Key Points
Vitamin A levels below the median at day 30 posttransplant are associated with increased cumulative incidence of GI GVHD in children.
Potential mechanisms include increased intestinal permeability and increased lymphocyte homing to the intestine.
Abstract
Vitamin A promotes development of mucosal tolerance and enhances differentiation of regulatory T cells. Vitamin A deficiency impairs epithelial integrity, increasing intestinal permeability. We hypothesized that higher vitamin A levels would reduce the risk of graft-versus-host disease (GVHD) through reduced gastrointestinal (GI) permeability, reduced mucosal injury, and reduced lymphocyte homing to the gut. We tested this hypothesis in a cohort study of 114 consecutive patients undergoing allogeneic stem cell transplant. Free vitamin A levels were measured in plasma at day 30 posttransplant. GI GVHD was increased in patients with vitamin A levels below the median (38% vs 12.4% at 100 days, P = .0008), as was treatment-related mortality (17.7% vs 7.4% at 1 year, P = .03). Bloodstream infections were increased in patients with vitamin A levels below the median (24% vs 8% at 1 year, P = .03), supporting our hypothesis of increased intestinal permeability. The GI mucosal intestinal fatty acid–binding protein was decreased after transplant, confirming mucosal injury, but was not correlated with vitamin A levels, indicating that vitamin A did not protect against mucosal injury. Expression of the gut homing receptor CCR9 on T-effector memory cells 30 days after transplant was increased in children with vitamin A levels below the median (r = −0.34, P = .03). Taken together, these data support our hypothesis that low levels of vitamin A actively promote GI GVHD and are not simply a marker of poor nutritional status or a sicker patient. Vitamin A supplementation might improve transplant outcomes.
Introduction
Graft-versus-host disease (GVHD) is the major cause of nonrelapse morbidity and mortality after allogeneic hematopoietic stem cell transplant (HSCT).1,2 Acute GVHD can be difficult to treat, especially if refractory to frontline steroid therapy, and mortality is high. Risk factors for acute GVHD include recipient age, donor and recipient HLA matching, and intensity of preparative regimen, none of which can be easily modified to reduce risk. GVHD is initiated by cytokine release from tissues damaged during the preparative regimen, particularly the gut mucosa.1 Damaged gut epithelium allows translocation of bacterial molecules into the bloodstream, activating T cells and promoting proliferation and differentiation, which lead to increased trafficking of T cells to target organs and worsening of tissue destruction and inflammation associated with gastrointestinal (GI) GVHD.1
We have been working to identify pretransplant host factors that increase risk of GVHD and could be improved before transplant. We considered vitamin A as a candidate variable in this search because it modifies gut permeability in other clinical settings, is essential for development of mucosal tolerance, and regulates lymphocyte trafficking to the gut.3-12
Vitamin A is an essential nutrient, ingested in the diet as preformed retinol. Levels in the blood are homeostatically regulated to maintain a narrow range, which is accomplished through cosecretion of retinol bound to its specific carrier protein, retinol-binding protein (RBP), from the liver.4 RBP is an acute-phase reactant that decreases with inflammation.13 Dietary vitamin A is hydrolyzed to the active metabolite, retinoic acid, by cells that express retinaldehyde dehydrogenase, present in large numbers in the gut mucosa, which are commonly damaged during HSCT. Retinoic acid promotes secretion of interleukin 22 (IL-22), known to promote epithelial cell proliferation and healing, restore tight junctions, and increase mucus production from goblet cells.14
Vitamin A is required for normal immune function and for the development of immune tolerance in the intestine.4 In human studies and in animal models, vitamin A deficiency increases gut permeability, which can be improved by vitamin A supplementation.5,15,16 Dendritic cells (DCs) within the intestine are constantly encountering new antigens and presenting them to naïve T cells. The presence of vitamin A and transforming growth factor-β shifts the differentiation of those naïve T cells toward regulatory T cells (Tregs) and away from T helper 17 cells, facilitating mucosal tolerance.4 In mouse models, vitamin A deficiency leads to lower levels of CD4+ Foxp3+LAP+ Tregs in gut lymphoid tissues and spleen,17 along with increased levels of inflammatory cytokines.6 Vitamin A supplementation increased the frequencies of tissue Tregs in this animal model. In addition, T-cell homing to the intestine is regulated by vitamin A through regulation of intestinal homing molecules on the surface of the T cells, such as CCR9, modifying risk of gut inflammation.9
We hypothesized that higher vitamin A levels would reduce the risk of GVHD, especially GI GVHD, through improved intestinal permeability, increased secretion of IL-22, reduced mucosal injury, and reduced lymphocyte homing to the gut.
Methods
Patients and transplant procedures
Patient and transplant characteristics are summarized in Table 1. Children were enrolled in a prospective cohort study, the Cincinnati Children’s Hospital Medical Center Bone Marrow Transplant repository. All participants were eligible to participate and signed consent for prospective blood, stool, and urine collection for biological studies of complications of transplantation. The institutional review board approved the study.
Patient and transplant demographics (n = 124)
| Characteristic . | Value . |
|---|---|
| Age, y | |
| Median | 8 |
| Range | 0.4-32 |
| Diagnosis, n (%) | |
| Malignancy | 33 (26) |
| Immune deficiency | 44 (36) |
| Bone marrow failure | 47 (38) |
| Conditioning regimens, n (%) | |
| Myeloablative | 72 (58) |
| Reduced intensity | 52 (42) |
| Stem cell source, n (%) | |
| BM | 104 (84) |
| PBSC | 14 (11) |
| CB | 6 (5) |
| Cell dose | |
| Median | 6.8 × 108 cells/kg |
| Range | 15.4 × 106-32.75 × 108 cells/kg |
| Donor, n (%) | |
| Related | 47 (38) |
| Unrelated | 77 (62) |
| Match, n (%) | |
| BM and PBSC | |
| 10/10 | 92 (74) |
| 9/10 | 23 (19) |
| 8/10 | 3 (2) |
| CB | |
| 6/6 | 2 (1) |
| 5/6 | 2 (1) |
| 4/6 | 2 (1) |
| GVHD prophylaxis, n (%) | |
| CSA ± other | 108 (87) |
| Tacrolimus ± other | 5 (4) |
| Ex-vivo T-cell depletion | 9 (7) |
| Other | 2 (2) |
| Characteristic . | Value . |
|---|---|
| Age, y | |
| Median | 8 |
| Range | 0.4-32 |
| Diagnosis, n (%) | |
| Malignancy | 33 (26) |
| Immune deficiency | 44 (36) |
| Bone marrow failure | 47 (38) |
| Conditioning regimens, n (%) | |
| Myeloablative | 72 (58) |
| Reduced intensity | 52 (42) |
| Stem cell source, n (%) | |
| BM | 104 (84) |
| PBSC | 14 (11) |
| CB | 6 (5) |
| Cell dose | |
| Median | 6.8 × 108 cells/kg |
| Range | 15.4 × 106-32.75 × 108 cells/kg |
| Donor, n (%) | |
| Related | 47 (38) |
| Unrelated | 77 (62) |
| Match, n (%) | |
| BM and PBSC | |
| 10/10 | 92 (74) |
| 9/10 | 23 (19) |
| 8/10 | 3 (2) |
| CB | |
| 6/6 | 2 (1) |
| 5/6 | 2 (1) |
| 4/6 | 2 (1) |
| GVHD prophylaxis, n (%) | |
| CSA ± other | 108 (87) |
| Tacrolimus ± other | 5 (4) |
| Ex-vivo T-cell depletion | 9 (7) |
| Other | 2 (2) |
BM, bone marrow; CB, cord blood; CSA, cyclosporine; PBSC, peripheral blood stem cell.
One hundred and twenty four patients were included in the study; however, patient samples were available for 114 patients in all time points discussed here. Median patient age was 8 years (range, 0.4-32 years). The majority of patients was transplanted for a nonmalignant disease and received bone marrow as the cell source. Myeloablative conditioning regimens were more frequent than a reduced intensity conditioning regimen. Clinical data were abstracted from the institutional transplant database and from the medical record.
Vitamin A, RBP, IL-22, and I-FABP levels
Blood samples and clinical data were collected prospectively, once weekly, from admission until 100 days posttransplant. Free vitamin A levels were measured in patient plasma at day 30 posttransplant (a time when onset of GVHD is frequent) in 114 patients using the Human Vitamin A enzyme-linked immunosorbent assay (ELISA) kit from MyBiosource as directed by manufacturer instructions. Retinol binding protein levels were measured in patient plasma at day 30 posttransplant using the Human RBP4 ELISA kit (R&D systems, Minneapolis, USA) as directed by manufacturer instructions. Intestinal fatty acid–binding protein (I-FABP) levels were measured in patient plasma at day 7 posttransplant using the Human FABP2 ELISA kit (R&D Systems), as directed by manufacturer instructions. IL-22 levels were measured in patient plasma at day 30 posttransplant using the Human IL-22 ELISA kit (R&D Systems), as directed by manufacturer instructions.
Flow cytometry
Frozen peripheral blood mononuclear cells collected at day +30 were obtained from the Cincinnati Children’s Hospital Medical Center HSCT repository. Cell numbers were sufficient for analysis in 42 transplant recipients, in whom vitamin A levels had already been determined. After thawing, samples were incubated with fluorochrome-conjugated monoclonal antibodies directed against CD3, CCR9, CD4 (Biolegend, San Diego, CA), CD45, CD8, and CD45RA (BD Biosciences, San Jose, CA). Samples were analyzed by flow cytometry on a FACS Canto II flow cytometer (BD Biosciences). Results were analyzed using FACSDiva (BD Biosciences). Effector memory T-cell populations (TEM) were defined as CD3+ CD8+ or CD4+ lymphocytes lacking CD45 RA and CCR7 expression. The absolute CD8+ T-cell count was calculated from the percentage of CD3+ CD8+ lymphocytes within the lymphocyte gate, multiplied by the absolute lymphocyte count. The absolute lymphocyte count was obtained from the complete blood count from the same day of the frozen peripheral blood mononuclear cell sample collection in the clinical hematology laboratory at Cincinnati Children’s Hospital Medical Center. Absolute CCR9+ CD8+ TEM cell counts were calculated as percentages of the absolute CD8+ T-cell count.
Statistical analysis
Categorical data are described as frequency (percent). Continuous data are described as median (range). Cumulative incidence of grade 2-4 GVHD, GI GVHD, blood stream infections, and transplant-related mortality (TRM) were assessed as univariate outcomes divided into those above and below the median vitamin A levels using the Gray method of competing risk. Kaplan-Meier calculations were used to estimate probability of survival at specific time points. Relapse was treated as a competing event for each outcome. Additionally, for grades 2-4 GVHD, GI GVHD, and blood stream infections, death from any cause was considered a competing event. For grades 2-4 GVHD and TRM, a multivariate analysis was conducted using the Fine and Gray method for competing events. Results from the multivariate analysis are summarized as odds ratio (OR) and 95% Wald confidence intervals. Spearman rank correlation was used to assess associations between CCR9 expression and vitamin A levels. All calculations were carried out using R, version 3.2.4 (Vienna, Austria).
Results
Vitamin A levels and transplant outcomes
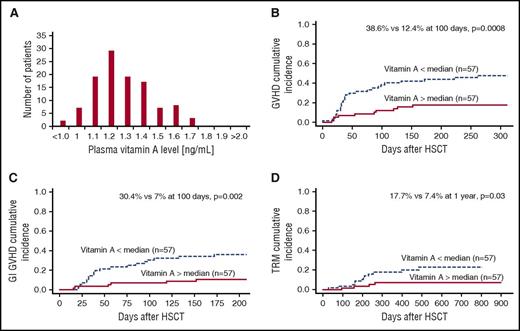
Free vitamin A levels at day 30 posttransplant were distributed normally in a narrow range, reflecting the homeostatic regulation of vitamin A (Figure 1A). We chose to measure free vitamin A because it is the biologically active form. Normal ranges for free vitamin A are not easily found; however, the normal distribution found in our patient population allowed us to focus on transplant recipients with levels above and below the median, rather than a subset of outliers with vitamin A deficiency, increasing the biological relevance of our findings. The median vitamin A level at day 30 posttransplant was 1.30 ng/mL (range, 1.0-1.7 ng/mL). We did consider whether vitamin A levels reflected overall nutritional status. We looked to see if vitamin A levels were correlated with vitamin D levels, but found no correlation (data not shown, P = .18), suggesting that levels of vitamin A were independent of nutritional status.
Vitamin A levels and transplant outcomes. (A) Free vitamin A levels in patient plasma 30 days posttransplant are in a narrow range and normally distributed with a median value of 1.296 ng/mL. (B) Vitamin A levels below the median are associated with increased incidence of grades 2-4 GVHD (38.6% vs 12.4% at 100 days, P = .0008). (C) Vitamin A levels below the median are associated with increased incidence of GI GVHD (30.4% vs 7% at 100 days, P = .002). (D) TRM was also increased in patients with vitamin A levels below the median compared with patients with vitamin A levels above the median (17.7% vs 7.4% at 1 year, P = .03).
Vitamin A levels and transplant outcomes. (A) Free vitamin A levels in patient plasma 30 days posttransplant are in a narrow range and normally distributed with a median value of 1.296 ng/mL. (B) Vitamin A levels below the median are associated with increased incidence of grades 2-4 GVHD (38.6% vs 12.4% at 100 days, P = .0008). (C) Vitamin A levels below the median are associated with increased incidence of GI GVHD (30.4% vs 7% at 100 days, P = .002). (D) TRM was also increased in patients with vitamin A levels below the median compared with patients with vitamin A levels above the median (17.7% vs 7.4% at 1 year, P = .03).
The overall incidence of grades 2-4 GVHD was 32.2% (n = 40) and that of GI GVHD was 25% (n = 31), with a median onset of 38 days (range, 15-261 days). The cumulative incidence of grades 2-4 GVHD was significantly higher in children with a vitamin A level below the median compared with those with levels above the median (38.6% vs 12.4% at 100 days, P = .0008, Figure 1B). The rate of GI GVHD was similarly greater (30.4% vs 7% at 100 days, P = .002, Figure 1C). Furthermore, the cumulative incidence of stage 3-4 GI GVHD was also increased in patients with vitamin A levels below the median compared with those with levels above the median (23% vs 7% at 100 days, P = .03, data not shown). In a multivariate analysis including diagnosis, stem cell source, and HLA match, vitamin A level remained an independent risk factor for GVHD with a threefold increase in incidence (OR, 3.3; P = .003; Table 2). Treatment-related mortality was also increased in children with lower than median vitamin A levels (17.7% vs 7.4% at 1 year, P = .03, Figure 1D). Vitamin A level below the median at day 30 posttransplant was an independent risk factor for TRM in multivariate analysis (OR, 3.07; P = .06; Table 2).
Multivariate analysis of development of GVHD and TRM
| . | GVHD OR (95% CI) . | P value . | TRM OR (95% CI) . | P value . |
|---|---|---|---|---|
| Vitamin A level | 3.32 (1.5-6.7) | .003 | 3.07 (0.9-10.1) | .06 |
| Malignancy | 1.9 (1-3.7) | .06 | 1.89 (0.6-5.8) | .27 |
| HLA match | 1.42 (0.5-3.9) | .5 | 1.11 (0.2-6.7) | .9 |
| Stem cell source | ||||
| BM | 1.8 (0.5-6) | .3 | 1.74 (0.2-13.7) | .6 |
| CB | 1.7 (0.1-24.2) | .7 | 3.73 (0.08-164.9) | .5 |
| PBSC | BV | NA | BV | NA |
| . | GVHD OR (95% CI) . | P value . | TRM OR (95% CI) . | P value . |
|---|---|---|---|---|
| Vitamin A level | 3.32 (1.5-6.7) | .003 | 3.07 (0.9-10.1) | .06 |
| Malignancy | 1.9 (1-3.7) | .06 | 1.89 (0.6-5.8) | .27 |
| HLA match | 1.42 (0.5-3.9) | .5 | 1.11 (0.2-6.7) | .9 |
| Stem cell source | ||||
| BM | 1.8 (0.5-6) | .3 | 1.74 (0.2-13.7) | .6 |
| CB | 1.7 (0.1-24.2) | .7 | 3.73 (0.08-164.9) | .5 |
| PBSC | BV | NA | BV | NA |
BV, baseline value; NA, not available.
RBP and transplant outcomes
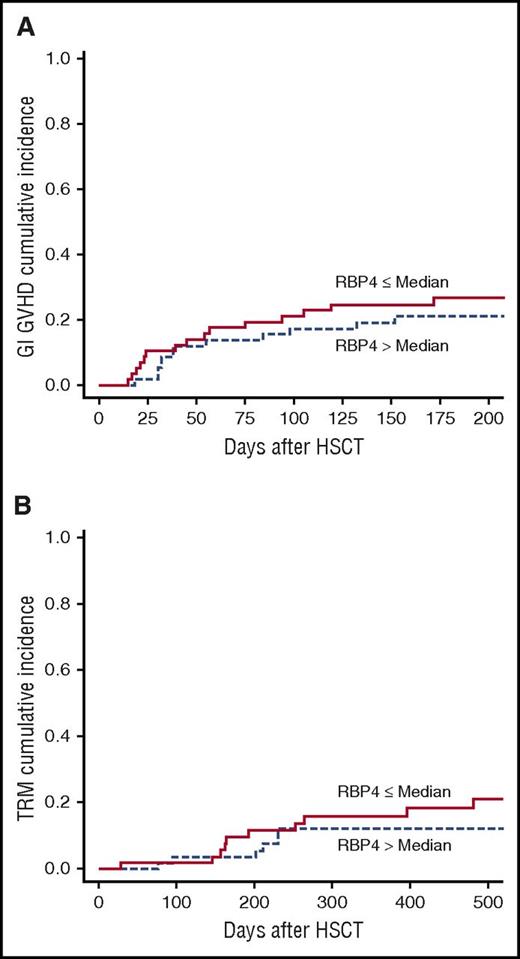
Our initial findings of an association between low vitamin A levels and occurrence of GVHD could perhaps be explained by a nonspecific response to inflammation reducing levels of RBP, a known negative acute phase reactant. To address this concern, we next measured RBP4 levels, expecting that if our data reflected a nonspecific inflammatory response, low RBP4 levels would be associated with increased incidence of GI GVHD and TRM. However, we found that the incidence of GI GVHD was similar in patients with RBP4 levels below the median compared with those with RBP4 levels above the median (P = .47, Figure 2A). Moreover, there was no change in TRM with regard to RBP4 levels (P = .33, Figure 2B).
RBP levels and transplant outcomes. RBP4 levels measured in plasma day 30 posttransplant had no effect on transplant outcomes including cumulative incidence of GI GVHD (A) and TRM (B).
RBP levels and transplant outcomes. RBP4 levels measured in plasma day 30 posttransplant had no effect on transplant outcomes including cumulative incidence of GI GVHD (A) and TRM (B).
Vitamin A and GI permeability
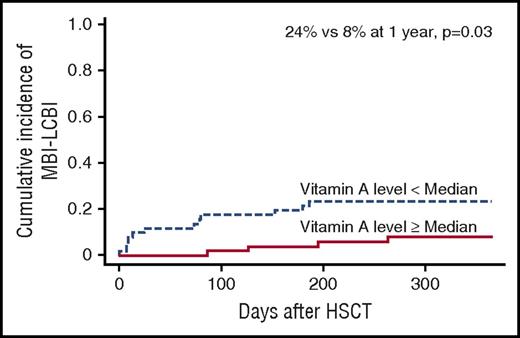
We hypothesized that patients with lower vitamin A levels would have increased intestinal permeability, leading to increased GI GVHD. Direct measurement of intestinal permeability, typically done using oral administration of high volumes of oral sugars, is not feasible in children undergoing HSCT because of the presence of mucositis and the inability of most children to tolerate oral intake. Instead, we tested our hypothesis by examining the frequency of mucosal barrier injury-laboratory confirmed bloodstream infections (MBI-LCBI) in patients with vitamin A levels above and below the median. MBI-LCBIs are defined by the National Healthcare Safety Network as bloodstream infections with specific intestinal organisms, but no other organisms, along with 1 of the following criteria: recipient of allogeneic HSCT within the past year with documented grade 3 or 4 GI GVHD or ≥1 L of diarrhea in a 24-hour period, or if any patient is neutropenic, defined as an absolute neutrophil count or a total white blood cell count <500 cells/mm3 within a 7-day period, which includes the date of the positive blood specimen.18 In agreement with the hypothesis, the incidence of MBI-LCBI was increased in patients with vitamin A levels below the median compared with those with vitamin A levels above the median (24% vs 8% at 1 year, P = .03; Figure 3), supporting our hypothesis of increased intestinal permeability.
Cumulative incidence of MBI-LCBIs. Incidence is higher in patients with vitamin A levels below the median at day 30 posttransplant compared with those with vitamin A levels above the median (24% vs 8% at 1 year, P = .03).
Cumulative incidence of MBI-LCBIs. Incidence is higher in patients with vitamin A levels below the median at day 30 posttransplant compared with those with vitamin A levels above the median (24% vs 8% at 1 year, P = .03).
Vitamin A and IL-22
We hypothesized that vitamin A might improve bowel epithelial function by promoting secretion of IL-22. We found no association between vitamin A levels in blood and IL-22 levels in blood (r = −0.03, P = .73), data not shown.
Vitamin A protection from mucosal damage
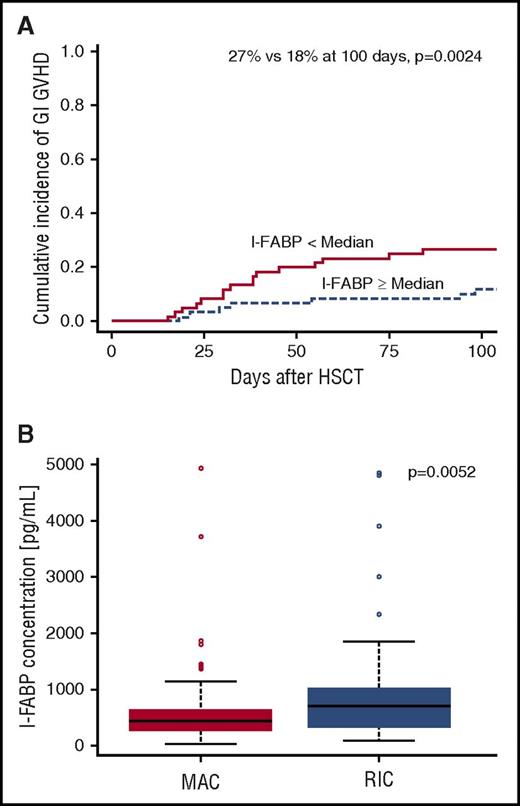
We next hypothesized that vitamin A might reduce risk of GI GVHD by reducing the extent of mucosal injury associated with the preparative regimen. To test this hypothesis, we measured serum levels of I-FABP, levels of which decrease in proportion to gut mucosal injury.19 We found that I-FABP levels below the median at day 7 were associated with an increased incidence of GI GVHD (27% vs 12% at 100 days, P = .002; Figure 4A). Moreover, I-FABP levels were lower and mucosal injury was greater in children receiving a myeloablative preparative regimen compared with a reduced intensity regimen (444 vs 696 pg/mL, P = .005; Figure 4B). However, vitamin A levels were not correlated with I-FABP levels (r = 0.05, P = .6), suggesting that vitamin A does not protect against mucosal injury.
Mucosal damage leads to increased incidence of GI GVHD and is increased in patients receiving a myeloablative conditioning regimen (MAC) compared with those receiving a reduced intensity conditioning regimen (RIC). (A) Cumulative incidence of GI GVHD is increased in patients with more mucosal damage as measured by I-FABP levels below the median (27% vs 18% at 100 days, P = .0024). (B) I-FABP levels are lower at day 7 posttransplant in patients who received MAC vs RIC, which correlates with increased mucosal damage.
Mucosal damage leads to increased incidence of GI GVHD and is increased in patients receiving a myeloablative conditioning regimen (MAC) compared with those receiving a reduced intensity conditioning regimen (RIC). (A) Cumulative incidence of GI GVHD is increased in patients with more mucosal damage as measured by I-FABP levels below the median (27% vs 18% at 100 days, P = .0024). (B) I-FABP levels are lower at day 7 posttransplant in patients who received MAC vs RIC, which correlates with increased mucosal damage.
CCR9 expression
Last, we hypothesized that vitamin A reduces risk of GI GVHD by reducing lymphocyte homing. We addressed this hypothesis by performing flow cytometry to measure the expression of CCR9 on TEM cells at day 30 posttransplant in a subset of our patients. We included patients with ≥1000 T-cell events during analysis (n = 42). We found that vitamin A levels below the median were associated with increased absolute CCR9 expression on CD8+ TEM cells (r = −0.34, P = .03; Table 3). These data support our hypothesis of increased intestinal homing of CD8+ TEM cells in patients with vitamin A levels below the median compared with those above the median.
Correlation of vitamin A levels and CCR9 expression on TEM cells (n = 42)
| Vitamin A . | ||
|---|---|---|
| CCR9 expression . | Correlation . | P value . |
| CD8 TEM | −0.34 | .03 |
| CD8 Naive | −0.21 | .19 |
| CD4 TEM | −0.11 | .58 |
| CD4 Naive | −0.27 | .09 |
| Vitamin A . | ||
|---|---|---|
| CCR9 expression . | Correlation . | P value . |
| CD8 TEM | −0.34 | .03 |
| CD8 Naive | −0.21 | .19 |
| CD4 TEM | −0.11 | .58 |
| CD4 Naive | −0.27 | .09 |
Discussion
We found that patients with vitamin A levels below the median at day 30 posttransplant had an increased incidence of GI GVHD and TRM compared with those with vitamin A levels above the median. Importantly, we found a normal distribution of vitamin A levels, so our findings likely reflect normal variation in physiologic activity of vitamin A, rather than a small group of outliers with pathological vitamin A deficiency.
Mouse data regarding the role of retinoic acid in transplantation are challenging, with some findings suggesting retinoic acid could ameliorate GVHD, and other data suggesting the opposite. Vitamin A and its active metabolite, retinoic acid, are important regulators of T-cell responses in murine models. Retinoic acid inhibits γ interferon (IFN-γ) production from T cells in vitro,20 and T cells from vitamin A–deficient mice overproduce IFN-γ.21 Retinoic acid inhibits T helper 17 cells in vitro and in vivo.22 Moreover, in vitro, retinoic acid inhibits IL-17 production and induces expression of the transcription factor Foxp3, associated with regulatory T cells, and IL-10 production.23,24 Several lines of experimental evidence also support a beneficial effect of vitamin A and retinoic acid on the host response to infection.25-27 In the gut, mechanisms that account for the anti-infective effects of vitamin A include support of B-cell function and T cell–dependent B-cell antibody responses.28 Trichinella spiralis infection of the gut in vitamin A–deficient mice resulted in T cells that produced IFN-γ but not IL-4 and, as a result, reduced the rate of parasite clearance.25,26 Furthermore, retinoic acid treatment reduced colonic inflammation caused by dextran sodium sulfate and infection, attributed to reduced production of IL-17 and IFN-γ.29,30 Further evidence of an anti-inflammatory role for retinoic acid in the GI tract comes from studies of APCmin+/− mice, a model of the human disorder familial adenomatous polyposis with increased GI inflammation and tumorigenesis, similar to humans with FAP. APCmin+/− mice placed on a vitamin A–deficient diet had increased GI inflammation and tumorigenesis that could be reversed by vitamin A repletion.31 Taken together, these data suggest that vitamin A and retinoic acid regulate T-cell function to limit inflammation following chemical and infectious injury in the gut. These data are in agreement with our findings in human allogeneic transplant, in which higher free vitamin A levels were associated with reduced GVHD and improved survival.
Contradictory murine evidence, suggesting that higher levels of vitamin A would be expected to be pro-inflammatory and worsen GVHD, also exists. Murine transplantation studies have identified retinoic acid and retinoic acid receptor signaling as critical factors in the pathogenesis of GVHD, as our data also suggest. In the mouse model, however, exogenous retinoic acid administration exacerbated GVHD, and genetic ablation of retinoic acid signaling in donor cells markedly reduced GVHD.32-34 A mouse model of induced dietary vitamin A deficiency showed that vitamin A deficiency altered GVHD tropism, reducing intestinal GVHD, although hepatic GVHD was perhaps worsened.9 None of the mouse transplantation models directly reflects our clinical study, however, in which we measured circulating levels of free vitamin A at day 30 after transplant. Our data show a close to normal distribution of vitamin A levels, and we compared levels above and below the median (likely mostly physiologically normal levels), rather than actively induced vitamin A deficiency. Moreover, no vitamin A supplementation was attempted in our study. These different results may indicate that the physiological effects of vitamin A depend on clinical context. Conversion of dietary vitamin A to the active metabolite, retinoic acid, requires access to cells expressing retinaldehyde dehydrogenase, which are present in limited tissues, mostly in the GI tract, which suffers severe damage during HSCT. Moreover, cells must express retinoic acid receptors to be responsive to ligand, and modulation of receptor expression is known to modify response. Clinical context, and perhaps expression of other molecules such as CYP26,35 likely modifies the effect of locally available levels of vitamin A, and situations of active deficiency or supplementation may show different responses in different tissues and different clinical settings, perhaps explaining the complex findings in mouse models.
Our study has identified 2 possible mechanisms by which higher vitamin A levels might reduce the incidence of GI GVHD. We found that the frequency of bloodstream infections tripled in children with vitamin A levels below the median, in agreement with increased permeability of the mucosal barrier. We recognize, however, that we did not directly measure GI permeability because of the challenge of giving a large oral load of sugars to children receiving HSCT. We cannot exclude the possibility that increased bloodstream infections occurred as a result of increased GVHD, rather than as a result of lower levels of vitamin A.
We also hypothesized that altered lymphocyte homing might modify risk of GVHD because retinoic acid has been shown to alter the expression of lymphocyte homing molecules. In support of our hypothesis, we found decreased expression of CCR9 on effector memory T cells in patients with higher than median vitamin A levels. Reduced CCR9 expression is in agreement with our clinical finding of reduced GVHD with higher levels of vitamin A, but contrasts with previous mouse and human reports that indicate that retinoic acid increases expression of CCR9.8,36 The expression of CCR9 on T cells has been studied in the context of supplementation or deficiency of vitamin A. In our study, no patient was deficient of vitamin A. Our observation could reflect differential changes in lymphocyte subset trafficking to the intestinal tract with small variations in vitamin A levels above or below the median.
Previous reports have shown that retinoic acid primes DCs to express CD103 and produce retinoic acid themselves, which induces CCR9 on T cells and amplifies transforming growth factor -β–mediated development of Foxp3+ Tregs.7 Cytokine context appears to be important in this report because the presence of TGF-β during retinoic acid DC-driven T-cell priming specifically favored the induction of Foxp3+ Tregs over IL-10+ Tregs. Moreover, experiments with naïve CD4+ T cells stimulated by anti-CD3 and anti-CD28 antibodies in the absence of DCs emphasized that retinoic acid specifically induces IL-10 in the face of inflammatory mediators, emphasizing the importance of both cytokine and cellular environments in modifying response to retinoic acid. The HSCT conditioning regimen causes significant damage to the GI mucosa, removing many of the intestinal epithelial cells and GI-associated lymphoid cells that express retinaldehyde dehydrogenase and convert vitamin A into active retinoic acid, perhaps modifying the responses reported in healthy mice and in in vitro human studies. In addition, responses to retinoic acid may be modified in the setting of HSCT by the intensely pro-inflammatory cytokine environment that occurs after the tissue damage associated with the pretransplant conditioning regimen. We are pursuing additional studies of changes in gene expression in the gut mucosa to try and better understand the effect of retinoic acid in the local cytokine and cellular environment of the gut immediately after HSCT.
Our study has both strengths and weaknesses. A weakness of our study is that it was performed in a largely pediatric transplant population, with the majority of patients receiving transplant for a nonmalignant disorder. Our results may not be generalizable to adult transplant populations, where the majority of transplants are performed to treat cancer. We were unable to measure intestinal permeability directly, as we would have wished to, because children tolerate high doses of oral sugars poorly immediately after transplant. Last, vitamin A plays a key role in intestinal immunity and tolerance, and, in this study, we did not directly address local retinoic acid activity in gut-associated lymphoid tissue and intestinal epithelial cells.
In summary, we found that patients with lower levels of vitamin A have an increased incidence of GI GVHD and TRM. Our results suggest that there may be a direct effect of lower levels of vitamin A causing increased intestinal permeability and increased CD8+ TEM homing to the intestine, although the relationship between permeability and GVHD may be complex. We are currently performing a pilot study of vitamin A supplementation37,38 to maintain levels in the 75th through 95th percentiles for age in the early days after transplant, before a randomized study to establish clinical benefits of vitamin A before HSCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.T.L. designed experiments, performed experiments, collected data, and wrote the manuscript. P.K. designed experiments, performed flow cytometry, was involved in clinical trial and patient management, and wrote the manuscript. C.E.D. provided infection data, was involved in clinical trial and patient management, and wrote the manuscript. S.J., M.S.G., G.W., C.T., and A.C.T.-C. were involved in clinical trial and patient management, and wrote the manuscript. A.L. performed statistical analysis and wrote the manuscript. K.E.L. designed experiments. S.M.D. designed experiments, was involved in clinical trial and patient management, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dana T. Lounder, Division of Bone Marrow Transplant and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnett Ave, MLC 7015, Cincinnati, OH 45229-3026; e-mail: dana.lounder@cchmc.org.