelofibrotic transformation. In perspective, this parameter might be used not only for improving the diagnostic or prognostication precision, but also for evaluating response to potentially disease-modifying drugs as they become available for patients with MPNs.
Clinical significance of genetic data: beyond clonal markers
The WHO classification of classical MPNs utilizes driver mutations essentially as clonal markers.19 The specific nosologic entity is primarily defined on the basis of clinical and pathologic features: for instance, ET is defined as a combination of thrombocytosis and megakaryocytic proliferation, whereas PV is defined as a combination of erythrocytosis, suppressed endogenous erythropoietin production, and panmyelosis. The genetic lesion, for example, JAK2 (V617F), is then used as a marker of clonality, as shown in Tables 1 and 2.
The genetic characterization of MPNs that took place in the last few years has shown that: (1) a single somatic mutation may be associated with different phenotypes depending on additional genetic or acquired factors; (2) the different driver mutations have distinct clinical effects; (3) the mutational landscape of MPNs goes beyond the 3 classical driver genes.12,43,52
A number of observations are consistent with the notion that JAK2 (V617F)-mutant ET and PV represent a continuum in which it may be difficult to define boundaries.53,54 Although JAK2 (V617F)-homozygous subclones are found both in ET and PV patients, the expansion of a dominant homozygous subclone occurs almost exclusively in PV patients.55 Polycythemic transformation of ET is not uncommon in clinical practice,20,54 and it has been clearly shown that homozygosity for human JAK2 (V617F) in knock-in mice results in a phenotypic switch from an ET-like to PV-like phenotype.56 Thus, although there is no question that JAK2 (V617F)-mutant PV is a more aggressive disease than JAK2 (V617F)-mutant ET, it is also clear that these disorders are strictly related. In this context, it would be important to better define the relationships between ET, PV, and the so-called masked PV.22
Clonal progression of CALR-mutant MPNs appears to be mainly associated with a progressive expansion of a mutant heterozygous clone that eventually becomes fully dominant in the bone marrow.10,54 Activation of megakaryocytes by mutant CALR and/or cooperating mutations is likely responsible for transition from ET to myelofibrosis.57-59 Clonal progression of MPL-mutant MPNs may be associated with acquired copy-neutral loss of heterozygosity of chromosome 1p, involving transition from heterozygosity to homozygosity for the MPL mutation.60
As underlined by Vainchenker and Kralovics, PMF has both myeloproliferative and myelodysplastic features, and may derive from various combinations of MPN driver mutations, cooperating mutations, and bone marrow microenvironment-related abnormalities.12 Lundberg et al have shown that about one-third of MPN patients, mainly those with PMF, carry somatic mutations in non-MPN-driver genes, and that the presence of 2 or more somatic mutations represents a negative prognostic factor.61 A more recent study has found that more than half of patients with PV or ET harbor mutations in non-MPN-driver genes, and that the presence of some of these mutations adversely affects overall, leukemia-free, and myelofibrosis-free survival.62 Ortmann et al focused on MPN patients carrying both JAK2 and TET2 mutations, and studied the clinical effect of the order in which mutations are acquired.63 As compared with TET2-first patients, JAK2-first patients were more likely to present with PV and to have a thrombotic event. More recently, the same group64 has shown that DNMT3A mutations also may occur early or late in patients with an MPN, and that mutation order influences phenotype: overall, mutations in either DNMT3A or TET2 are associated with an ET phenotype when acquired prior to JAK2 (V617F).
Table 3 highlights the mutant genes, including MPN drivers and comutated non-MPN-driver genes, that play a role in the pathogenesis of MPNs and contribute to their variability in terms of phenotype and clinical outcome. Combinations of these mutant genes can also generate hybrid myeloid neoplasms, as illustrated by the atypical neoplasms with a hybrid CML/MPN phenotype associated with both BCR-ABL1 rearrangement and CALR mutation.65-67 It can be anticipated that mutational analysis of all of these genes is likely to become a fundamental component of innovative diagnostic approaches and prognostic models for MPN.
Clinical use of genetic data
In our opinion, a future classification of MPNs should more efficiently integrate clinical features, morphology, and genetics along the following lines:
the driver mutation should be included in the definition of the specific MPN as shown in Table 1 (“Relationships between genotype, phenotype, and clinical outcome”). JAK2 (V617F)-mutant ET has a high risk of thrombosis and may progress to PV.54 By contrast, CALR-mutant ET has a lower risk of thrombosis, but a relatively high risk of myelofibrotic transformation (especially the condition associated with type 1 mutation).54,68 Furthermore, CALR-mutant PMF has relatively indolent clinical course with respect to JAK2 (V617F)- or MPL-mutant PMF, and especially as compared with triple-negative PMF (see “Risk stratification in PMF”);
as massive parallel sequencing is increasingly used in clinical settings, somatic mutations in non-MPN-driver, comutated genes should be considered to define subgroups with distinct clinical features. For instance, in an interesting study, PMF patients with wild-type CALR and ASXL1 mutation had a median survival of 2.3 years, whereas patients with CALR mutation and wild-type ASXL1 had a median survival of 10.4 years69 ; and
molecular profiling based on massive parallel sequencing also provides the assessment of the variant allele frequency, or mutant allele burden. In turn, this may allow for definition of mutation order in patients carrying both driver and non-MPN-driver mutations.63,64
Risk stratification
MPN patients are at variable risk of vascular complications, including arterial or venous thrombosis, and bleeding. In addition, a portion of patients with ET and especially of those with PV may develop secondary myelofibrosis, whereas all MPN patients may progress to a blast phase that is indistinguishable from AML, sometimes preceded by a myelodysplastic phase.
Risk of vascular complications
So far, risk-adapted therapy in PV and ET has been essentially based on the estimate of the likelihood of thrombotic complications. Age over 60 years and a previous thrombotic event represent the 2 main risk factors for thrombosis, and these are also the criteria currently used to stratify PV and ET patients into low risk (no risk factors) and high risk (at least 1 risk factor).70 Extreme thrombocytosis (platelet [PLT] count ≥1500 × 109/L) represents a risk factor for bleeding because it may be associated with an acquired von Willebrand syndrome, defined by a combination of reduced ristocetin cofactor activity and normal von Willebrand factor antigen level.
Risk stratification in ET
Table 4 includes 3 prognostic scoring systems that have been developed for patients with ET. According to the European LeukemiaNet recommendations, age ≥60 years, history of vascular complications, and extreme thrombocytosis are the 3 risk factors used to classify patients with ET at low and high risk of vascular complications in order to decide a cytoreductive treatment.20
The International Prognostic Score for Essential Thrombocythemia (IPSET)-thrombosis also takes into account the major cardiovascular risk factor and the JAK2 (V617F) mutation, that is, additional factors that have been found to be independent predictors of thrombosis.71 This model stratifies patients into 3 groups: low, intermediate, and high risk, with a respective thrombosis risk of 1.03%, 2.35%, and 3.56% of patients per year. Other studies have shown that MPN driver mutations variably impact on the risk of thrombosis in patients with ET, and that JAK2 (V617F) is a strong thrombophilic factor. Indeed, patients with CALR-mutant ET have a much lower risk of thrombosis than patients with JAK2-mutant ET and those with PV.54 A recent practice-relevant revision of IPSET-thrombosis based on 1019 patients with WHO-defined ET has led to a 4-group risk stratification72 : very-low risk (age <60 years, no thrombosis history, and absence of JAK2 mutation); low risk (age <60 years, no thrombosis history, and presence of JAK2 mutation); intermediate risk (age ≥60 years, no thrombosis history, and absence of JAK2 mutation); and high risk (age ≥60 years or thrombosis history, and presence of JAK2 mutation).
The IPSET prognostic model was developed in an international study on 867 subjects with WHO-defined ET.73 Age ≥60 years, prior thrombosis, and leukocytosis were found to significantly affect survival, and these variables were used to construct a model that allocated patients into 3 risk categories with significantly different survival (Table 4). Gangat et al had previously identified age ≥60 years, anemia, and leukocytosis as independent risk factors for inferior survival in ET.74 Leukocytosis also has been previously found to be an independent predictor of thrombosis75-77 : however, it is currently unclear how this information should be used in clinical decision-making.
Risk stratification in PV
As shown in Table 5, the conventional thrombosis score for PV is based on age ≥60 years and history of thrombosis: the presence of either factors defines high-risk patients and represents an indication to a cytoreductive treatment.
In an international study on 1545 PV patients, survival was found to be adversely affected by older age, leukocytosis, venous thrombosis, and abnormal karyotype.78 A prognostic model that included the first 3 risk factors identified 3 risk groups with median survivals ranging from 11 to 29 years (Table 5).
We have previously reported that the JAK2 (V617F) allele burden varies considerably in PV, and that this contributes at least in part to determining both phenotypic manifestations and clinical course of this disorder: in particular, a JAK2 (V617F) allele burden >50% was found to represent a risk factor for progression to myelofibrosis.79
Risk stratification in PMF
Three prognostic models are currently used in clinical practice for prognostication and clinical decision-making in PMF: their main features are summarized in Table 6.
The International Prognostic Scoring System (IPSS) was developed in 2009 by the International Working Group for Myeloproliferative Neoplasm Research and Treatment (IWG-MRT) in a study on patients evaluated at time of initial diagnosis. The IPSS utilizes 5 independent predictors of inferior survival: age >65 years, hemoglobin <10 g/dL, WBC count >25 × 109/L, circulating blasts ≥1%, and presence of constitutional symptoms. As shown in Table 6, scores define 4 risk groups (low, intermediate 1, intermediate 2, and high) with corresponding median survival of 11.3, 7.9, 4.0, and 2.3 years.80
The IWG-MRT later developed the Dynamic International Prognostic Scoring System (DIPSS, Table 6), which uses the same prognostic variables but assigns 2 points instead of 1 to hemoglobin <10 g/dL.81 DIPSS can be applied at any time during the disease course, and modification of the DIPSS risk during follow-up of PMF patients can also predict different risks of leukemic transformation.82
DIPSS was further refined by incorporating 3 additional DIPSS-independent risk factors (Table 6): unfavorable karyotype, PLT count lower than 100 × 109/L, and transfusion requirement.83 The new prognostic model, DIPSS-plus, identifies 4 risk groups with median survival ranging from 15 to 1.3 years.83
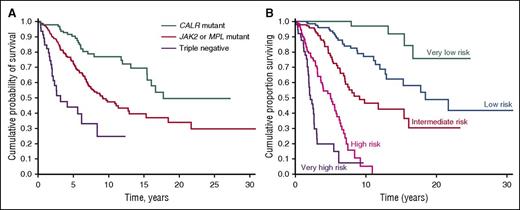
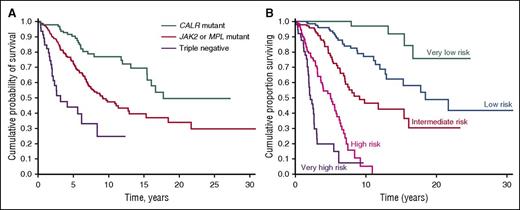
Although the prognostic models for PMF currently used in clinical practice are essentially based on clinical and hematologic parameters, recent observations indicate that the driver mutation has an independent effect on overall survival and risk of leukemic transformation.16,84,85 In our study of 617 PMF patients, median overall survival was 17.7 years in CALR-mutated, 9.2 years in JAK2-mutated, 9.1 years in MPL-mutated, and 3.2 years in triple-negative patients (Figure 4A). Notably, triple-negative patients had much higher incidence of leukemic transformation compared with either CALR-mutated or JAK2-mutated patients.85 The unfavorable clinical outcome of triple-negative PMF patients has been confirmed in a more recent study.86 As a proof of concept, based on the above-mentioned study of 617 PMF patients, we developed a prognostic model that includes JAK2, CALR, and MPL mutation status in addition to the IPSS variables. As shown in Figure 4B, this model stratifies PMF patients into 5 subgroups with significantly different overall survival: this underlines the importance of integrating clinical and genetic data in order to improve the prognostication precision of MPNs.
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation or a clinical-molecular prognostic model that includes IPSS variables and driver mutation. (A) Patients stratified according to their driver mutation. This analysis illustrates the prognostic significance of the driver mutation: although all patients have a similar PMF phenotype (that is, bone marrow megakaryocytic proliferation with atypia, fibrosis grades 2/3, and abnormal stem cell trafficking), their outcome is largely determined by the driver mutant gene. (B) Patients stratified according to a clinical-molecular prognostic model. As a proof of concept, this analysis illustrates the potential of integrating clinical and molecular data for improving the prognostication precision in clinical practice and in designing clinical trials. The clinical-molecular prognostic model depicted here includes JAK2, CALR, and MPL mutation status in addition to the IPSS variables. Modified from Rumi et al85 with permission.
Kaplan-Meier analysis of survival of PMF patients stratified according to their driver mutation or a clinical-molecular prognostic model that includes IPSS variables and driver mutation. (A) Patients stratified according to their driver mutation. This analysis illustrates the prognostic significance of the driver mutation: although all patients have a similar PMF phenotype (that is, bone marrow megakaryocytic proliferation with atypia, fibrosis grades 2/3, and abnormal stem cell trafficking), their outcome is largely determined by the driver mutant gene. (B) Patients stratified according to a clinical-molecular prognostic model. As a proof of concept, this analysis illustrates the potential of integrating clinical and molecular data for improving the prognostication precision in clinical practice and in designing clinical trials. The clinical-molecular prognostic model depicted here includes JAK2, CALR, and MPL mutation status in addition to the IPSS variables. Modified from Rumi et al85 with permission.
PMF is an MPN of high genetic complexity, characterized by various combinations of driver and cooperating mutations.12 Mutations in ASXL1, EZH2, SRSF2, or IDH1/2 have been shown to represent unfavorable prognostic factors, as these genetic lesions identify PMF patients who are at risk for premature death or leukemic transformation independent of the conventional prognostic scoring systems.87 In addition, the number of detrimental mutations represents an additional unfavorable prognostic factor per se, with 2 or more mutations being associated with shortened leukemia-free survival.88 Ideally, a clinical/molecular prognostic model should include not only the MPN-driver mutations, as shown in Figure 4B, but also cooperating mutations in order to capture all clinically relevant genetic data. In addition, we have recently found that higher grades of bone marrow fibrosis correlate with unique clinical and molecular aspects, and represent an independent adverse variable in patients with PMF.86
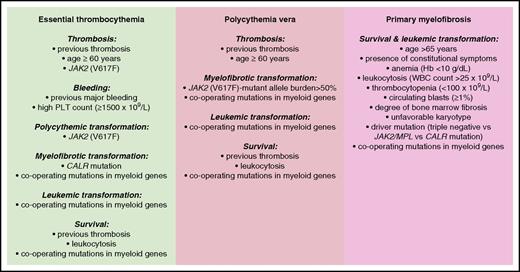
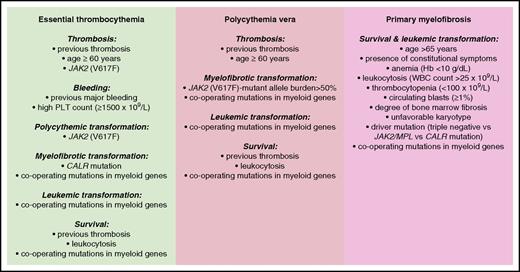
The main risk factors so-far identified in patients with MPNs are schematically illustrated in Figure 5.
Conventional and molecular risk factors for patients with MPNs. Information is from studies discussed in the “Risk stratification” section.
Conventional and molecular risk factors for patients with MPNs. Information is from studies discussed in the “Risk stratification” section.
Response evaluation
Currently defined response criteria
Response criteria for ET, PV, and PMF have been recently revised within collaborative projects of the IWG-MRT and the European LeukemiaNet89,90 : these criteria are primarily aimed at objectively assessing the value of new drugs for MPNs, and therefore appear particularly useful in clinical trial settings. In addition, simple scoring methods may be used to assess symptom burden among MPN patients, in both clinical practice and trial settings.91
Currently available treatments for ET patients are mainly aimed at minimizing the risk of thrombosis and/or bleeding. Response criteria cannot be defined in ET patients receiving low-dose aspirin, whereas the objective of treatment in those receiving cytoreductive drugs is to lower the PLT count below 450 × 109/L. However, the actuarial probability of thrombosis under cytoreductive treatment is influenced by leukocytosis and not by PLT count, indicating that this latter is not of crucial relevance in response evaluation, whereas correction of leukocytosis is likely more important.92
So far, prevention of thrombosis has also been the main goal of treatment in PV. Low-risk patients are treated with low-dose aspirin and phlebotomy, whereas high-risk patients are also given a cytoreductive treatment: in all patients, the objective of treatment is to lower hematocrit below 45%, a value that involves a significantly lower rate of cardiovascular death and major thrombosis compared with higher hematocrit values.93
Molecular analysis of response
So far, none of the available drugs for treatment of MPNs has shown clear evidence of a disease-modifying activity.94 However, a few studies have examined at molecular level the effect of compounds that can target the mutant myeloproliferative clone. These studies have shown that pegylated or regular interferon α can induce significant reduction in mutant allele burden in patients with JAK2 (V617F)-mutant ET or PV,95,96 and in those with CALR-mutant ET.97,98 More recently, ropeginterferon α-2b, a compound with longer elimination half-life, has been shown to induce complete molecular responses in about 20% of the PV patients treated.99 Ruxolitinib has been found to induce complete molecular responses in patients with PMF,100 and in patients with ET or PV who were refractory or intolerant to hydroxyurea.101
In order to evaluate response to drugs targeting the myeloproliferative clone, mutational analysis of hematopoiesis should include both MPN drivers and comutated myeloid genes. In PV patients, interferon α was found to target JAK2 (V617F)-mutant clones without affecting TET2-mutant cells,102 whereas in patients with CALR-mutant ET, interferon treatment was less effective in subjects with concomitant mutations in genes like TET2, ASXL1, IDH2, and TP53.98
Conclusions
In the past 10 years, there has been an impressive improvement in our understanding of the genetic basis of MPNs, but so far this has mainly translated into better diagnostic approaches to these disorders. Genetic data are currently used for developing innovative prognostic and predictive models, and to monitor disease response to innovative drugs that can target the mutant clone.
Acknowledgments
The authors thank all patients for participating in their studies, and the Associazione Italiana per la Ricerca sul Cancro (AIRC; Milan, Italy) for funding their research on MPNs.
This work was supported by the AIRC (MFAG 2014 Id.15672, and Special Program Molecular Clinical Oncology 5×1000, #1005).
Authorship
Contribution: E.R. and M.C. conceived and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mario Cazzola, Department of Hematology Oncology, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.

![Figure 1. Representative bone marrow biopsies from patients with MPNs. (A) ET: Normocellular marrow, proliferation of giant megakaryocytes with hyperlobulated nuclei, scattered or in loose clusters (hematoxylin and eosin [H&E], original magnification ×40). (B) PV: Hypercellular marrow with erythroid proliferation and scattered pleomorphic megakaryocytes (H&E, original magnification ×20). (C) PMF: Hypercellular marrow with granulocytic proliferation and large megakaryocytes with atypical bulbous nuclei (H&E, original magnification ×40). (D) Overt PMF: Hypercellular marrow, proliferation of atypical megakaryocytes forming dense clusters, and dilated vessels with intraluminal hematopoiesis (H&E, original magnification ×40). (E) Overt PMF (collagen fibrosis): Bands of collagen fibrosis within hematopoietic lacunae (Masson trichrome staining, original magnification ×40). Courtesy of Emanuela Boveri, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/6/10.1182_blood-2016-10-695957/4/m_blood695957f1.jpeg?Expires=1771160176&Signature=FTfMKLy~QmaQSWtuifFQsi9hqfkB43xCcjHqqAXRGY46YnH-ukFU6E-BLpKTzsEr2AA2jXI8Q7VC7OqrFqkQ7Ws~qo-z5tplhtiolaRmo~ywCrqc2ebqLWvAPO23OwpTzu5YnlgnzNHRhCOJWELm~5TeQ8vrbQ79MWXkJd438aqZxluAlH4vW-6nC8m9ON4pL50h0mh8lbz6vd72peUb5ftNFMxkMr2Fvx3RSQ0o0o9~qXObKrAE1lGtVBY0-2QkSNbjrlequq2JoHN5PVk5fpkCSjV194x3hYxOJcVRNZF~xmrrixBPYCSqT7n6JL~BA0-xtieYpQWzFVFtgSQioA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Representative bone marrow biopsies from patients with MPNs. (A) ET: Normocellular marrow, proliferation of giant megakaryocytes with hyperlobulated nuclei, scattered or in loose clusters (hematoxylin and eosin [H&E], original magnification ×40). (B) PV: Hypercellular marrow with erythroid proliferation and scattered pleomorphic megakaryocytes (H&E, original magnification ×20). (C) PMF: Hypercellular marrow with granulocytic proliferation and large megakaryocytes with atypical bulbous nuclei (H&E, original magnification ×40). (D) Overt PMF: Hypercellular marrow, proliferation of atypical megakaryocytes forming dense clusters, and dilated vessels with intraluminal hematopoiesis (H&E, original magnification ×40). (E) Overt PMF (collagen fibrosis): Bands of collagen fibrosis within hematopoietic lacunae (Masson trichrome staining, original magnification ×40). Courtesy of Emanuela Boveri, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo, Pavia, Italy.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/6/10.1182_blood-2016-10-695957/4/m_blood695957f1.jpeg?Expires=1771162168&Signature=pGiB9ZWV-Xod3zt9wL6vthmcy5RzPoq2DV3BvLTtSAvfNMkCFvYPusF1zTG8mF9hFPEbwse3~lDtbEpmLzqvFiKGQyZ7AnnGDtwE1wUnBsZAppvVu38q61DspeiXtRf52j~KYW0srIc6QvBjNxciRW4KsvRt~5o~JbU8g~Cj5cID9mLdWKyJXsIkQ-0FQdM4GHAD3RMGnodLPdH-Ec5YTL8zvgVpR50lTsgEKDC7dxO13k1MYqCRDOYr~ZQaz92MKAdF~Wq~11HS33VX5FCbUwZQ5Id3PLl6pnivLlx35H0uBuiUq~c8Xv0TOINUork7kQFDfVxy8sRaZQNKMtK7yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)