To the editor:
The inhibitor of Bruton’s tyrosine kinase (BTK) ibrutinib has transformed the treatment of chronic lymphocytic leukemia (CLL); many patients with previously untreatable disease may now enter durable remissions.1,2 Nevertheless, the kinome of ibrutinib is broad, resulting in toxicities including bleeding, arthralgia, diarrhea, hypertension, and atrial fibrillation.3-6 Up to 20% of patients discontinue ibrutinib due to toxicity.7-9 More selective BTK inhibitors (BTKis) include ONO/GS-4059, acalabrutinib, and BGB-3111. Preliminary data indicate that these drugs have comparable activity to ibrutinib, but with reduced toxicities.10-12 However, long-term follow-up and response data have not yet been reported. We provide an updated, 3-year follow-up of treatment efficacy, safety, and laboratory correlates, including baseline mutational profiling of CLL patients in the phase 1 ONO/GS-4059 extension study.
The ONO/GS-4059 POE001 phase 1 clinical study (NCT01659255) was conducted to determine the safety and tolerability of ONO/GS-4059 in patients with relapsed/refractory (R/R) B-cell malignancies. Between September 2012 and January 2015, 90 patients were enrolled and treated with ONO/GS-4059. Patients continuing to respond or those who have stable disease could enroll in the long-term extension study (ONO/GS-US-1787, NCT02457559). In the CLL cohort (comprising 28 patients), treatment consisted of 9 cohorts receiving 20 mg once daily to 600 mg once daily or a twice-daily regimen of 300 mg. Each site had Institutional Ethical Committee approval. Informed consent was obtained from all patients transferring to the extension study. DNA was extracted from peripheral blood from 27/28 CLL patients before trial therapy. Targeted sequencing was performed using the Illumina next-generation sequencing (NGS) platform from Sistemas Genomicos (Valencia, Spain), using a predesigned CLL panel (supplemental Table 1, available on the Blood Web site). Reads were aligned against the human reference genome version GRCh37/hg19. Filtering was performed using Picard tools (https://broadinstitute.github.io/picard/) and SAMtools (http://samtools.sourceforge.net/). Confirmatory Sanger sequencing was performed on identified sequence variants and annotated using the Ensembl database (www.ensembl.org). Only sequence variants leading to a change in amino acid composition and not reported in the single nucleotide polymorphism database were scored as mutations. Sanger sequencing was used to determine IGHV status. Statistical analysis was performed on the modified intention-to-treat population (patients with ≥1 dose of study drug). Kaplan-Meier methodology was used to calculate progression-free survival (PFS). The date of definitive progression was the time point at which progression was first identified by radiographic, imaging, or clinical data, or death. Patients were censored if no PFS event was observed.
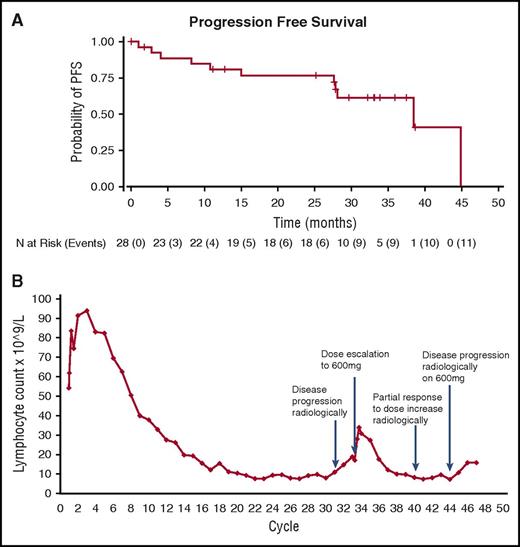
All 28 patients enrolled with R/R CLL were evaluable for efficacy and safety. The median number of prior treatments was 4 (range 2-9); 5 patients were primarily fludarabine refractory, and 1 patient had received a prior PI3K inhibitor. Eleven patients (39%) were refractory to their last line of therapy. None had received prior BTKi treatment. Anticoagulant therapy was permitted; 6/28 patients were on anticoagulant therapy during the study. At the time of updated analysis (June 8, 2016), 11 patients (39.3%) had discontinued treatment. (In comparison, at 3 years, 47% had discontinued treatment with Ibrutinib.13 ) Reasons for discontinuation were death (n = 3), disease progression (n = 4), adverse events (AEs) (n = 3), and sponsor decision due to extended drug interruption (n = 1); in 1 patient with AE, disease progression occurred concurrently (supplemental Table 1). Subjects remaining on study were receiving doses of ONO/GS-4059 ranging from 40 mg once daily to 600 mg once daily or 300 mg twice daily. No maximum tolerated dose in patients with CLL was identified. The median duration on study at censoring was 32.5 months. Estimated median PFS was 38.5 months (Figure 1A), and median overall survival was 44.9 months.
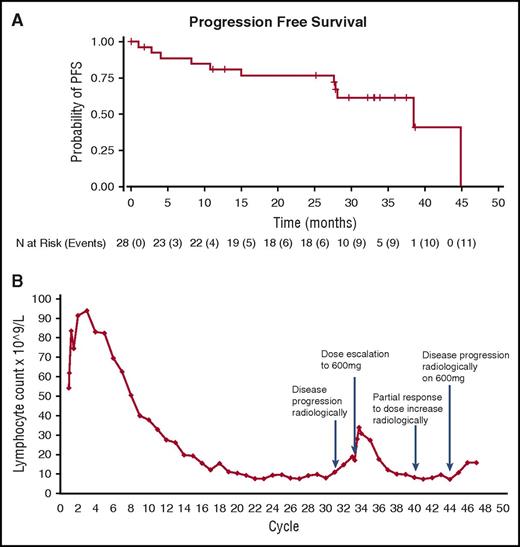
Updated results with ONO/GS-4059 in patients with CLL. (A) Updated PFS curve for CLL patients. Mean duration on study was 26.6 months and estimated median PFS was 38.5 months. (B) Case example: Recurrent lymphocytosis shown in TP53 mutant CLL patient following an initial lymph nodal response for 31 cycles on 40 mg OD ONO/GS-4059; the patient subsequently responded for a further 12 months to 600 mg OD, with a second lymphocytosis (1.74-fold increase; initial 1.81-fold increase) and lymph nodal response.
Updated results with ONO/GS-4059 in patients with CLL. (A) Updated PFS curve for CLL patients. Mean duration on study was 26.6 months and estimated median PFS was 38.5 months. (B) Case example: Recurrent lymphocytosis shown in TP53 mutant CLL patient following an initial lymph nodal response for 31 cycles on 40 mg OD ONO/GS-4059; the patient subsequently responded for a further 12 months to 600 mg OD, with a second lymphocytosis (1.74-fold increase; initial 1.81-fold increase) and lymph nodal response.
Responses (complete or partial) were initially observed in 24/25 (96%) evaluable patients.10 Ibrutinib can result in prolonged lymphocytosis (duration >1 year), reported in 20% of patients in the phase 1b/2 trial14 and associated with 13q deletion. In our study, 23 patients (82%) exhibited lymphocytosis; mean fold increase above baseline was 4.5-fold.10 In all instances, lymphocytosis following ONO/GS-4059 resolved by cycle 6. As with other BTKis,11,15 changes in serum levels of CCL3 and CCL4 showed a significant decrease at day 8, consistent with B-cell receptor signaling pathway blockade; tumor necrosis factor-α, interleukin-10 (IL-10), IL-6, and IL-8 also showed a significant decrease 8 days after treatment initiation (supplemental Figure 1A). Similar to data reported with acalabrutinib11 but in contrast to ibrutinib,16 immunoglobulin levels did not change significantly with long-term therapy with ONO/GS-4059 (supplemental Figure 1B).
Targeted NGS mutational data at time of trial entry, along with IGHV mutation and interphase FISH data, are shown in supplemental Table 1. Twenty-one of 25 patients exhibited unmutated IGHV gene segments. Seven of 21 patients with unmutated IGHV gene segments have discontinued treatment. One patient with mutated IGHV utilizing VH3-21 progressed. Although no formal correlative analysis was possible due to small sample size, no differences in response or PFS according to chromosome 17p deletion or TP53 mutation were observed. Seven of 10 patients with TP53 mutation remain on therapy; of the 3 that discontinued study treatment, 1 progressed, 1 was withdrawn due to an AE, and 1 died of septicemia. The patient with the TP53 mutation who progressed with a TP53 mutation in the DNA binding domain (DeltaL252T253) had an initial response for 31 cycles on a dose of 40 mg once daily ONO/GS-4059. Because this patient lacked BTK and PLCG2 mutations (data not shown), the dose of ONO/GS-4059 was increased to 600 mg once daily. The patient responded for a further 12 months, associated with a second lymphocytosis comparable to that seen initially (1.74-fold increase; initial 1.81-fold increase) and lymph nodal response (Figure 1B). One of 3 patients with ATM mutation has progressed on study (930 days). Eight patients had SF3B1 mutations, 5 of whom have discontinued treatment (1 due to progression). NOTCH1 mutations were found in 7 patients, 3 of whom have come off study (1 due to progression). As previously reported, NOTCH1 and SF3B1 were mutually exclusive.17 No mutations appeared to predict shorter PFS with ONO/GS-4059, but the sample sizes were too small for statistical analysis. Mutations not previously identified in CLL include a mutation in MEK1 (E203K) in 1 patient with early progression, previously reported in metastatic melanoma resistant to vemurafenib,18 and a POT1 mutation E67K. No mutations in MYD88, PLCG2, or BRAF were observed.
ONO/GS-4059 continued to be well tolerated. Extended follow-up did not reveal new safety or toxicity concerns, and updated treatment-emergent AEs (TEAEs; frequency ≥15%) are shown in Table 1. Most TEAEs were grade 1 or 2. The most common AEs were bruising (35.7% all grades), neutropenia (35.7% all grades), and anemia (32.1% all grades). Only 1 grade 3 bleeding event (3.6%; hematoma) occurred on study in a patient not receiving anticoagulation therapy. Twelve patients (42.9%) had greater than or equal to grade 3 infections. There were no greater than or equal to grade 3 events reported for other AEs of interest with the BTKi class, including hypertension and atrial fibrillation. One patient had an AE of weight gain; 14 patients (50%) had a grade 1 to 3 weight gain. Similar weight gain has been reported with acalabrutinib.11
Interestingly, no cases of Richter transformation have been reported in patients receiving ONO/GS-4059. Richter transformation in patients receiving ibrutinib tends to occur early.8,19
In conclusion, these data strongly support the ongoing evaluation of ONO/GS-4059 in CLL. Patients with high-risk CLL genetics responded with minimal toxicity. Identification of significant differences in toxicity profiles between BTKis awaits direct comparative studies. However, the tolerability of ONO/GS-4059 shown here with extended follow-up may confer advantages, particularly in the context of combination therapies and in ibrutinib-intolerant patients.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors extend their thanks to the patients and their families and to ONO Pharma UK for their help with data analysis.
NGS (Sistemas Genomicos; https://www.sistemasgenomicos.com) was funded by the Cancer Research UK Leicester Centre and supported by the Leicester Experimental Cancer Medicine Centre. This study was funded by Gilead Sciences, Inc. Editorial support was provided by Impact Communication Partners, Inc.
Contributions: H.S.W. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; S.J. designed and performed research, collected, analyzed, and interpreted data; S.A.R. designed and performed research, analyzed data, and wrote the manuscript; G.C., L.K., and C.V.H. performed research and collected data; F.M. performed research, collected, analyzed, and interpreted data, and wrote the manuscript; S. Macip collected, analyzed, and interpreted data; C.J. and N.S. performed research; C.H. performed research and analyzed and interpreted data; P.Q. performed research, collected data, and wrote the manuscript; C.F. performed research, collected data, and wrote the manuscript; Y.Y. analyzed and interpreted data and performed statistical analysis; S. Mitra analyzed and interpreted data and wrote the manuscript; G.S. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; and M.J.S.D. designed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: H.S.W. received consulting fees from AbbVie, research funding from Gilead Sciences, Inc, and honoraria from AbbVie and Gilead Sciences, Inc; S.A.R. received consulting fees from Pharmacyclics and Janssen, research funding from Janssen, and honoraria from Acerta Pharma; G.C. received consulting fees from Celgene and Roche and honoraria from Celgene, Gilead Sciences, Inc, Janssen, Roche, and Sanofi; F.M. received consulting fees from Gilead Sciences, Inc and Servier, honoraria from Celgene, Gilead Sciences, Inc, Janssen, and Roche, and holds membership on the Board of Directors of Celgene, Gilead Sciences, Inc, and Roche; L.K. received honoraria from Gilead Sciences, Inc; C.H. is employed by and received consulting fees from Bristol-Myers Squibb, Gilead Sciences, Inc, and Takeda and received research funding from Roche; C.V.H. received research funding from Acerta Pharma; C.F. received consulting fees from AbbVie, Gilead Sciences, Inc, Janssen, and Roche and holds membership on the Board of Directors of Roche; Y.Y. and S. Mitra are employed by and hold stock options in Gilead Sciences, Inc; M.J.S.D. received consulting fees from AbbVie and Roche, research funding from Gilead Sciences, Inc and Ono Pharmaceuticals, and honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Martin J. S. Dyer, University of Leicester, Henry Wellcome Building, Room 3/57, Lancaster Rd, Leicester LE1 9HN, United Kingdom; e-mail: mjsd1@le.ac.uk.