Abstract
There has been a major revolution in the management of patients with myeloproliferative neoplasms (MPN), and in particular those with myelofibrosis and extensive splenomegaly and symptomatic burden, after the introduction of the JAK1 and JAK2 inhibitor ruxolitinib. The drug also has been approved as second-line therapy for polycythemia vera (PV). However, the therapeutic armamentarium for MPN is still largely inadequate for coping with patients’ major unmet needs, which include normalization of life span (myelofibrosis and some patients with PV), reduction of cardiovascular complications (mainly PV and essential thrombocythemia), prevention of hematological progression, and improved quality of life (all MPN). In fact, none of the available drugs has shown clear evidence of disease-modifying activity, even if some patients treated with interferon and ruxolitinib showed reduction of mutated allele burden, and ruxolitinib might extend survival of patients with higher-risk myelofibrosis. Raised awareness of the molecular abnormalities and cellular pathways involved in the pathogenesis of MPN is facilitating the development of clinical trials with novel target drugs, either alone or in combination with ruxolitinib. Although for most of these molecules a convincing preclinical rationale was provided, the results of early phase 1 and 2 clinical trials have been quite disappointing to date, and toxicities sometimes have been limiting. In this review, we critically illustrate the current landscape of novel therapies that are under evaluation for patients with MPN on the basis of current guidelines, patient risk stratification criteria, and previous experience, looking ahead to the chance of a cure for these disorders.
Introduction: myeloproliferation beyond William Dameshek
The myeloproliferative neoplasms (MPN) essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF) were all first described in the late 19th or early 20th centuries. Parallels in their clinical features were first recognized by Dameshek, who coined the term myeloproliferative (MP). Landmark descriptions of JAK2V617F mutation, followed by those in JAK2 exon 12, MPL exon 10, and CALR exon 9, triggered reclassification as neoplasms (thus MPN) and promoted revised diagnostic criteria, and ultimately new treatments. Many of these aspects are touched on elsewhere in this review series; here we describe current therapies for ET, PV, and PMF (hereafter, MF is used for both PMF and MF arising after PV and ET), focusing on emerging aspects within this diverse field.
Clinical needs and risk-adapted conventional treatment options for MPN
PV and ET
Clinical manifestations of PV and ET are similar: both are primarily characterized by a high risk for thrombosis, hemorrhage, evolution to MF, acute myeloid leukemia (AML), and less commonly, myelodysplasia (MDS). Life expectancy is reduced in high-risk patients with PV, and with contemporary care, median survival is 10.9 years,1 yet for low-risk ET, some investigators report a standardized mortality ratio of 1,2 although others suggest younger patients, as a result of a longer duration of disease, accumulate more risk for transformation.2,3 PV and ET are managed with aspirin, venesection (PV), and selected use of hydroxycarbamide (HC), interferon α (IFNa), or anagrelide. First-line therapy remains suboptimal, with ongoing risk for thrombosis, hemorrhage, impaired quality of life, and risk for transformation. For example, despite treatment in high-risk PV, residual thrombosis risk is 2.93 per 100 patient-years,4 with overall risk for polycythemia vera (PPV)-MF (6%, 14%, and 26%) and AML (2%, 5%, and 10%) at 10, 15, and 20 years, respectively.5 In the setting of ET, 2 large randomized studies, PT-1 and ANAHYDRET, demonstrated either superiority of HC vs anagrelide in PT-16 or, in the case of ANAHYDRET, noninferiority of anagrelide.7 This causes some confusion, however: in the ANAHYDRET study, World Health Organization criteria were used to define ET, aspirin was not used, and the study was only powered for noninferiority. A recent large phase 4 study, EXELs, has reached the same conclusion as PT-1; that is, anagrelide therapy is associated with increased risk for arterial thrombosis, bleeding, and MF transformation, but less venous thrombosis than HC therapy.8
Some aspects merit specific consideration. These are achieving the target hematocrit for PV, use of aspirin, pegylated IFN, and treatment target. Although retrospective work suggested a target hematocrit of 0.45,9 this was only recently confirmed in a randomized trial.10 Some clinicians prefer to use a lower target of 0.42 for women, but this lacks an evidence base. Benefits of aspirin in PV were demonstrated in the ECLAP study11 and always inferred for ET; however, a recent analysis in low-risk CALR-positive ET suggests perhaps aspirin may have more harm than benefit.12 In this retrospective study, CALR vs JAK2 mutated patients treated with aspirin had the same risk for thrombosis, but more hemorrhage, than CALR-mutated patients not treated with aspirin. Potential confounding factors include the information that CALR-mutated patients have higher platelet counts, and thus potentially an underlying acquired von Willebrand disease defect. However, these data suggest that our strategies for this simple therapy merit further review.
Historically, the utility of IFNa has been restricted by poor tolerability; however, alternative formulations of interferon, such as Pegasys (pegylated interferon alfa-2a) and AOP2014 (peg-proline interferon alfa-2b) are currently in randomized trials. Much interest lies in the ability of IFNa to induce molecular responses in a portion of patients.13 Completing the large studies that are required for the endpoints that are really important to assess in this field, such as effects on thrombosis, transformation, and survival, is critical.14
MF
MF is associated with greatest symptom burden and largest effect on quality of life among the MPN, and has the worst prognosis. In the 1131 consecutive patients with PMF diagnosed between 1980 and 2007 and included in the development of the International Prognostic Score System (IPSS), overall median survival was 5.7 years (95% confidence interval, 5.1-5.6 years).15 Stratification according to the period of diagnosis (pre/post-1995) provided evidence of improving survival; however, this applied only to lower-risk patients, suggesting better supportive care but lack of disease-modifying effects.16
IPSS scores are used to facilitate decisions regarding allogeneic stem cell transplantation (HSCT),17 but are not useful for deciding about conventional management, which is rather problem-oriented. This “uncoupling” of prognostic scores from patient medical needs and therapeutic choices outside HSCT is exemplified by the absence of splenomegaly, 1 of the most relevant clinical manifestations and most common reasons for instituting therapy, among the variables of the IPSS scores. The inclusion of spleen size in future prognostic scores is suggested by the results of the COMFORT trials, which identified larger baseline spleen volume to associate with an increased risk for death.18
In the future, refinement of IPSS scores is likely, as disease with a CALR mutation, especially type 1/type 1-like,19-21 is associated with longer survival than JAK2, MPL, and so-called triple-negative disease.22 The high-molecular-risk category has been defined by the presence of a mutation in ASXL1, EZH2, SRSF2, IDH1, or IDH2.23 Prognosis is also informed by specific cytogenetic abnormalities.24,25 Integration of these variables in risk scores might improve precision of prognostic criteria, although how this will affect therapeutic decisions and correlate with medical needs remains to be ascertained. For example, ASXL1 or triple-negative mutation status in a Dynamic International Prognostic Score System (DIPSS) intermediate-1 patient might make them a putative candidate for HSCT, as such a patient would have a worse prognosis when the molecular status is considered.17
The only potentially curative approach for MF is HSCT. However, high transplant-related mortality (20% to 40%) renders the procedure unattractive, age-related comorbidities are virtually always present, and potential family donors are usually old. Recent consensus criteria (Table 1) were generated by the European Leukemia Net and the European Bone Marrow Transplant group, aiming to bridge clinical practice and clinical research, particularly with regard to the choice of the intensity, the modalities of the conditioning regimen, and the effect of ruxolitinib.17 Future improvements in transplant procedures and better preparation of the patient might result in an increasing usage of transplant at earlier disease stages, hopefully translating into better outcome. At this time, less than 10% of patients with MF are even referred for HSCT.26
Recommendations on selection and preparation measures for patients with MF referred to HSCT, as developed by the European Leukemia Net/International Working Group–Myeloproliferative Neoplasms Research and Treatment panel
| Recommended . | Indicated* . | Not recommended . |
|---|---|---|
| Patient selection | ||
| Patients with intermediate-2-risk or high-risk disease according to IPSS, DIPSS, or DIPSS-plus, and age <70 y | Patients with intermediate-1-risk disease and age <65 y if they present with either refractory, transfusion-dependent anemia or more than 2% blasts in peripheral blood or adverse cytogenetics | Patients with low-risk disease |
| Patients with intermediate-1-risk disease if they are triple negative, ASXL1 positive, or both | Patients in blast transformation | |
| Patients in blast transformation after achieving a partial or complete remission of leukemia with debulking therapy | ||
| Pretransplant management | ||
| Iron chelation therapy in severely iron overloaded patients only | Ruxolitinib treatment of patients with a symptomatic spleen and/or constitutional symptoms | Splenic irradiation–splenectomy (case-by-case decision) |
| Recommended . | Indicated* . | Not recommended . |
|---|---|---|
| Patient selection | ||
| Patients with intermediate-2-risk or high-risk disease according to IPSS, DIPSS, or DIPSS-plus, and age <70 y | Patients with intermediate-1-risk disease and age <65 y if they present with either refractory, transfusion-dependent anemia or more than 2% blasts in peripheral blood or adverse cytogenetics | Patients with low-risk disease |
| Patients with intermediate-1-risk disease if they are triple negative, ASXL1 positive, or both | Patients in blast transformation | |
| Patients in blast transformation after achieving a partial or complete remission of leukemia with debulking therapy | ||
| Pretransplant management | ||
| Iron chelation therapy in severely iron overloaded patients only | Ruxolitinib treatment of patients with a symptomatic spleen and/or constitutional symptoms | Splenic irradiation–splenectomy (case-by-case decision) |
Not a strong recommendation, but case-by-case approach.
Conventional medical options in the pre-JAK inhibitor (JAKi) era were limited. In early stages of the disease, there are some data from case-series to support the use of IFNa.27,28 HC is recommended for control of leukocytosis, thrombocytosis, and splenomegaly, although data from controlled studies are lacking; effect on symptoms is minimal and was no different from placebo in the COMFORT studies.29 Splenectomy may be indicated for selected patients with symptomatic, treatment-resistant splenomegaly; for transfusion-dependent anemia; or for symptomatic portal hypertension when balanced against risks; that is, 5% to 10% mortality and 30% to 40% risks for thrombosis, bleeding, or infection. Splenectomy is not recommended in the presence of severe thrombocytopenia because of low efficacy and because this often heralds leukemic transformation.30,31 Splenic irradiation is rarely used, and its effect is short-lived and may be accompanied by severe prolonged cytopenias. Local radiotherapy is indicated for localized extramedullary hematopoiesis and in MF-associated pulmonary hypertension.
Anemia, a strong predictor of inferior survival, occurs in more than one third of the patients at presentation and worsens progressively. Corticosteroids, erythropoietin, and danazol32 are used, with transient improvements in 20% to 40%.33 The immunomodulator thalidomide is now rarely used because of toxicity and modest efficacy,34 and lenalidomide is recommended only in the presence of 5q abnormality.35 A phase 1-2 study of pomalidomide reported responses in up to 36% of the patients in association with prednisone36 ; unfortunately, a phase 3 study failed confirm these results.37
JAK inhibition targeted therapy for MPN
Ruxolitinib the first approved targeted therapy in MPN
MF
The JAK1/JAK2 inhibitor ruxolitinib was evaluated in the COMFORT-I and COMFORT-II studies, delivering meaningful results for spleen volume reduction and improvement in symptoms and quality of life.38,39 Dose-limiting myelosuppression, particularly in the first 3 months of therapy, was common. Increased risk for infection has been documented, ranging from common bacterial/viral ailments to rare severe infections such as progressive multifocal leukoencephalopathy (reviewed in O’Sullivan et al40 ). Follow-up data demonstrated that clinical benefits were generally maintained and, importantly, provided compelling evidence that ruxolitinib is associated with a survival advantage compared with placebo or conventional therapy. This was confirmed in comparison with historical cohorts and for molecularly defined higher-risk disease.41-43 A Cochrane review concluded that evidence of survival benefit was lacking but is now out of date with regard to the latest trial data, as it included studies published up to October 2012 only.44
Speculation continues as to the reason for survival advantage with ruxolitinib: improvements in clinical status, for example, reversal of cachexia and control of systemic inflammation, are potential reasons.45,46 Long-term therapy may halt bone marrow fibrosis, and gradual decreases in mutant allele burden are seen.47,48 Complete histopathological and/or molecular remission has rarely been observed.48,49 Finally, recent data suggest that the likelihood of treatment success and durability, that is, the likelihood of achieving spleen response and time to treatment failure, diminishes incrementally with the presence of multiple adverse prognostic mutations.50 Experience to date may suggest that timely initiation of ruxolitinib, for example, in intermediate-1 or low-risk MF, might maximize potential longer-term benefits. Available data support the use of ruxolitinib in selected patients with intermediate-1-risk disease,51-53 and the ReTHINK trial is underway for patients with lower-risk disease and adverse mutational profile.54 At this time, a careful evaluation of benefit–risk ratio is required before initiating ruxolitinib in patients with lower-risk disease outside a clinical trial, where the main indication might, for example, be rapidly progressive splenomegaly or severe symptoms unresponsive to standard therapy and that are not of prognostic significance; for example, pruritus.
PV
Ruxolitinib has been evaluated in 3 phase 3 studies, RESPONSE, RESPONSE2, and RELIEF, showing that in the second line after HC failure/intolerance, ruxolitinib effectively controls blood count, spleen size, and symptoms, and interestingly, there may be molecular responses in some patients.55-58 In RESPONSE, there was a reduction in thrombotic events (6 vs 1), although not a preplanned endpoint, which thus cannot be overinterpreted; transformation to PPV-MF and AML occurred on both arms of the study. Selection of patients resistant to HC is important because those patients with cytopenias in particular have an increased risk for death (hazard ratio, 3.5; 95% confidence interval, 1.5-8.3; P = .003). Cytopenia at the lowest dose required to achieve a response was also an independent risk factor for transformation to acute leukemia (hazard ratio, 20.3; 95% confidence interval, 5.4-76.5; P < .001).59,60 Problems with these studies is the selection of the endpoints: control of hematocrit, reduction of spleen size, and as a result of restricted alternative therapies, the comparison of ruxolitinib against drugs the patient already failed. Nonetheless, ruxolitinib presents a valuable treatment of PV resistant and/or intolerant of HC and could be tested earlier in disease to assess the effect on major manifestations and disease course. No new safety signals emerged here, but an excess of herpes zoster reactivation and nonmelanoma skin cancers were observed, the latter perhaps as a result of prior HC exposure, but nonetheless meriting attention.
Essential thrombocythemia
In a phase 2 study, 39 ET patients refractory/intolerant to HC were treated with ruxolitinib for a median of 205.6 weeks. At data cut off, 61.5% of patients continued ruxolitinib, and there was some efficacy in blood count and symptom and spleen reduction.61 However, there remains a question of the efficacy of ruxolitinib vs other conventional therapies. The primary analysis of the ET arm of the randomized phase 2 MAJIC study showed equivalence for ruxolitinib compared with best available therapy in the primary endpoint of complete hematological response; further data are awaited.62
Other JAK inhibitors
Unfortunately, several other JAKi have been halted in development; for example, fedratinib was withdrawn because of the development of Wernicke’s encephalopathy, despite having positive results from a phase 3 clinical trial.63 Pacritinib and momelotinib are discussed here; other drugs of interest include NS01864 and INCB039110.65 None are currently being evaluated in PV or ET.
Pacritinib was evaluated in phase 3 trials PERSIST-1 and PERSIST-2. Results are available from PERSIST-166 ; myelosuppression was not as marked as anticipated, and 23% of transfusion-dependent patients became transfusion-independent. Pacritinib is potentially attractive for cytopenic patients with MF; indeed, the PERSIST-2 study enrolled only patients with platelets less than 100 × 109/L. In February 2016, pacritinib was put onto a clinical hold because of concern about survival, increased bleeding, and cardiac events; hopefully, it will become clear whether these concerns are valid or not.
Momelotinib, a JAK1 and JAK2 inhibitor, produced anemia responses in addition to improvements in spleen and symptom burden.67,68 Intriguingly, multivariate analysis suggested best responses occurred in CALR+/ASXL1− patients.69 Peripheral neuropathy was reported and may be important in deciding where momelotinib sits in the therapeutic algorithm. Results of phase 3 studies (SIMPLIFY-1, comparing momelotinib with ruxolitinib, and SIMPLFY-2, assessing momelotinib in patients who have failed ruxolitinib) are expected soon.
JAK inhibitors in combination
There is significant interest in combining JAKi with traditional and experimental agents. The rationale for such combination therapy could be to maximize the dose of ruxolitinib by, for example, maintaining the platelet count with danazol, maintaining hemoglobin with pomalidomide, or improving outcome (eg, better spleen response). Multiple studies with immunomodulatory agents (IMiDs), androgens, histone deacetylase inhibitors (HDACi), phosphatidylinositol 3-kinase (PI3K) inhibitors, smoothened inhibitors, and before HSCT and so on have been initiated (Table 2).70-78 Criteria used for judging success are complex and not validated, so there is a risk of missing real signals.
Selected data for combination studies with ruxolitinib
| Drug/acronym . | Eligible pts . | Number of pts . | Other findings (eg, MTD) . | Benefits . | Toxicity . |
|---|---|---|---|---|---|
| Ruxolitinib and buparlisib (BKM120) | Intermediate-/high-risk MF spleen >5 cm; both ruxolitinib-naive (arm A) and previously treated (arm B) | Arm A: 22 pts (11 in both escalation and expansion phase) | MTD: ruxolitinib 15 mg twice daily/buparlisib 60 mg daily | Expansion phase at week 24, 45% (5/11) of pts vs 18% (2/11) of pts in arm A and arm B, respectively, achieved a ≥35% reduction from BL in spleen volume | Grade 3/4 nonhematological AEs in >1 pt: anxiety (arm A, n = 2/22), multiorgan failure (arm B, n = 2/20) as a result of AML and progressive disease |
| HARMONY Study74 | Arm B: 20 pts (9 in escalation and 11 in expansion phase) | At week 24, a median (range) change of −3.35 (−26.9 to 2.7) was observed in JAK2V617F allele burden from BL in arm A and 0.60 (−12.6 to 24.7) in arm B at MTD | Grade 3/4 hematologic AEs included anemia (arm A, n = 2/22; arm B, n = 7/20) and thrombocytopenia (arm A, n = 5/22; arm B, n = 6/20) | ||
| Bone marrow fibrosis at 24 wk, 4 pts (n = 3 in arm A; 1 in arm B) and 19 pts (n = 9 in arm A; 10 in arm B) showed an improvement and stabilization, respectively; 2 pts in arm A (0 in arm B) experienced worsening of bone marrow fibrosis | 3 deaths reported at MTD in arm B; 2 deaths attributed to myeloid leukemia and 1 to multiorgan failure secondary to progressive MF | ||||
| Ruxolitinib and sonidegib (LDE225)72 | Intermediate-/high-risk MF spleen >5 cm not previously treated with a JAK inhibitor or HPI | 27 pts were treated at the RP2D | RP2D: sonidegib 400 mg once daily, ruxolitinib 20 mg twice daily | End of week 24, 12 pts (44.4%), ≥35% reduction in spleen volume | 5 pts discontinued treatment as a result of an AE, and 1 pt each as a result of pt decision and death (multiorgan failure unrelated to study treatment occurring 2 d after |
| 15 pts (55.6%) achieved a ≥35% reduction in spleen volume at any time on treatment | |||||
| ≥50% reduction in spleen length at any time achieved by 25 pts (92.6%), with 15 pts (55.6%) having a nonpalpable spleen | Overall, the most common AEs regardless of causality (all grade; grade 3/4) were anemia (52%; 33%) and muscle spasms (48%; 4%) | ||||
| The mean change in JAK2 V617F allele burden was −9.0% (range, −56.5% to 7.0%) from BL to the end of week 24 | AEs requiring dose adjustment or interruption in 17 pts (63%), most common being increased CK (19% [n = 5]) and myalgia (19% [n = 5]) | ||||
| BM fibrosis: 2 pts improved (grade 3 to grade 2), 8 pts remained stable, and 3 pts had a worsening from BL by the end of week 24 | |||||
| Ruxolitinib and panobinostat76 | Intermediate-1, Intermediate-2, or high-risk MF and spleen ≥5 cm by palpation; no prior JAKi | 61 pts (escalation phase, n = 38; expansion phase, n = 23) | RP2D: ruxolitinib 15 mg twice daily, panobinostat 25 mg three times a week | In expansion phase at week 24 (87%; 20/23) and at week 48 (74%; 17/23); 57% (13/23) and 39% (9/23) of pts achieved a ≥35% reduction from baseline in spleen volume | For 34 pts treated at the RP2D, 65% remained on treatment, and 21% discontinued because of AEs |
| BM fibrosis (n = 12 evaluable): 4 had improved fibrosis at wk 48, 6 had no change, and 2 worsened | Most common grade 3/4 hematologic AEs (at RP2D), regardless of causality: | ||||
| JAK2 V617F allele burden (n = 17 evaluable): 5 (29%) had a ≥20% reduction by wk 48 | Anemia (32%), thrombocytopenia (29%) | ||||
| Inflammatory markers (IL-18, MMP-9, and MPO) normalized | Grade 3/4 nonhematologic AEs included diarrhea (18%), asthenia (12%), and fatigue (9%). | ||||
| Three deaths (resulting from progression of underlying disease, myocardial infarction, and hypoxic cardiac arrest) attributed as unrelated | |||||
| Ruxolitinib and interferon COMBI trial70 | PV or MF evidence of active disease | 30 pts, PMF (n=10) OR PV (n=20) ± prior treatment with IFNa2 | Initial therapy was IFNa2 45 μg × 1 sc/week (Pegasys) or 35 μg × 1 sc/week (PegIntron) + ruxolitinib (Jakavi) 20 mg × 2/d | Overall, complete response was achieved as best response in 19 pts (63.3%), and partial response or major response in 8 pts (26.7%); only 3 pts (10%) had no response to treatment | 3 pts discontinued treatment because of an AE; one patient died of transformation to AML shortly after initiation of CT and was not included in this interim analysis |
| Palpable splenomegaly in 7 pts at baseline was significantly reduced by week 2; hct control without phlebotomy was achieved by week 4 in 78% of pts (7 of 9) who at baseline had an elevated hct, only 3 pts required a total of 3 phlebotomies after initiation of CT | Anemia (n = 15, 2 grade 3), granulocytopenia (n = 13, 2 grade 3), or thrombocytopenia (n = 6, 1 grade 3) | ||||
| JAK2V617F% declined significantly | Eleven severe AEs requiring hospitalization: pneumonia (n = 3); fever (n = 2); lipotymia, hematemesis, phlebitis, herpes zoster, angina pectoris, and arterial hypertension (n = 1 pt each) | ||||
| Ruxolitinib and danazol75 | Patients with MF with anemia | 14 patients (9 had prior JAKi) | Ruxolitinib, 10 mg twice-daily or 5 mg twice-daily in combination with tapered danazol up to 200 mg orally three times a day | 4/5 JAKi-naive pts had stable or increasing hgb levels | Nine pts discontinued: 2 progressive disease, 2 adverse event, 2 preference, 1 stem cell transplant, 1 unrelated death, 1 comorbidity. |
| 5/9 pts on prior JAKi, 5 (55.5%) and 7 (77.7%) patients had stable or increasing hgb or plt levels. | Grade ≥3 anemia in 7, neutropenia in 2 leukopenia in 1 and thrombocytopenia in 2 pts | ||||
| Symptoms 4 had at least 50% improvement from baseline on MPN-SAF TSS | Grade ≥3 electrolyte abnormalities in 3, infection in 2, edema in 1, hypertension in 1, and intracranial hemorrhage in 1 pt | ||||
| Overall treatment response: stable disease in 10 pts, clinical improvement in 3, all of which were spleen responses, and progressive disease in 1 |
| Drug/acronym . | Eligible pts . | Number of pts . | Other findings (eg, MTD) . | Benefits . | Toxicity . |
|---|---|---|---|---|---|
| Ruxolitinib and buparlisib (BKM120) | Intermediate-/high-risk MF spleen >5 cm; both ruxolitinib-naive (arm A) and previously treated (arm B) | Arm A: 22 pts (11 in both escalation and expansion phase) | MTD: ruxolitinib 15 mg twice daily/buparlisib 60 mg daily | Expansion phase at week 24, 45% (5/11) of pts vs 18% (2/11) of pts in arm A and arm B, respectively, achieved a ≥35% reduction from BL in spleen volume | Grade 3/4 nonhematological AEs in >1 pt: anxiety (arm A, n = 2/22), multiorgan failure (arm B, n = 2/20) as a result of AML and progressive disease |
| HARMONY Study74 | Arm B: 20 pts (9 in escalation and 11 in expansion phase) | At week 24, a median (range) change of −3.35 (−26.9 to 2.7) was observed in JAK2V617F allele burden from BL in arm A and 0.60 (−12.6 to 24.7) in arm B at MTD | Grade 3/4 hematologic AEs included anemia (arm A, n = 2/22; arm B, n = 7/20) and thrombocytopenia (arm A, n = 5/22; arm B, n = 6/20) | ||
| Bone marrow fibrosis at 24 wk, 4 pts (n = 3 in arm A; 1 in arm B) and 19 pts (n = 9 in arm A; 10 in arm B) showed an improvement and stabilization, respectively; 2 pts in arm A (0 in arm B) experienced worsening of bone marrow fibrosis | 3 deaths reported at MTD in arm B; 2 deaths attributed to myeloid leukemia and 1 to multiorgan failure secondary to progressive MF | ||||
| Ruxolitinib and sonidegib (LDE225)72 | Intermediate-/high-risk MF spleen >5 cm not previously treated with a JAK inhibitor or HPI | 27 pts were treated at the RP2D | RP2D: sonidegib 400 mg once daily, ruxolitinib 20 mg twice daily | End of week 24, 12 pts (44.4%), ≥35% reduction in spleen volume | 5 pts discontinued treatment as a result of an AE, and 1 pt each as a result of pt decision and death (multiorgan failure unrelated to study treatment occurring 2 d after |
| 15 pts (55.6%) achieved a ≥35% reduction in spleen volume at any time on treatment | |||||
| ≥50% reduction in spleen length at any time achieved by 25 pts (92.6%), with 15 pts (55.6%) having a nonpalpable spleen | Overall, the most common AEs regardless of causality (all grade; grade 3/4) were anemia (52%; 33%) and muscle spasms (48%; 4%) | ||||
| The mean change in JAK2 V617F allele burden was −9.0% (range, −56.5% to 7.0%) from BL to the end of week 24 | AEs requiring dose adjustment or interruption in 17 pts (63%), most common being increased CK (19% [n = 5]) and myalgia (19% [n = 5]) | ||||
| BM fibrosis: 2 pts improved (grade 3 to grade 2), 8 pts remained stable, and 3 pts had a worsening from BL by the end of week 24 | |||||
| Ruxolitinib and panobinostat76 | Intermediate-1, Intermediate-2, or high-risk MF and spleen ≥5 cm by palpation; no prior JAKi | 61 pts (escalation phase, n = 38; expansion phase, n = 23) | RP2D: ruxolitinib 15 mg twice daily, panobinostat 25 mg three times a week | In expansion phase at week 24 (87%; 20/23) and at week 48 (74%; 17/23); 57% (13/23) and 39% (9/23) of pts achieved a ≥35% reduction from baseline in spleen volume | For 34 pts treated at the RP2D, 65% remained on treatment, and 21% discontinued because of AEs |
| BM fibrosis (n = 12 evaluable): 4 had improved fibrosis at wk 48, 6 had no change, and 2 worsened | Most common grade 3/4 hematologic AEs (at RP2D), regardless of causality: | ||||
| JAK2 V617F allele burden (n = 17 evaluable): 5 (29%) had a ≥20% reduction by wk 48 | Anemia (32%), thrombocytopenia (29%) | ||||
| Inflammatory markers (IL-18, MMP-9, and MPO) normalized | Grade 3/4 nonhematologic AEs included diarrhea (18%), asthenia (12%), and fatigue (9%). | ||||
| Three deaths (resulting from progression of underlying disease, myocardial infarction, and hypoxic cardiac arrest) attributed as unrelated | |||||
| Ruxolitinib and interferon COMBI trial70 | PV or MF evidence of active disease | 30 pts, PMF (n=10) OR PV (n=20) ± prior treatment with IFNa2 | Initial therapy was IFNa2 45 μg × 1 sc/week (Pegasys) or 35 μg × 1 sc/week (PegIntron) + ruxolitinib (Jakavi) 20 mg × 2/d | Overall, complete response was achieved as best response in 19 pts (63.3%), and partial response or major response in 8 pts (26.7%); only 3 pts (10%) had no response to treatment | 3 pts discontinued treatment because of an AE; one patient died of transformation to AML shortly after initiation of CT and was not included in this interim analysis |
| Palpable splenomegaly in 7 pts at baseline was significantly reduced by week 2; hct control without phlebotomy was achieved by week 4 in 78% of pts (7 of 9) who at baseline had an elevated hct, only 3 pts required a total of 3 phlebotomies after initiation of CT | Anemia (n = 15, 2 grade 3), granulocytopenia (n = 13, 2 grade 3), or thrombocytopenia (n = 6, 1 grade 3) | ||||
| JAK2V617F% declined significantly | Eleven severe AEs requiring hospitalization: pneumonia (n = 3); fever (n = 2); lipotymia, hematemesis, phlebitis, herpes zoster, angina pectoris, and arterial hypertension (n = 1 pt each) | ||||
| Ruxolitinib and danazol75 | Patients with MF with anemia | 14 patients (9 had prior JAKi) | Ruxolitinib, 10 mg twice-daily or 5 mg twice-daily in combination with tapered danazol up to 200 mg orally three times a day | 4/5 JAKi-naive pts had stable or increasing hgb levels | Nine pts discontinued: 2 progressive disease, 2 adverse event, 2 preference, 1 stem cell transplant, 1 unrelated death, 1 comorbidity. |
| 5/9 pts on prior JAKi, 5 (55.5%) and 7 (77.7%) patients had stable or increasing hgb or plt levels. | Grade ≥3 anemia in 7, neutropenia in 2 leukopenia in 1 and thrombocytopenia in 2 pts | ||||
| Symptoms 4 had at least 50% improvement from baseline on MPN-SAF TSS | Grade ≥3 electrolyte abnormalities in 3, infection in 2, edema in 1, hypertension in 1, and intracranial hemorrhage in 1 pt | ||||
| Overall treatment response: stable disease in 10 pts, clinical improvement in 3, all of which were spleen responses, and progressive disease in 1 |
AE, adverse events; hbg, hemoglobin; hct, hematocrit; HPI, hedgehog pathway inhibitor; Int, intermediate; MPN-SAF TSS, myeloproliferative neoplasms symptom assessment form total symptom score; MTD, maximum tolerated dose; RP2D, recommended phase 2 dose; plt, platelets; pt(s), patient(s); sc, subcutaneously.
Emerging therapies beyond JAK inhibitors
Drugs acting on the PI3K/Akt/mTOR pathway
Receptor-mediated activation of PI3K promotes a cascade of events involving Ak-strain transforming (Akt) activating the mechanistic target of rapamycin (mTOR) kinase complexes, mTORC1 and mTORC2; a pathway indirectly activated by JAK2/STAT.79-81 Different PI3K/Akt/mTOR inhibitors dose-dependently inhibited JAK2V617F-mutated cell lines and reduced clonogenic activity of hematopoietic progenitors from patients with MPN.82 BEZ235, a PI3K/mTOR inhibitor, showed activity in JAK2V617F-mutated Hel cells83 and induced apoptosis in PMF progenitor cells synergizing with fedratinib and TG101209.84 In JAK2V617F knock-in mice, combination of ruxolitinib and BEZ235 was most effective in spleen reduction and ameliorating spleen histopathology.83 No trial has been initiated in MF. Buparlisib (BKM120), an inhibitor of PI3K, is under evaluation in combination with ruxolitinib for patients with MF (Table 2). Everolimus (RAD-001), an mTORC1 inhibitor used in prevention of organ rejection, effectively inhibited proliferation of JAK2V617F-mutated cell lines, alone and synergistically with JAK1/JAK2i.82 In a phase 1/2 study in patients with MF, the response rate was 23%.85 At present, there is no ongoing trial in MF. One limitation of pure mTORC1 inhibitors is that their effects may be prevented by feedback upregulation of Akt.86 Other potentially interesting PI3K/Akt/mTOR inhibitors have not yet been evaluated in MPN.
Drugs acting as farnesyl transferase inhibitors
In vitro, the FT inhibitor tipifarnib (Zarnestra) inhibited proliferation of MF progenitors,87 and a phase 2 trial in MF, although showing benefit (33% response rate for hepatosplenomegaly, 38% for anemia), was limited because of myelosuppression and disease progression.88 This drug target is not actively being evaluated in MF.
HDACi
HDACi also affect nonhistone targets (eg, Bcl2, p53, telomerase); this may be important for both efficacy and toxicity. Valproic acid, in clinical use as an anticonvulsant, has weak HDACi activity but clinical efficacy in cancer; there is one report of its success in the treatment of MF.89 Vorinostat (suberanilohydroxamic acid, Zolinza) showed effectiveness in reducing splenomegaly, pruritus, and thrombocytosis/leukocytosis, but had significant adverse effects in ET/PV90 ; a study is ongoing in MF. Panobinostat has been shown to promote JAK2V617F degradation synergistically with TG101209.91 A phase 1 study of the drug used alone in MF demonstrated clinical effects at low doses given during a long period of time with minimal adverse effects.92 There are currently 2 phase 1/2 trials assessing the safety and tolerability of the combination of panobinostat and ruxolitinib (Table 2). In vitro, givinostat inhibits the clonogenic potential of JAK2V617F-mutated progenitor cells at concentrations 10 times lower than JAK2 wild-type cells,93 and in a phase 2a study of patients with ET/PV, clinical activity was observed in about half.94 Subsequently, 44 patients with PV refractory/intolerant to HC were randomly assigned to receive 2 different doses of givinostat with HC,95 and responses were reported in about half of the patients without a dose–response relationship. A phase 1b/2 dose-escalation study to assess MTD and efficacy of givinostat is ongoing. Pracinostat was evaluated in a phase 2 study96 in 22 patients with MF; modest activity was noted, with 27% having reduction in splenomegaly and 36% deriving some clinical benefit.
Hypomethylating agents
Hypermethylation of CpG islands causing inactivation of tumor suppressor genes has been seen in leukemic transformation of MF,97 but rarely in “chronic phase” MF.98 Decitabine is effective in MDS and has been used successfully in 1 sporadic case of chronic phase MF.99 Its use might be beneficial in patients who show signs of leukemic transformation and for whom HSCT is not an option.100,101 A phase 2 study of azacitidine showed limited clinical activity, with 23% of patients with MF having a response after a median duration of 5 months, and myelosuppression was a major toxicity.102 There may be more efficacy, however, when there is evidence of an acute leukemic transformation.103
Telomerase inhibitors
High telomerase activity is characteristic of cancer cells and is also detected in MF.104,105 Imetelstat, a telomerase inhibitor, was used as second-line therapy in 18 patients with ET, reporting rapid (median, 6.1 weeks) and complete hematological response in 89% of the patients and concomitant reductions of mutation allele burden.106 In an independent study, 33 patients with intermediate-2 or high-risk MF, 48% of whom pretreated with JAK inhibitor, received imetelstat at 1- to 3-week infusion intervals. Complete or partial response was observed in 21%, with median duration of 10 to 18 months.107 Reversal of BM fibrosis was reported in 3 of 4 patients in complete response, and there were reductions of JAK2V617F allele burden. Interestingly, none of the JAK2 wild-type patients had a response compared with 28% of JAK2V617F-mutated patients, whereas ASXL1 mutations were associated with lack of response and SF3B1 or U2AF1 mutations predicted response. Most common adverse events were myelotoxicity and elevations of liver enzymes, which tended to recover in most patients. A randomized phase 2 study (IMBARK) in patients with MF relapsed or refractory to ruxolitinib is ongoing, whereas clinical development in ET has been stopped. The facts that no changes in telomere length were observed in patients with MF receiving imetelstat, and that the initial length of telomeres did not predict the clinical response, raise intriguing questions about the mechanism of action of imetelstat.
Hedgehog pathway inhibitors
Abnormalities in hedgehog signaling were detected in the BM and spleen of GATA-1low myelofibrotic mice,108 and combination of JAK2 and hedgehog inhibitor was more active than ruxolitinib alone.109 In a phase 1b study in MF, the SMO inhibitor sonidegib was combined with ruxolitinib, but no improved efficacy was recorded and there was some toxicity (Table 2).72 Other hedgehog inhibitors currently in clinical trials include saridegib (IPI-926), which had negative results110 ; erismodegib (LEE225), together with ruxolitinib; and PF 04449913.
Angiogenesis inhibitors
Antifibrotic agents
The pathogenesis of bone marrow fibrosis in MPN is ascribed to inflammatory and fibrogenic cytokines released by clonal cells, particularly megakaryocytes and monocytes. In turn, this altered microenvironment might favor maintenance/expansion of the malignant clone.116 PRM-151 is a recombinant form of human pentraxin-2, an endogenous regulator of tissue repair that was shown to prevent and/or reverse fibrosis in preclinical models because of its ability to inhibit fibrocyte differentiation. Interestingly, clonal fibrocytes producing collagen and fibronectin were abundantly present in the bone marrow of patients with MF; mice xenografted with cells from patients with MF develop a lethal MF-like disease that was slowed by PRM-151.117 In patients with MF, plasma levels of PTX-2 are reduced compared with in healthy controls, and are inversely correlated with fibrosis grade. In a phase 2 study in MF, PRM-151, in combination with ruxolitinib, was well tolerated, and 35% and 70% of the patients had 1 or more grade reduction of fibrosis at 24 and 72 weeks, respectively. Increases in hemoglobin and reduction of transfusions were also seen, whereas spleen volume reduction and symptomatic improvement occurred in a minority. A phase 2 study (PROMOTE) in patients intolerant/refractory/ineligible for ruxolitinib is ongoing. In a phase 1 trial, fresolimumab, a monoclonal antibody neutralizing all isoforms of transforming growth factor β, induced reduction in transforming growth factor β levels in the 2 evaluable patients, who also had some improvement of anemia, but no significant changes in fibrosis.118 Lysyl oxidase (LOX) and lysyl oxidase like (LOXL) are amine oxidases involved in crosslinking and stabilization of collagen and elastin fibers. LOX levels have been found elevated in patients with MF.119 In GATA-1low mice, LOX inhibition led to decrease of bone marrow fibrosis.120 Simtuzumab, a humanized antibody against LOXL2, was used in 54 patients with MF; treatment was well tolerated, but no clear signal of response was observed.121
Other emerging therapies: CRISPR and immunotherapy
CRISPR/Cas9 is a novel genome editing technology that allows for targeting and modifying specific sites in the genome and that is becoming a major avenue for gene therapy.122,123 Using induced pluripotent stem cells as a model, specific target of the JAK2V617F mutated allele over the wild-type was demonstrated,124 speaking in support of its potential for treating these diseases. However, this is still far from being clinically applicable.
There is yet no evidence that the immunotherapeutic approach might be effective in MPN, outside the settings of HSCT. In theory, the novel protein terminal originated by CALR frameshift mutations might serve as a cancer-associated epitope for either antibody- or cell-mediate immune targeting. Similarly, immune checkpoint inhibitors are potentially worthwhile to be evaluated, although we lack any information about the expression of their targets, such as PD1 and PDL1, in MPN125 ; a study is currently beginning in MF.
Conclusions
MPN present the clinician with a number of significant therapeutic challenges, and although much progress has been made in recent times, as always, there is more work to be done. As we consider the breadth of these conditions, we reflect that there are different needs for the various types of MPN at different stages or risk levels. As a key principle, it is critical to be able to achieve an accurate diagnosis. At the present time, we retain the entities described in previous centuries: ET, PV, and PMF. Yet, even with perfect application of current diagnostic criteria, there is marked clinical heterogeneity. Perhaps with increasing availability of rapid reliable biological profiling techniques, we might be better able to identify subgroups of patients with more homogeneous clinical behavior who might respond best to specific therapies. Such technologies could also be used perhaps to monitor patients through the course of their disease to judge when they might need a change of therapeutic strategy. In a similar manner, better-validated endpoints for trials and important targets for use in standard clinical practice are required.
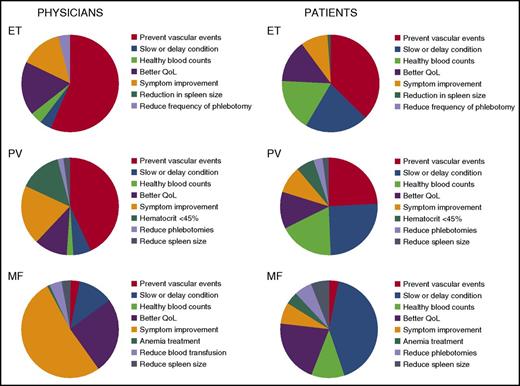
There is now a significant body of evidence to suggest that clinicians should focus attention not only on the conventional aspects of disease (ie, management of vascular risk and judicious use of aspirin and agents such as HC, IFNa, JAK inhibitors, etc) but also on the symptoms and effect on quality of life of these diseases. In these chronic conditions, the apparent and sometimes striking mismatch between physician perception of and the patient’s stated priority for management demonstrated in the landmark study is a matter for reflection and review if proven in a wider study (Figure 1).126 Another interesting disconnection in our therapeutic strategies for these illnesses is well demonstrated in MF, where prognostic data are used only in deciding whether to consider transplantation or not.
Priorities for MPN treatment, perceptions of physicians and patients. A figure redrawn from the data presented in the landmark study.126 QoL, quality of life.
Priorities for MPN treatment, perceptions of physicians and patients. A figure redrawn from the data presented in the landmark study.126 QoL, quality of life.
We have reflected critically on the current gaps in our knowledge and ability to better coordinate patient needs with basic and translational science; however, we must also look back on the past decade as one during which this field made great advances, including the notable achievement of delivering a novel targeted therapy that not only prolongs but, critically, also improves the quality of life for patients. This has not been possible without a great deal of positive collaboration among patients, clinicians, basic researchers, and pharma, and it is these links that will surely deliver the advances that are still required.
Acknowledgment
This work was supported by a special grant from Associazione Italiana per la Ricerca sul Cancro (project 1005) (A.M.V.).
Authorship
Contribution: A.M.V. and C.N.H. cowrote this paper.
Conflict-of-interest disclosure: C.N.H. declares receiving research funding from Novartis Pharmaceuticals; speaker funding from CTI, Novartis, Sanofi, Baxalta, Gilead, Shire, and Incyte; and advisor fees from CTI, Novartis, Gilead, and Baxalta. A.M.V. declares receiving funding from Novartis Pharmaceuticals, speaker funding from Janssen and Shire, and advisory fees from Novartis.
Correspondence: Claire N. Harrison, Department of Haematology, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, United Kingdom; e-mail claire.harrison@gstt.nhs.uk.