Key Points
Therapeutic enoxaparin was associated with a greater than threefold increased risk of major ICH in patients with glioma.
The PANWARDS risk score was a sensitive predictor of major ICH in glioma.
Venous thromboembolism occurs in up to one-third of patients with primary brain tumors. Spontaneous intracranial hemorrhage (ICH) is also a frequent occurrence in these patients, but there is limited data on the safety of therapeutic anticoagulation. To determine the rate of ICH in patients treated with enoxaparin, we performed a matched, retrospective cohort study with blinded radiology review for 133 patients with high-grade glioma. After diagnosis of glioma, the cohort that received enoxaparin was 3 times more likely to develop a major ICH than those not treated with anticoagulation (14.7% vs 2.5%; P = .036; hazard ratio [HR], 3.37; 95% confidence interval [CI], 1.02-11.14). When enoxaparin was analyzed as a time-varying covariate, anticoagulation was associated with a >13-fold increased risk of hemorrhage (HR, 13.26; 95% CI, 3.33-52.85; P < .0001). Overall survival was significantly shorter for patients who suffered a major ICH on enoxaparin compared with patients not receiving anticoagulation (3.3 vs 10.2 months; log-rank P = .012). We applied a validated ICH prediction risk score PANWARDS (platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke), and observed that all major ICHs on enoxaparin occurred in the setting of a PANWARDS score ≥25, corresponding with a sensitivity of 100% (95% CI, 63% to 100%) and a specificity of 40% (95% CI, 25% to 56%). We conclude that caution is warranted when considering therapeutic anticoagulation in patients with high-grade gliomas given the increased risk of ICH and poor prognosis after a major hemorrhage on anticoagulation. The PANWARDS score may assist clinicians in identifying the patients at greatest risk of suffering a major intracranial hemorrhage with anticoagulation.
Introduction
Intracranial hemorrhage frequently occurs in patients with primary brain tumors, such as malignant glioma, even in the absence of anticoagulation.1 To complicate the issue, glioma is also one of the most prothrombotic tumors with >30% of patients ultimately diagnosed with venous thromboembolism.2,3 The administration of therapeutic anticoagulation in the setting of brain metastases and venous thromboembolism (VTE) does not appear to increase the risk of intracranial hemorrhage (ICH).4,5 However, the risk-benefit profile of therapeutic anticoagulation in glioma is less certain. In a recent meta-analysis, the pooled risk of ICH among the 5 studies that reported outcomes for glioma was significantly higher than in controls not receiving anticoagulation.5 An issue complicating the interpretation of previous studies is the lack of an a priori definition of ICH. Intracranial hemorrhage technically spans the spectrum from trace radiologic evidence of blood products to overt hemorrhage with mass effect. The diagnosis of ICH in the setting of glioma is additionally confounded by surgical interventions, with postoperative hemorrhage representing normal sequelae of the procedure. To better assess the safety of therapeutic anticoagulation in glioma, we performed a matched, retrospective cohort study with blinded radiology review using predefined ICH criteria.
Beyond tumor diagnosis, there is minimal data to assist treating clinicians in the decision to administer therapeutic anticoagulation in patients with brain tumors.4 The platelets, albumin, no congestive heart failure, warfarin, age, race, diastolic blood pressure, stroke (PANWARDS) risk score was previously developed in noncancer cohorts to predict the development of ICH on therapeutic anticoagulation.6 Whether this risk score is useful to predict ICH in patients with brain tumors is unknown. In this study, we report that the administration of therapeutic anticoagulation is associated with a significant increase in risk of major ICH, and the PANWARDS score is a sensitive discriminator for the eventual development of major ICH in patients with glioma.
Methods
Study design
The protocol was approved by the Institutional Review Board at the Dana-Farber/Harvard Cancer Center. Data were extracted from the electronic medical record database at Beth Israel Deaconess Medical Center from 2000 to 2016. Cases were identified by using International Classification of Diseases, Ninth Revision and Tenth Revision codes for “malignant neoplasm of brain,” excluding secondary neoplasms. The electronic medical record was then manually reviewed for each case to ensure eligibility. Matched controls for each enoxaparin case were then selected by using a “round-robin” scoring algorithm that ranked controls according to age at diagnosis, year of diagnosis, and sex. Each enoxaparin case was successfully matched with at least 1 control and, if available, a second control. Inclusion criteria for all cases included: a diagnosis of World Health Organization grade III or IV glioma, including glioblastoma, anaplastic astrocytoma, and anaplastic oligodendroglioma; ≥2 radiographic brain images available for review; and care received at Beth Israel Deaconess Medical Center for at least 2 months. Inclusion criteria for the enoxaparin arm included therapeutic anticoagulation with enoxaparin for a diagnosis of venous thromboembolism. Exclusion criteria included therapeutic anticoagulation with any medication in the control cohort. Patients were recorded as having hypertension if hypertension was recorded in their past medical history and they required pharmacologic treatment. Patients were defined as having chronic kidney disease if they had a glomerular filtration rate of <60 mL/min per 1.73 m2. Antiangiogenic agents refer to vascular endothelial growth factor inhibitors, such as sorafenib, bevacizumab, vandetanib, and cabozantinib.
Intracranial hemorrhage
For each case, all available radiology reports, including magnetic resonance imaging of the brain and computed tomography scan of the head from time of diagnosis were reviewed to identify instances of hemorrhage. If a report indicated hemorrhage or blood products, information surrounding the event, such as indication for imaging and subsequent management, was collected from the electronic medical record. Any bleed that occurred within 4 weeks after neurosurgery was excluded from analysis. The primary radiographic image of each instance of hemorrhage was then reviewed by a neuro-oncologist blinded to cohort allocation to confirm the presence of hemorrhage, identify the type of hemorrhage, and calculate the bleed volume using the one-half ABC technique.7,8 Intracranial hemorrhages were classified as trace, measurable, and major. Trace hemorrhages were too small to be measured or measured <1 mL in volume. Measurable ICHs were classified as those that measured ≥1 mL. Major ICHs were defined as any hemorrhage that was ≥10 mL in volume, required surgical intervention, or was associated with clinical symptoms, such as nausea and vomiting, focal neurologic deficit, or change in cognitive function.4,8,9
Prediction of ICH
The PANWARDS risk score for ICH was calculated as previously described.6 The variables include: platelet count (× 109): <125 (11 points), 125-149 (8 points), 150-174 (5 points), and 175-199 (3 points); albumin (g/dL): <3.0 (18 points), 3.0-3.49 (14 points), 3.5-3.99 (9 points), and 4.0-4.49 (5 points); no history of congestive heart failure (6 points); age (years): 55-64 (4 points), 65-74 (8 points), 75-84 (13 points), ≥85 (17 points); race: African American (18 points) and Asian (9); diastolic blood pressure (mm Hg): 50-69 (4 points), 70-89 (9 points), 90-109 (13 points), and ≥110 (17 points); and previous history of stroke or transient ischemic attack (5 points). A score was calculated by using the recorded data closest to initiation of anticoagulation (within 30 days), and a score was generated only if data were available for at least 6 of the 7 variables.
Statistical analysis
The primary endpoint of the study was major ICH from the time of diagnosis of glioma. The initial sample size estimates were based on an anticipated rate of ICH of 16% in the enoxaparin cohort and 2% in the control cohort.10 Therefore, the target analysis was 50 patients treated with enoxaparin and 100 controls (one-sided α of 0.05% and power of 0.84). Categorical variables were compared between groups by using Fisher’s exact test and the Wilcoxon rank-sum test for continuous variables. For analysis of bleeding endpoints, if a patient had >1 bleed, data from the most significant bleed (major > measurable > trace) was used, or if the 2 bleeds were in the same category, then the first bleed chronologically was used. The cumulative incidence of bleeding was compared by using the Gray test with death (without ICH) as a competing risk.11 To additionally assess the effect of anticoagulation on the rate of ICH, enoxaparin was considered as a time-varying covariate in a Fine-Gray model (Stata Corp, Houston, TX). Because patients in the enoxaparin cohort initiated anticoagulation during the course of the study, the time-dependent covariate represented the change in enoxaparin status for the cohort. Hazard ratios (HRs) for the bleeding rates were obtained by using the Fine-Gray method for competing risks regression.12 Cox proportional hazards models were used to obtain HRs for overall survival (OS). Two-sided P values <.05 were considered statistically significant. Patients who were still alive were censored at the date of last contact. Patients who were discharged to hospice were considered deceased at the date of last contact. Patients who presented with a bleed were counted as events at time 0 in the initial (day 0) analysis. A landmark analysis was performed, in which the day after diagnosis was treated as time 0 (day +1) so that only hemorrhages occurring after the initial diagnosis of glioma were assessed. The PANWARDS prediction score was analyzed by a receiver operating curve (ROC) to generate an area under the curve (AUC) and a z statistic was calculated to test the null hypothesis that the AUC equals 0.5 (SigmaStat, San Jose, CA).
Results
There was a total of 133 patients with primary brain tumors included in the study: 50 in the enoxaparin cohort and 83 in the control cohort. Patient characteristics are described in Table 1. The most common diagnosis was glioblastoma (84%) followed by anaplastic oligodendroglioma (10%) and anaplastic astrocytoma (5%). The 2 cohorts were similar in terms of age, sex, and glioma treatment (ie, radiation and surgery) along with aspirin or use of antiangiogenic therapeutics. There were significantly more individuals with hypertension in the controls than those receiving enoxaparin (49% vs 26%, P = .01). In the enoxaparin cohort, 29 (58%) received anticoagulation for pulmonary embolism and 21 (42%) for isolated deep vein thrombosis. The majority of patients (76%) received enoxaparin at 1 mg/kg twice daily. Two patients (4%) received enoxaparin at 1.5 mg/kg, 8 patients (16%) received enoxaparin at less than therapeutic dosing, and in 2 patients (4%), the weight was not recorded.
Demographics and patient characteristics
| Patient characteristics . | Enoxaparin (N = 50), n (%) . | Control (N = 83), n (%) . | P . |
|---|---|---|---|
| Male | 33 (66) | 48 (58) | .37 |
| Age at diagnosis, y (range) | 62 (26-89) | 61 (24-82) | .84 |
| Type of glioma | .18 | ||
| Anaplastic astrocytoma | 5 (10) | 2 (2) | |
| Anaplastic oligodendroglioma | 4 (8) | 10 (12) | |
| Glioblastoma | 41 (82) | 71 (86) | |
| Hypertension | 13 (26) | 41 (49) | .01 |
| Chronic kidney disease | 1 (2) | 2 (2) | 1.00 |
| Glioma treatment | |||
| Involved field radiation | 49 (98) | 82 (99) | 1.00 |
| Stereotactic radiosurgery | 19 (38) | 20 (24) | .12 |
| Surgical resection | 33 (66) | 56 (67) | 1.00 |
| Any antineoplastic drug | 48(96) | 81 (98) | .63 |
| Antiangiogenic agents | 23 (46) | 42 (51) | .72 |
| Aspirin use | 5 (10) | 11 (13) | .78 |
| Patient characteristics . | Enoxaparin (N = 50), n (%) . | Control (N = 83), n (%) . | P . |
|---|---|---|---|
| Male | 33 (66) | 48 (58) | .37 |
| Age at diagnosis, y (range) | 62 (26-89) | 61 (24-82) | .84 |
| Type of glioma | .18 | ||
| Anaplastic astrocytoma | 5 (10) | 2 (2) | |
| Anaplastic oligodendroglioma | 4 (8) | 10 (12) | |
| Glioblastoma | 41 (82) | 71 (86) | |
| Hypertension | 13 (26) | 41 (49) | .01 |
| Chronic kidney disease | 1 (2) | 2 (2) | 1.00 |
| Glioma treatment | |||
| Involved field radiation | 49 (98) | 82 (99) | 1.00 |
| Stereotactic radiosurgery | 19 (38) | 20 (24) | .12 |
| Surgical resection | 33 (66) | 56 (67) | 1.00 |
| Any antineoplastic drug | 48(96) | 81 (98) | .63 |
| Antiangiogenic agents | 23 (46) | 42 (51) | .72 |
| Aspirin use | 5 (10) | 11 (13) | .78 |
ICH at time of glioma diagnosis
A total of 61 ICHs were recorded in both arms over the entire study. Significantly more ICHs in the control cohort occurred at the time of glioma diagnosis (46%, 16 of 38 patients who experienced hemorrhage) compared with the enoxaparin cohort (13%, 3 of 23; P = .023). To test the underlying hypothesis that anticoagulation does not influence the development of ICH in the setting of glioma, only hemorrhages that occurred after initial glioma diagnosis (day + 1) were used for cumulative incidence analyses.
Cumulative incidence of ICH
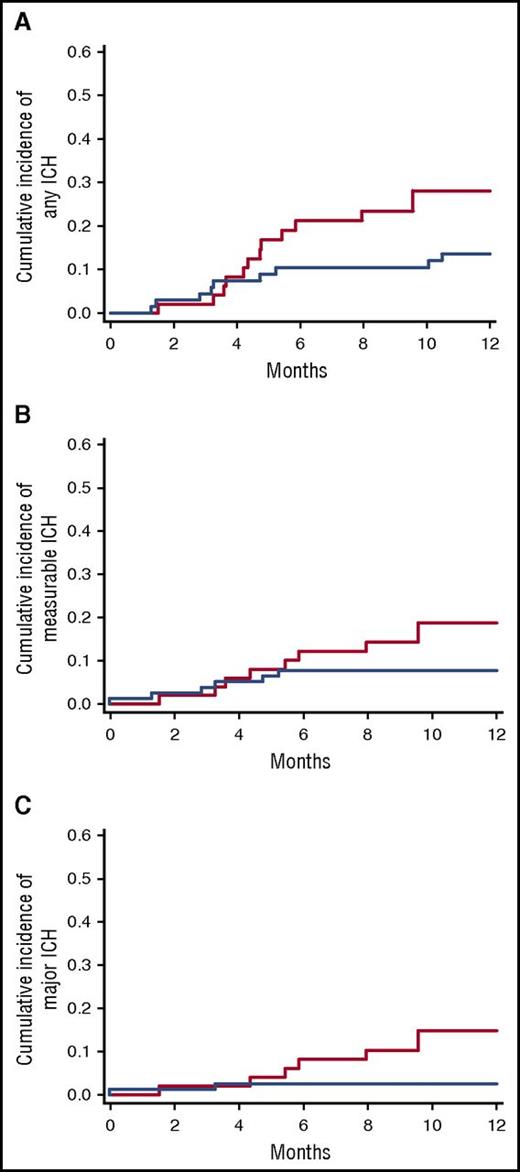
As shown in Figure 1, the 1-year incidence of any hemorrhage was 28.1% in the enoxaparin cohort vs 13.6% in the control cohort (HR, 1.39; 95% confidence interval [CI], 0.75-2.55; P = .37) after the diagnosis of glioma. The 1-year incidence of measurable ICH was 18.8% in the enoxaparin cohort and 7.8% in the control cohort (HR, 2.16; 95% CI, .99-4.74; P = .05). Notably, the administration of therapeutic enoxaparin was associated with a greater than threefold (HR, 3.37; 95% CI, 1.02-11.14) increased risk of developing a major ICH at 1 year (14.7% vs 2.5%, P = .036).
Cumulative incidence of ICH in patients with glioma. (A) The 1-year incidence of any ICH was 28.1% in the enoxaparin cohort and 13.5% in the control cohort after glioma diagnosis (Gray test P = .37); (B) measurable ICH was 18.8% compared with 7.8%, respectively, (Gray test P = .05); and (C) major ICH was 14.7% vs 2.5%, respectively (Gray test P = .036). The enoxaparin cohort is shown in red, and the control cohort is shown in blue.
Cumulative incidence of ICH in patients with glioma. (A) The 1-year incidence of any ICH was 28.1% in the enoxaparin cohort and 13.5% in the control cohort after glioma diagnosis (Gray test P = .37); (B) measurable ICH was 18.8% compared with 7.8%, respectively, (Gray test P = .05); and (C) major ICH was 14.7% vs 2.5%, respectively (Gray test P = .036). The enoxaparin cohort is shown in red, and the control cohort is shown in blue.
Variables predictive of ICH
Univariate Fine-Gray competing risk regression was performed to identify variables predictive of measurable/major ICH in patients with glioma. As shown in Table 2, the rates of major ICH did not differ significantly for age, hypertension, glioma treatment, or administration of antiangiogenic agents. The rates of major hemorrhage also were not significantly different for glioblastoma compared with other high-grade glioma histology (HR, 0.59; 95% CI, 0.17-2.17; P = .45).
Univariable Fine-Gray competing risk analysis for the development of major ICH
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis, y | 1.04 | 1.00-1.08 | .05 |
| Hypertension | 1.03 | 0.33-3.21 | .96 |
| Surgical resection | 0.94 | 0.29-3.09 | .92 |
| Stereotactic radiosurgery | 0.74 | 0.20-2.70 | .65 |
| Aspirin use | 0.63 | 0.08-4.84 | .66 |
| Antiangiogenic agents | 0.95 | 0.31-2.90 | .92 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age at diagnosis, y | 1.04 | 1.00-1.08 | .05 |
| Hypertension | 1.03 | 0.33-3.21 | .96 |
| Surgical resection | 0.94 | 0.29-3.09 | .92 |
| Stereotactic radiosurgery | 0.74 | 0.20-2.70 | .65 |
| Aspirin use | 0.63 | 0.08-4.84 | .66 |
| Antiangiogenic agents | 0.95 | 0.31-2.90 | .92 |
ICH relative to the initiation of enoxaparin
The cumulative incidence of major hemorrhage after the start of enoxaparin was 17.03% (95% CI, 7.83% to 29.21%) at 1 year. The cumulative incidence of major hemorrhage was 14.36% at 1 year (95% CI, 5.11% to 28.18%) when enoxaparin was only initiated after glioma-directed therapy, which addresses the question regarding the incidence of ICH in treated brain tumors. To further evaluate the association between enoxaparin administration and development of ICH, we considered enoxaparin as a time-varying covariate in a Fine-Gray model. During exposure to therapeutic anticoagulation, there was an ∼13-fold increased risk of developing a major ICH (HR, 13.29; 95% CI, 3.33-52.85; P < .0001). To address potential surveillance bias, we analyzed the rate of imaging after the start of enoxaparin compared with the rate of imaging in those not receiving anticoagulation (ie, both the control cohort along with the enoxaparin patients before the start of anticoagulation). The median rate of imaging after the initiation of enoxaparin was 0.90 images per month, which was similar to the 0.97 images per month in the non-anticoagulated groups of patients (rank-sum P = .56).
OS
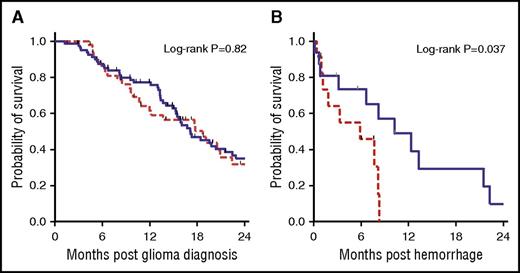
The median survival was similar in the 2 cohorts at 18.7 months in the enoxaparin cohort and 17.1 months in the control cohort (P = .82) (Figure 2). However, the diagnosis of ICH while receiving enoxaparin was associated with a significantly shorter survival time than for those not on anticoagulation (3.3 months in the enoxaparin cohort vs 10.2 months in the control cohort; log-rank P = .012). This effect remained significant even after adjusting for time of glioma diagnosis until hemorrhage as well as age at diagnosis (adjusted HR, 3.74; 95% CI. 1.24-11.30; P = .02).
Kaplan-Meier survival curves of OS and postbleed survival. (A) OS did not differ between the enoxaparin and control cohorts (log-rank P = .82). (B) Survival was significantly shorter after the diagnosis of a major ICH for patients on enoxaparin compared with patients not on anticoagulation (3.3 vs 10.2 months; log-rank P = .037). The enoxaparin cohort is represented by the red dashed line, and the control cohort is shown represented by blue solid line.
Kaplan-Meier survival curves of OS and postbleed survival. (A) OS did not differ between the enoxaparin and control cohorts (log-rank P = .82). (B) Survival was significantly shorter after the diagnosis of a major ICH for patients on enoxaparin compared with patients not on anticoagulation (3.3 vs 10.2 months; log-rank P = .037). The enoxaparin cohort is represented by the red dashed line, and the control cohort is shown represented by blue solid line.
Prediction of ICH in glioma
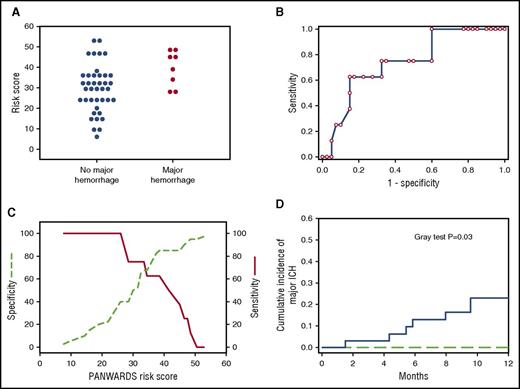
We evaluated the performance of the PANWARDS risk score previously developed to predict the development of ICH among patients receiving warfarin or rivaroxaban during treatment of atrial fibrillation.13 We performed a ROC analysis for major ICH among glioma patients receiving anticoagulation; the calculated area under the curve was 0.74 ± 0.09 (P = .03), supporting the discriminatory function of the risk score. The sensitivity and specificity plots relative to risk score are shown in Figure 3. All major ICHs occurred in the setting of risk scores of ≥25 with a corresponding sensitivity of 100% (95% CI, 63% to 100%) and a specificity of 40% (95% CI, 25% to 56%). The cumulative incidence of major ICH at 1 year in glioma patients receiving anticoagulation with scores ≥25 was 23% (95% CI, 9.91-39.41) compared with 0% for those with lower scores (P = .03).
Prediction of major ICH by using PANWARDS scores. (A) Dot plot of PANWARDS risk scores for patients on enoxaparin diagnosed with a major ICH (red circles) and without a major ICH (blue circles). (B) ROC curve comparing 1-specificity and sensitivity for major hemorrhage according to PANWARDS score (AUC, 0.74; P = .03). (C) The sensitivity (red solid line) and specificity (green dashed line) plots of major ICH according to PANWARDS risk score. (D) The 1-year cumulative incidence of major ICH in patients with scores ≥25 was 23% compared with 0% for scores <25 (Gray test P = .03). Patients with a PANWARDS risk score of ≥25 are represented by the blue solid line, and patients with score <25 are represented by the green dashed line.
Prediction of major ICH by using PANWARDS scores. (A) Dot plot of PANWARDS risk scores for patients on enoxaparin diagnosed with a major ICH (red circles) and without a major ICH (blue circles). (B) ROC curve comparing 1-specificity and sensitivity for major hemorrhage according to PANWARDS score (AUC, 0.74; P = .03). (C) The sensitivity (red solid line) and specificity (green dashed line) plots of major ICH according to PANWARDS risk score. (D) The 1-year cumulative incidence of major ICH in patients with scores ≥25 was 23% compared with 0% for scores <25 (Gray test P = .03). Patients with a PANWARDS risk score of ≥25 are represented by the blue solid line, and patients with score <25 are represented by the green dashed line.
Discussion
The risk-to-benefit assessment driving the decision to treat VTE can simplistically be reduced to the likelihood of preventing a life-threatening pulmonary embolism vs the likelihood of causing a life-threatening hemorrhagic event. In the setting of malignancy, the rate of recurrent VTE is threefold greater than major hemorrhage, thus justifying aggressive secondary thromboprophylaxis.14,15 We evaluated whether the administration of low–molecular weight heparin in the setting of primary and secondary brain tumors negatively impacted outcomes. At least in the setting of brain metastases, therapeutic anticoagulation appears safe; although the 1-year rates of major ICH exceeded 20%, the development of ICH was not influenced by the administration of therapeutic enoxaparin (1 year cumulative incidence of major ICH of 21% vs 22%, P = .87).4 In the current study, we focused exclusively on primary brain tumors (ie, glioma) and observed that the risk of major ICH after therapeutic anticoagulation was significantly higher compared with those who did not receive anticoagulation. This strong association was further supported by a time-varying covariate analysis confirming that time-on vs time-off enoxaparin resulted in an ∼13-fold increased risk of ICH.
The risk of major ICH on enoxaparin (HR, 3.37) is similar to the pooled risk of ICH among 5 studies (odds ratio, 3.53) in a recent meta-analysis.5 None of the prior studies included a blinded radiology review, a rigorous definition of ICHs, nor specifically matched the cohorts by using an algorithm to minimize selection and ascertainment biases. Although the rates of ICH appear to be negatively influenced by the administration of therapeutic low–molecular weight heparin, our study was not designed to specifically address whether alternative approaches to treatment of venous thromboembolism improve outcomes. Khoury et al10 previously compared the rates of ICH only among patients with glioblastoma diagnosed with venous thromboembolism. Among the 97 patients receiving anticoagulation, the rate of ICH was 15.5% compared with 2.6% among the 73 patients not receiving anticoagulation. There was a trend toward improved OS with the administration of anticoagulation vs no anticoagulation (P = .07) for the treatment of VTE, an effect which was not dependent on the placement of an inferior vena cava filter.10 The decision to anticoagulate is a nonrandom event, thus it is difficult to discern whether the comorbidities that influenced the decision to pursue anticoagulation similarly impacted mortality.
OS was not significantly different among the 2 cohorts we analyzed. This may point to a potential therapeutic benefit of therapeutic anticoagulation because the diagnosis of VTE historically has been associated with a poorer prognosis relative to cancer patients without the diagnosis of VTE.16,17 However, caution is warranted in drawing conclusions regarding OS comparisons in retrospective cohort studies due to the potential for immortal time bias because individuals in the VTE cohort need to live long enough to develop the incident thrombotic event. A concerning finding in this study was the observation that enoxaparin negatively impacted clinical outcomes, with the median survival time shortened by ∼70% (3.3 months vs 10.2 months) for ICHs on enoxaparin compared with spontaneous ICH. This effect remained significant even when adjusting for potentially confounding variables, such as time from tumor diagnosis and age. These findings are also in line with noncancer populations, whereby ICHs on warfarin are associated with worsened survival and outcomes relative to spontaneous ICHs.18,19 For instance, among the 435 consecutive patients with ICH, the 3-month mortality for those on warfarin was 52% compared with 25.8% not on anticoagulation.19 In populations for which the annual risk of ICH is <0.5%, such as atrial fibrillation, the potential deleterious impact of anticoagulation on ICH outcomes is less concerning than in cohorts where the anticipated rates of ICH range from 10% to 30%, such as glioma.
Several validated risk scores predict the likelihood of developing major hemorrhages on therapeutic anticoagulation. In general, these prediction scores are skewed toward the prediction of more common gastrointestinal hemorrhages.20,-22 Hankey and colleagues6 developed a nomogram to specifically predict the likelihood of developing ICH for patients on anticoagulation. We analyzed the glioma data set using this nomogram, and it proved useful in predicting major ICHs among patients with glioma receiving therapeutic enoxaparin. Notably, there were no major ICHs among patients with glioma and risk scores <25 (sensitivity of 100% and specificity of 40% for major ICH). To the best of our knowledge, this study is the first application of any risk score for the prediction of ICHs in patients with brain tumors and provides a framework for clinical decision-making. Although it is likely that there are unique risk factors predictive of ICH in patients with brain tumors compared with healthy individuals, additional studies are needed to determine whether optimization of the scoring system is possible by incorporating cancer-specific characteristics, such as histology, size, location, and antineoplastic therapies.
The question is whether the increased risk of ICH with therapeutic anticoagulation in glioma warrants a recalibration of the management of venous thromboembolism in glioma.23 The alternatives to consider include conservative management with or without placement of an inferior vena cava (IVC) filter, reduced-intensity anticoagulation (with or without IVC filter placement), or direct oral anticoagulants (DOACs). Conservative management alone after venous thromboembolism is associated with poor outcomes. For instance, among 11 glioma patients who did not receive anticoagulation or IVC filter placement, 4 were later treated for progressive or recurrent VTE.24 The routine placement of IVC filters is generally discouraged due to the high rate of recurrent VTE that can exceed 25% along with an ∼5% rate of mechanical complications.25,26 However, recent evidence suggests, at least in high bleeding-risk patients, that IVC filters may reduce pulmonary embolism–related mortality.27 Anticoagulation is commonly administered along with the placement of IVC filters,24,28 but whether reduced-intensity anticoagulation in this setting is efficacious is not known. Among the 3222 cancer patients followed in the Registro Informatizado de Enfermedad TromboEmbólica registry who received less aggressive anticoagulation with low–molecular weight heparin (ie, below the recommended dosing of 150 IU/kg per day), they not only experienced less major hemorrhages, but paradoxically less fatal pulmonary emboli.29 DOACs are known to result in less frequent major hemorrhages relative to warfarin and improved bleeding-related mortality in noncancer cohorts.30 Studies are currently underway evaluating the use of direct oral anticoagulants, such as edoxaban, for the treatment of thromboembolism in cancer.31 Fortunately, these studies do not specifically exclude patients with primary brain tumors. In a recent study, the 3-month mortality rate after a diagnosis of ICH in patients treated with DOACs was 28%,32 but the case-fatality rate appears to be similar to that of warfarin-treated patients in atrial fibrillation trials.13
There are several limitations to the current study that warrant discussion. Cohorts were not allocated randomly, which may introduce selection biases. We used an automated, “round-robin” scoring algorithm to match controls based on baseline characteristics to the enoxaparin cohort as an attempt to best control for these potential biases. Because ICH can be a presenting finding of glioma, which is likely to influence the eventual decision to anticoagulate, we only included ICHs that occurred after the initial diagnosis of glioma in the primary analyses. Notably, none of the patients in the enoxaparin cohort were on anticoagulation at the time of glioma diagnosis, which further validates this approach. We were also unable to conclude that therapeutic anticoagulation increased the overall rate of any intracranial (trace) hemorrhage or measurable ICHs in the enoxaparin cohort relative to controls which is likely due to the limited sample size. Nonetheless, there was sufficient power for the most salient conclusion that major ICHs were significantly greater in enoxaparin-treated patients. To more thoroughly evaluate predictive variables and modifiers, a larger study involving external institutions will be required.
Given the greater than threefold increased risk of major ICH in patients with glioma on enoxaparin and the poor prognosis after a major ICH, we believe caution is warranted when considering therapeutic anticoagulation in this setting. Using a previously validated nomogram, we identified patients at greatest risk of developing ICH. Key elements of this nomogram include thrombocytopenia, advanced age, and uncontrolled hypertension. which are established risk factors for the development of ICH33,-35 and are reasonable variables to factor in when considering full-dose or alternative anticoagulation strategies (ie, reduced-intensity enoxaparin along with IVC filter placement). More research is needed to establish which factors best predict the development of ICH in patients with primary brain tumors and whether modified anticoagulant approaches improve outcomes.
Presented as an oral abstract at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3 December 2016.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.M. and J.I.Z. were the principal authors; C.M., E.J.U., M.P., D.N., G.M.W., and J.I.Z. designed the study; C.M. and E.J.U. performed the data collection; G.M.W. performed the cohort matching algorithms; C.M., M.P., D.N., and J.I.Z. analyzed the data; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: J.I.Z. received research funding from Quercegen Pharma. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Zwicker, Division of Hematology and Oncology, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215; e-mail: jzwicker@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal