The gene p53 was originally identified in 1979 as a tumor suppressor in a multitude of human cancers (eg, colorectal, lung, and breast), where it functions as a suppressor of cancer proliferation by means of the induction of cell cycle arrest and programmed apoptosis in response to a variety of cellular stress signals. Loss of p53 or the expression of p53 gain-of-function mutations can enhance the metastatic potential of tumor cells.1,2 However, p53 has subsequently been found to be involved in a variety of fundamental gene processes, including gene transcription, the cell cycle, the abovementioned programmed cell death, DNA replication, and DNA repair. Now, for example, p53 is known to regulate and mediate vascular remodeling and wound healing,1 with effects on adhesion molecules and, relevant to fibrinolysis, the expression of plasminogen activator inhibitor-1.3,4
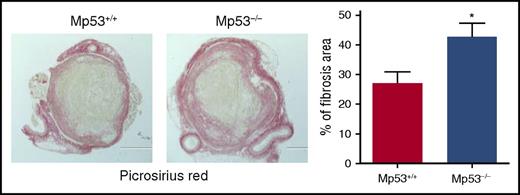
Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each genotype are shown. The intrathrombus collagen area was quantitated as a measure of fibrosis, as described in “Methods” in the article by Mukhopadhyay et al. All values represent the mean ± SEM (n = 4-5). *P < .05, p53 Mp53+/+ vs Mp53−/−. See Figure 5D in the article by Mukhopadhyay et al that begins on page 3245.
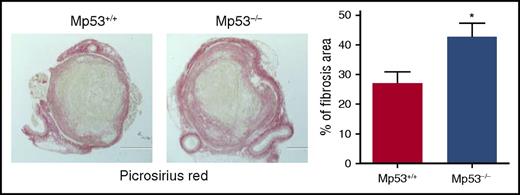
Histochemical analysis of intrathrombotic collagen content by Picrosirius Red staining at day 12 after vena cava ligation; original magnification ×100. Representative images from 4 to 5 independent mice of each genotype are shown. The intrathrombus collagen area was quantitated as a measure of fibrosis, as described in “Methods” in the article by Mukhopadhyay et al. All values represent the mean ± SEM (n = 4-5). *P < .05, p53 Mp53+/+ vs Mp53−/−. See Figure 5D in the article by Mukhopadhyay et al that begins on page 3245.
In this regard, in this issue of Blood, the article by Mukhopadhyay et al5 reports the novel finding that endogenous p53 activity in cells of myeloid lineage plays a significant role in macrophage polarization towards a tissue remodeling phenotype and thereby resolution of venous thrombus formed using a mouse vena cava ligation model. By resolution is meant inflammatory-mediated vascular remodeling that directly involves the thrombus. In particular, intrathrombus collagen content by Picrosirius Red staining was reduced by 30% to 35% in wild-type mice, as compared with Mp53−/− mice, that lack myeloid p53, at day 12 (see figure).
The authors further report that the genetic deficiency of p53 (Tp53−/− mice) or pharmacologic inhibition by daily intraperitoneal pifithrin-α, a small-molecule inhibitor of p53 function, leads to a significant reduction in the inflammatory state associated with major thrombus formation, the inflammatory state itself being a requirement for thrombus resolution. Conversely, pharmacologic augmentation of p53 activity using subcutaneous infusion of the antimalarial quinacrine, a p53 agonist, accelerates resolution of the “well-established” thrombus present 3 days following ligation. Treatment with quinacrine, a known stabilizer of p53 and inducer of both p53-dependent and p53-independent cell death, results in a 40% reduction in thrombus weight by day 12, as shown in Figure 6 in the article by Mukhopadhyay et al. The quinacrine effect, moreover, is specific to myeloid p53, since treatment of lysM-Cre/p53 mice, deficient in myeloid p53, has no effect on venous thrombus resolution.
Quinacrine, taken up into platelet-dense bodies and endothelial cells as well as leukocytes, may have other p53 cellular actions not presently described, if not ones that bear on thrombus resolution. Further, resolution of thrombus fibrin is not addressed explicitly, and the full implications of the discovery of the above role for p53 for venous thrombosis are not yet known. For example, might certain p53 mutations contribute to hypercoagulability? Can some measure of macrophage polarization become a biomarker for clot resolution? Since blood flow directs convective diffusion of platelets and procoagulant proteins and has shear stress–mediated effects on cellular function6,7 as well as receptor–ligand bond lifetimes,8 what might be the effect of blood flow on p53 function?
Nonetheless, the findings of Mukhopadhyay et al suggest a novel pathway to which treatment of venous thrombosis may conceivably be extended in the future. Beyond quinacrine and other acridine derivatives, perhaps other agonists will now be found that, alone or in combination with newer anticoagulants and/or systemic or catheter-directed exogenous thrombolytics, will have even greater and/or more rapid benefits in thrombus resolution while retaining an acceptable safety profile. Such therapies are needed if we are to avoid early and irreversible damage to the venous valves, which underlies a large part of venous incompetence and the presently high incidence (just under 50%) of postthrombotic syndrome in deep venous thrombosis.9 A p53-inclusive approach might also offer a safe treatment alternative for those patients (eg, with concurrent intracranial hemorrhage or recent major surgery) for whom anticoagulation or thrombolysis poses too grave a bleeding risk.
Conflict-of-interest disclosure: The author declares no competing financial interests.