To the editor:
The anti-CD38 monoclonal antibody (mAb) daratumumab is effective in multiple myeloma (MM) and is increasingly being used at first relapse in combination with bortezomib/dexamethasone or lenalidomide/dexamethasone, which are capable of inducing complete responses in a notable percentage of patients (19.2% and 43.1%, respectively).1,2 In addition, daratumumab is used as single agent in relapsed/refractory (R/R) myeloma after previous treatment with immunomodulatory drugs and proteasome inhibitors.3,-5 However, the rate of partial and complete remissions accomplished with daratumumab monotherapy is low (29.2%), and the median duration of response is limited (7.4 months), although a proportion of patients that achieve very good partial remissions or better may benefit long term.4,6 A clinical observation with daratumumab is that the expression of CD38 on pretreatment myeloma cells correlates with efficacy, and patients with higher CD38 expression have a higher chance to respond.7 Moreover, during daratumumab therapy, there are reduced CD38 expression levels on myeloma cells, favoring immune escape and disease progression.7 These observations suggest that an increase in density of CD38 molecules on myeloma cells could improve response rates and durability of responses to daratumumab treatment and prevent immune escape. Therefore, significant efforts are invested in defining agents that increase CD38 expression on myeloma cells and work synergistically with daratumumab.
Panobinostat (LBH589) is a pan–histone deacetylase inhibitor that possesses intrinsic antimyeloma activity and has been demonstrated to modulate the expression profile of genes involved in apoptosis and cell adhesion in myeloma and myeloid malignancies.8,-10 Intriguingly, there is precedence in B-cell lymphoma and acute myeloid leukemia showing that panobinostat induces epigenetic changes that lead to enhanced expression of CD20 and CD33 and subsequently enhanced efficacy of mAb immunotherapy.11,12 Therefore, we sought to determine the effect of panobinostat on CD38 expression on myeloma cells and the ensuing antimyeloma efficacy of daratumumab.
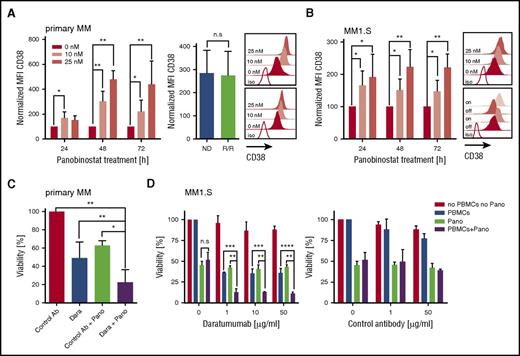
We treated primary myeloma cells (n = 12 patients) with titrated doses of panobinostat (0, 10, and 25 nM) and observed a uniform increase in CD38 expression in each case by flow cytometry. Upregulation of CD38 was already detectable after 24 hours, peaked after 48 hours of exposure to panobinostat, and was higher at the 25-nM than at the 10-nM dose (Figure 1A). At 48 hours, the mean fluorescence intensity (MFI) of CD38 expression was threefold (10-nM dose) and fivefold (25-nM dose) higher in panobinostat-treated than in untreated myeloma (P < .01). The increase in CD38 was equal in patients with newly diagnosed (n = 5) and in patients with R/R (daratumumab-naive) myeloma who had received previous treatment with immunomodulatory drugs and proteasome inhibitors (n = 7) and could be confirmed in 2 myeloma cell lines (ie, MM1.S and OPM-2) (Figure 1A-B; supplemental Figure 1A, available on the Blood Web site). The panobinostat-induced upregulation of CD38 was rapidly reversible and returned to baseline levels within 24 hours after withdrawing the drug (supplemental Figure 2). However, on reexposure to panobinostat, CD38 expression increased again and reached high expression levels similar to those at primary exposure (Figure 1B). The increase in CD38 expression after panobinostat treatment was specific for myeloma and did not occur in lymphoma cell lines (supplemental Figure 3). Consistent with previous work, panobinostat exerted a direct cytotoxic antimyeloma effect in both primary cells and cell lines (supplemental Figure 4).13,14
Augmented CD38 expression and daratumumab-mediated ADCC against primary myeloma and myeloma cell lines (MM1.S) after panobinostat treatment. (A) CD38 expression on primary myeloma cells (n = 12 patients) before and after panobinostat treatment. Left bar diagram shows CD38 expression as normalized MFI of panobinostat-treated vs untreated myeloma after 24, 48, and 72 hours of culture. Normalized MFI values were calculated as follows: MFIs 10 nM or 25 nM × 100/MFI untreated cells. Right bar diagram shows CD38 expression of new diagnosed (ND) vs R/R myeloma patients after 48 hours of treatment with 10 nM of panobinostat. Upper and bottom overlay histograms show CD38 expression from the patient with the highest and the patient with the lowest increase in CD38 expression after 48 hours of treatment with panobinostat at the indicated dose, respectively. Shaded histograms show staining with anti-CD38 mAb, and the white histogram shows staining with isotype control antibody. (B) CD38 expression on MM1.S myeloma cells (n = 5 experiments) before and after panobinostat treatment, in experiments performed analogous to panel A. The upper overlay histogram shows CD38 expression after 48 hours of treatment with panobinostat at the indicated dose in 1 representative case. The bottom overlay histogram shows CD38 expression on untreated MM1.S cells (off), 72 hours after panobinostat treatment (10 nM, on), 24 hours after subsequent removal of the drug (off), and after 72 hours of reexposition (on). Shaded histograms show staining with anti-CD38 mAb, and the white histogram shows staining with isotype control antibody. (C) ADCC against primary myeloma cells (n = 4 patients) with and without panobinostat treatment. Panobinostat pretreatment was performed for 48 hours at a dose of 10 nM, and then autologous PBMCs (effector/target ratio of 3:1) and daratumumab (0.1 µg/mL) or control antibody (1µg/mL) were added to induce ADCC. The percentage of live myeloma cells was determined after 4 hours by flow cytometry. The bar diagram shows the percentage of viable (7-AAD–) myeloma cells (CD38+/CD138+). (D) ADCC against MM1.S myeloma cells (n = 3 experiments) with and without panobinostat treatment. Panobinostat pretreatment was performed for 48 hours at a dose of 10 nM. PBMCs from healthy donors (effector/target ratio of 25:1) and control antibody or daratumumab were added at the indicated concentrations. MM1.S stably expressed firefly luciferase, and viability was analyzed after the addition of luciferin substrate by bioluminescence measurements after 20 hours. (A-D) Values are mean ± SD. P values between indicated groups were calculated by using a paired Student t test for expression levels and 2-way analysis of variance for ADCC assays. *P < .05; **P < .01; ***P < .001; ****P < .0001. Dara, daratumumab; n.s., not significant; Pano, panobinostat.
Augmented CD38 expression and daratumumab-mediated ADCC against primary myeloma and myeloma cell lines (MM1.S) after panobinostat treatment. (A) CD38 expression on primary myeloma cells (n = 12 patients) before and after panobinostat treatment. Left bar diagram shows CD38 expression as normalized MFI of panobinostat-treated vs untreated myeloma after 24, 48, and 72 hours of culture. Normalized MFI values were calculated as follows: MFIs 10 nM or 25 nM × 100/MFI untreated cells. Right bar diagram shows CD38 expression of new diagnosed (ND) vs R/R myeloma patients after 48 hours of treatment with 10 nM of panobinostat. Upper and bottom overlay histograms show CD38 expression from the patient with the highest and the patient with the lowest increase in CD38 expression after 48 hours of treatment with panobinostat at the indicated dose, respectively. Shaded histograms show staining with anti-CD38 mAb, and the white histogram shows staining with isotype control antibody. (B) CD38 expression on MM1.S myeloma cells (n = 5 experiments) before and after panobinostat treatment, in experiments performed analogous to panel A. The upper overlay histogram shows CD38 expression after 48 hours of treatment with panobinostat at the indicated dose in 1 representative case. The bottom overlay histogram shows CD38 expression on untreated MM1.S cells (off), 72 hours after panobinostat treatment (10 nM, on), 24 hours after subsequent removal of the drug (off), and after 72 hours of reexposition (on). Shaded histograms show staining with anti-CD38 mAb, and the white histogram shows staining with isotype control antibody. (C) ADCC against primary myeloma cells (n = 4 patients) with and without panobinostat treatment. Panobinostat pretreatment was performed for 48 hours at a dose of 10 nM, and then autologous PBMCs (effector/target ratio of 3:1) and daratumumab (0.1 µg/mL) or control antibody (1µg/mL) were added to induce ADCC. The percentage of live myeloma cells was determined after 4 hours by flow cytometry. The bar diagram shows the percentage of viable (7-AAD–) myeloma cells (CD38+/CD138+). (D) ADCC against MM1.S myeloma cells (n = 3 experiments) with and without panobinostat treatment. Panobinostat pretreatment was performed for 48 hours at a dose of 10 nM. PBMCs from healthy donors (effector/target ratio of 25:1) and control antibody or daratumumab were added at the indicated concentrations. MM1.S stably expressed firefly luciferase, and viability was analyzed after the addition of luciferin substrate by bioluminescence measurements after 20 hours. (A-D) Values are mean ± SD. P values between indicated groups were calculated by using a paired Student t test for expression levels and 2-way analysis of variance for ADCC assays. *P < .05; **P < .01; ***P < .001; ****P < .0001. Dara, daratumumab; n.s., not significant; Pano, panobinostat.
We sought to determine whether the increase in CD38 antigen density enabled superior antimyeloma activity of the anti-CD38 mAb daratumumab. We treated primary myeloma cells (n = 4 patients) with panobinostat for 48 hours at 10 nM, because this is the serum level that is achievable with currently approved dosing regimens.15 We then added daratumumab or control antibody as well as patient-derived autologous peripheral blood mononuclear cells (PBMCs) to initiate antibody-dependent cellular cytotoxicity (ADCC). We observed a significant increase in ADCC against panobinostat-treated compared with untreated myeloma in all patients (Figure 1C). On average, 78% of panobinostat-treated primary myeloma cells were eliminated by daratumumab within the 4-hour ADCC assay, whereas only 51% myeloma cells were eliminated without panobinostat treatment (P < .01) (Figure 1C). The synergistic antimyeloma efficacy of panobinostat and daratumumab was confirmed with myeloma cell lines (eg, after 4 and 20 hours, 45% vs 26% and 89% vs 64% of panobinostat-treated vs untreated MM1.S were eliminated [n = 3 experiments, P < .0001]) (Figure 1D; supplemental Figure 1C). Our data are consistent with the clinical observation that CD38 expression is associated with the antimyeloma efficacy of daratumumab and with preclinical work that showed antigen density is a critical variable for daratumumab-mediated ADCC.16 Previous work has shown that daratumumab has a low or absent potential to confer complement-dependent cytotoxicity against native primary myeloma cells and many myeloma cell lines.7,17 Despite the marked increase in CD38 expression after panobinostat treatment, we could still not induce complement-dependent cytotoxicity through daratumumab against myeloma cells, potentially due to concomitant upregulation of the complement inhibitors CD55 and CD59 on primary myeloma (supplemental Figure 5) and myeloma cell lines (supplemental Figure 5). In aggregate, however, we anticipate a strong gain of antimyeloma efficacy after panobinostat treatment because ADCC is the leading mode of action of daratumumab.7,17
CD38 is expressed on a variety of normal hematopoietic cells and is a marker of T-cell activation.18 We analyzed resting and activated CD8+ and CD4+ T cells at different time points after treatment with panobinostat and did not detect a difference in CD38 expression compared with untreated T cells at the 10-nM dose (supplemental Figure 7). These data indicate that panobinostat alters CD38 expression specifically in myeloma and will not lead to increased toxicity of daratumumab to CD38+ nonmyeloma cells.
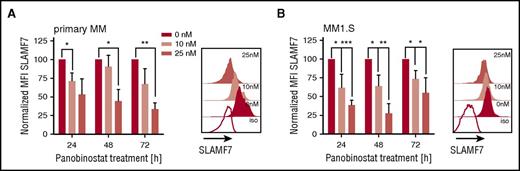
SLAMF7 is pursued as an alternative mAb target in myeloma.19 We analyzed expression of SLAMF7 on myeloma cells after panobinostat treatment, but in contrast to CD38, detected downregulation of SLAMF7 in both primary myeloma and myeloma cell lines at all tested doses and time points (Figure 2A-B; supplemental Figure 1B), suggesting the antimyeloma efficacy of anti-SLAMF7 mAbs may not be enhanced by panobinostat.
Downregulation of SLAMF7 on primary myeloma and myeloma cell lines (MM1.S) after panobinostat treatment. (A) SLAMF7 expression on primary myeloma cells (n = 3 patients) before and after panobinostat treatment. Left bar diagram shows SLAMF7 expression as normalized MFI of panobinostat-treated vs untreated myeloma after 24, 48, and 72 hours of culture. Normalized MFI values were calculated as follows: MFIs 10 nM or 25 nM × 100/MFI untreated cells. The overlay histogram shows SLAMF7 expression after 48 hours of treatment with panobinostat at the indicated dose in 1 representative case. Shaded histograms show staining with anti-SLAMF7 mAb, and the white histogram shows staining with isotype control antibody. (B) SLAMF7 expression on MM1.S myeloma cells (n = 4 experiments) before and after panobinostat treatment in experiments performed analogous to panel A. (A-B) Values are mean ± SD. P values between indicated groups were calculated by using a paired Student t test. *P < .05; **P < .01; ***P < .001.
Downregulation of SLAMF7 on primary myeloma and myeloma cell lines (MM1.S) after panobinostat treatment. (A) SLAMF7 expression on primary myeloma cells (n = 3 patients) before and after panobinostat treatment. Left bar diagram shows SLAMF7 expression as normalized MFI of panobinostat-treated vs untreated myeloma after 24, 48, and 72 hours of culture. Normalized MFI values were calculated as follows: MFIs 10 nM or 25 nM × 100/MFI untreated cells. The overlay histogram shows SLAMF7 expression after 48 hours of treatment with panobinostat at the indicated dose in 1 representative case. Shaded histograms show staining with anti-SLAMF7 mAb, and the white histogram shows staining with isotype control antibody. (B) SLAMF7 expression on MM1.S myeloma cells (n = 4 experiments) before and after panobinostat treatment in experiments performed analogous to panel A. (A-B) Values are mean ± SD. P values between indicated groups were calculated by using a paired Student t test. *P < .05; **P < .01; ***P < .001.
Collectively, we demonstrate that panobinostat induces a significant increase in CD38 expression on myeloma cells from patients with newly diagnosed and R/R disease. In addition, we show that the panobinostat-induced increase in CD38 expression can be exploited to enhance the antimyeloma efficacy of daratumumab through a substantial increase in ADCC. Our data suggest that panobinostat and daratumumab could be used synergistically to increase response rates and extend duration of responses in R/R myeloma compared with daratumumab monotherapy and that clinical trials are warranted to investigate the safety and efficacy of this combination treatment. Indeed, the clinical use of daratumumab in combination with lenalidomide and dexamethasone at first relapse has illustrated that the efficacy of daratumumab can be enhanced in combination regimens.2
Panobinostat has a known ability to modulate the transcriptional profile of myeloma cells,14 and our data demonstrate for the first time that this ability can be exploited to augment the efficacy of antibody immunotherapy in MM. Several HDAC inhibitors other than panobinostat with overlapping or complementary activity against class I and class II HDACs are in ongoing preclinical and clinical development.20 Our data encourage additional experimentation to identify single or even combinations of HDAC inhibitors that are capable of increasing the expression of CD38 and other myeloma antigens (eg, SLAMF7 and B-cell maturation antigen) to improve the efficacy of antibody and cellular immunotherapy in MM.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: E.G.-G. was supported by Instituto de Salud Carlos III PFIS - FI12/00189. S.D. was supported by Interdisziplinäres Zentrum für Klinische Forschung Würzburg and is a fellow of the Clinician Scientist Program of the Else-Kröner Forschungskolleg. M.H. was supported by the Young Scholar Program of the Bavarian Academy of Sciences (Junges Kolleg, Bayerische Akademie der Wissenschaften).
Contribution: E.G.-G. designed and performed experiments, analyzed data, and wrote the manuscript; T.G., C.P., and J.A.P.-S. designed experiments and analyzed data; S.D. and M.S. provided biological material and analyzed data; and M.H. and H.E. designed experiments, analyzed data, wrote the manuscript, and supervised the project.
Conflict-of-interest disclosure: C.P. is an employee of Novartis Pharmaceuticals. M.H. and H.E. received research funding from Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Michael Hudecek, Medizinische Klinik und Poliklinik II, Universitätsklinikum Würzburg, Oberdürrbacher Str 6, 97080 Würzburg, Germany; e-mail: hudecek_m@ukw.de.