Key Points
FZR1 loss causes increased sensitivity of B-ALL cells to oncogene- or chemotherapy-induced DNA damage.
Prolonged loss of FZR1 contributes to the development of treatment-resistant clones in mouse and human B-ALL.
Abstract
FZR1 (fizzy-related protein homolog; also known as CDH1 [cell division cycle 20 related 1]) functions in the cell cycle as a specific activator of anaphase-promoting complex or cyclosome ubiquitin ligase, regulating late mitosis, G1 phase, and activation of the G2-M checkpoint. FZR1 has been implicated as both a tumor suppressor and oncoprotein, and its precise contribution to carcinogenesis remains unclear. Here, we examined the role of FZR1 in tumorigenesis and cancer therapy by analyzing tumor models and patient specimens. In an Fzr1 gene-trap mouse model of B-cell acute lymphoblastic leukemia (B-ALL), mice with Fzr1-deficient B-ALL survived longer than those with Fzr1-intact disease, and sensitivity of Fzr1-deficient B-ALL cells to DNA damage appeared increased. Consistently, conditional knockdown of FZR1 sensitized human B-ALL cell lines to DNA damage–induced cell death. Moreover, multivariate analyses of reverse-phase protein array of B-ALL specimens from newly diagnosed B-ALL patients determined that a low FZR1 protein expression level was an independent predictor of a longer remission duration. The clinical benefit of a low FZR1 expression level at diagnosis was no longer apparent in patients with relapsed B-ALL. Consistent with this result, secondary and tertiary mouse recipients of Fzr1-deficient B-ALL cells developed more progressive and radiation-resistant disease than those receiving Fzr1-intact B-ALL cells, indicating that prolonged inactivation of Fzr1 promotes the development of resistant clones. Our results suggest that reduction of FZR1 increases therapeutic sensitivity of B-ALL and that transient rather than tonic inhibition of FZR1 may be a therapeutic strategy.
Introduction
FZR1 (fizzy-related protein homolog; also known as CDH1 [cell division cycle 20 related 1]) specifically activates the anaphase-promoting complex or cyclosome (APC/C), a ubiquitin ligase that plays a key regulatory role between late mitotic and G1 phases of the cell cycle. During late mitosis, FZR1 replaces CDC20, which is the activator of APC/C during early mitosis. This switch results in the ubiquitination of various cell cycle modulators, including PLK1, SKP2, and GMNN.1-5 FZR1 also contributes to the regulation of postmitotic6 and quiescent (G0)7,8 states.
In addition to functioning in cell cycle regulation,9,10 evidence suggests that FZR1 plays an important role in the DNA damage response (DDR). We previously demonstrated FZR1 is essential for DNA damage–induced G2 arrest in chicken B-cell lymphoma (DT40) cells, with loss of FZR1 resulting in an aberrant G2 checkpoint and sensitization of the cells to radiation.11 The induction of DNA double-strand breaks (DSBs) by radiation was also shown to trigger the nucleoplasmic localization of the phosphatase CDC14B, resulting in the dephosphorylation and activation of FZR1, G2 arrest, and DNA repair.12 We previously generated a tissue-specific conditional Fzr1 gene-trap (GT) mouse13 to overcome the embryonic lethality of homozygous Fzr1 knockout10 or GT.14 Our analysis of these animals revealed that the radiation-induced G2-M checkpoint is defective in Fzr1-deficient nonmalignant bone marrow (BM) cells, resulting in increased probability of mitotic catastrophe during radiation-induced stress.13
The expression of FZR1 has been found to be reduced or lost in various human cancers,12,15 suggesting FZR1 may function as a tumor suppressor. Fzr1 heterozygous GT mice were also shown to be more likely to develop epithelial tumors than wild-type (WT) mice,10 indicating that Fzr1 is a haploinsufficient tumor suppressor. On the other hand, observations that loss of Fzr1 induces cell death in neural and hematopoietic progenitor cells6,9,11,13,16 suggest that FZR1 may be essential for malignant cells to survive. The precise role of FZR1 in tumorigenesis has remained ill defined.
To determine whether FZR1 promotes or inhibits tumorigenesis in vivo, we have combined tissue-specific conditional Fzr1 GT mice13 with a MYC-induced B-cell acute lymphoblastic leukemia (B-ALL) model17 and compared phenotypes of Fzr1-intact vs Fzr1-deficient B-ALL mice. We also analyzed FZR1 expression in primary B-ALL cells derived from 224 adult patients with the use of a reverse-phase protein array (RPPA). Our results suggest that loss of FZR1 initially renders B-ALL cells less robust because of increased sensitivity to DNA damage; however, over time, FZR1 loss leads to the generation of resistant clones that give rise to progressive disease. We propose that FZR1 expression predicts chemosensitivity of B-ALL cells and that transient inhibition of FZR1 is a potential therapeutic strategy for B-ALL.
Materials and methods
Mice
All mouse experiments were performed under approval of the Institutional Animal Care and Use Committee of Keio University School of Medicine and in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. C57BL/6 mice (7 to 8 weeks of age) were obtained from Sankyo Labo Service (Tokyo, Japan). Fzr1+/GT (C57BL/6 background), Fzr1GT[f-cDNA]/GT[f-cDNA] (Fzr1f/f), and Mx1-Cre transgenic mice were obtained from TransGenic (Kumamoto, Japan) as previously described.13,14,18 Supplemental Materials and methods (available on the Blood Web site) describes additional details.
Retrovirus infection and BM transplantation
Murine Mycn complementary DNA was cloned into the retroviral vector pMXs-IG as described previously.17 Mx1-Cre expression was induced by intraperitoneal injection of 7- to 8-week-old Mx1-Cre(+);Fzr1f/f mice (with Mx1-Cre(−);Fzr1f/f mice as controls) with polyinosinic-polycytidylic acid (pIpC; 25 µg per gram bodyweight) (Sigma-Aldrich, St. Louis, MO) on 5 successive days. Both groups of mice were selected from among the same littermates for each experiment. The mice were injected intraperitoneally with 150 mg/kg 5-fluorouracil (Kyowa Hakko Kirin, Tokyo, Japan) at 4 weeks after the final pIpC injection, and bone marrow mononuclear cells (BM-MNCs) were isolated 2 days later with the use of Lympholyte-M (Cedarlane, Uden, The Netherlands). The cells were cultured for 2 days in α–minimum essential medium (Invitrogen, San Diego, CA) supplemented with 20% fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin, 55 mM 2-mercaptethanol, 100 ng/mL recombinant mouse stem cell factor (Peprotech, London, UK), and 50 ng/mL recombinant human interleukin-6 (Peprotech) before infection with a retrovirus encoding Mycn and GFP as described previously.17 For generation of recombinant retrovirus, the corresponding retroviral vector was introduced into Platinum-E packaging cells19 by transfection with the use of the Fugene HD reagent (Promega, Madison, WI) and incubation of the cells overnight at 37°C under 5% CO2. The medium was then replaced, and cells were incubated for an additional 48 hours before collection of culture supernatants. The supernatants were filtered with the use of a 0.45-µm cellulose acetate filter (Iwaki, Tokyo, Japan) for isolation of the recombinant retrovirus. Retroviral infection of BM-MNCs was performed for 3 days in 35-mm dishes coated with RetroNectin (Takara Bio, Tokyo, Japan). The cells were then collected by exposure to cell dissociation buffer (Invitrogen) for transplantation into mice. Infected (green fluorescent protein [GFP]+) cells (5 × 104) were injected IV into WT C57BL/6 mice that had been subjected to sublethal irradiation (4 Gy). For secondary and tertiary transplantation, BM-MNCs were harvested from moribund mice, and GFP+ cells (5 × 104) were transferred to WT recipients as for the primary transplants. When moribund, 2 primary transplant mice were selected from each of the Fzr1-deficient group and the Fzr1-intact group for injection of BM-MNCs into secondary recipients. Survival times of these mice at primary transplantation were 94 and 96 days in Fzr1-deficient and 90 and 94 days in Fzr1-intact mice.
Gene expression profiling
Total RNA was extracted from sorted GFP+ BM-MNCs using Trizol reagent (Invitrogen) and RNeasy Mini kits (QIAGEN Inc., Hilden, Germany) from GFP+ BM-MNCs. Biotin-labeled aRNA probes were synthesized from total RNA and subjected to hybridization with the Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA). Raw intensity data were normalized and analyzed using R statistical programming language (version 2.12.1; http://www.r-project.org) and robust multiarray analysis.20 Differentially expressed genes were identified using the Linear Models for Microarray Analysis Data program (http://www.bioconductor.org/packages/release/bioc/html/limma.html).21 Gene set enrichment analysis (GSEA)22 software version 2.0.7 with Molecular Signatures Database gene sets (version 3.0) was used to analyze genes ranked according to the Fzr1-deficient/Fzr1-intact transcript ratio. Gene sets with P values less than 0.05 plus false-discovery rates less than 0.25 were considered significant. The data from our microarray studies have been submitted to the Gene Expression Omnibus (accession number GSE69863; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=ctqrmmswzrwdfot&acc=GSE69863).
RPPA
Patient specimens were acquired during diagnostic assessment and analyzed in accordance with regulations and protocols approved by the MD Anderson Investigational Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Detailed methods and validation of proteomic profiling were described previously.23 Peripheral blood (PB) and BM specimens were collected from 224 B-ALL patients (207 newly diagnosed and 17 relapsed) who were evaluated at The University of Texas MD Anderson Cancer Center. All samples remained on ice until processing (typically within 2 hours of collection). All protein preparations were made from fresh (not cryopreserve), cells on the day of collection. Clinical details, including responses and outcomes, were collected for 172 of the patients with newly diagnosed disease (supplemental Table 1). Additional normal specimens were collected as controls, including CD34+ BM cells (N = 16), CD133+ BM cells (N = 5), and PB-MNCs (N = 9). Antibodies against FZR1 (LifeSpan BioSciences, Seattle, WA) and MYC (Cell Signaling Technology) were validated and used for RPPA analysis as described previously.23 The level of FZR1 expression for all 224 B-ALL specimens as determined by RPPA analysis was compared with that of normal BM and PB specimens. RPPA data for the 172 clinically annotated specimens of newly diagnosed B-ALL were used for analysis of clinical outcomes based on levels of FZR1 and MYC. The threshold for high vs low expression was set as the maximum expression level in normal CD34+ BM cells.
Results
Overexpression of Mycn in Fzr1-intact or Fzr1-deficient BM cells confers the B-ALL phenotype
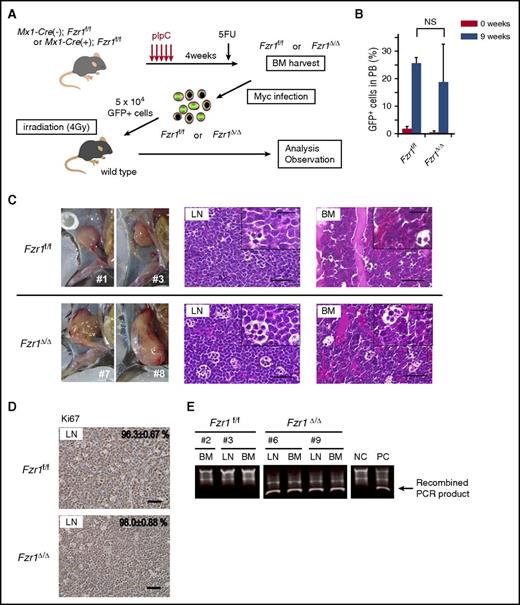
To determine the role of FZR1 in MYC-induced leukemogenesis, we examined the effect of Fzr1 loss on the development of B-ALL in mice. Fzr1-intact (Fzr1f/f) or Fzr1-deficient (Fzr1∆/∆) BM-MNCs of conditional Fzr1 GT mice were infected with a retrovirus harboring Mycn as well as an internal ribosome entry site and a GFP gene construct, and the infected cells were then injected into sublethally irradiated WT mice (Figure 1A). Diagnosis of B-ALL was determined by detection of GFP+/B220+/IgM−/CD3−/Gr1−/Mac1− cells in PB and BM or in lymph nodes (LNs) for animals with lymphadenopathy. Both groups of recipient mice manifested a similar B-ALL phenotype with similar incidence rates (70% in the Fzr1-intact group vs 80% in the Fzr1-deficient group; n = 20 mice for each group, P = .72). GFP+ cells were detected in PB 9 weeks after transplantation, and there was no significant difference in the percentage of such cells between the 2 groups (Figure 1B). Whereas all recipient mice manifested B-ALL in BM, 4 out of 7 mice in the Fzr1-intact group and 5 out of 7 mice in the Fzr1-deficient group had lymphadenopathy. There were no differences in leukemia cell morphology or immunophenotype (uniformly B220+/IgM−/CD3−/Gr1−/Mac1− uniform blast cells) between the recipients of Fzr1-intact or Fzr1-deficient cells (Figure 1C; supplemental Figure 1A). More than 95% of cells in all affected LNs were positive for Ki67 in both groups (Figure 1D), indicating that Fzr1 loss did not affect the proportion of proliferating cells.
Fzr1-intact and Fzr1-deficient B-ALL share similar pathological characteristics. (A) Experimental design for retrovirus-mediated introduction of Mycn into Fzr1-intact (Fzr1f/f) or Fzr1-deficient (Fzr1∆/∆) BM-MNCs and transplantation of the infected cells into WT recipient mice (see Materials and methods for experimental details). 5FU, 5-fluorouracil. (B) Flow cytometry–based determination of the proportion of GFP+ cells in the PB of recipient mice immediately and 9 weeks after transplantation of Mycn-overexpressing Fzr1-intact or Fzr1-deficient BM-MNCs. The data are presented as mean ± standard deviation (SD) (n = 3 mice per group). NS, not significant; P = .44. (C) Gross appearance of enlarged lymph nodes (left) as well as hematoxylin and eosin staining of affected LNs (middle) and BM (right) in mice given Fzr1-intact (top) or Fzr1-deficient (bottom) cells when they became moribund. Insets show higher-magnification views. Scale bars, 50 µm (low power) or 20 µm (high power). (D) Immunohistochemical staining of Ki67 in affected lymph nodes as in panel C. The percentages of Ki67+ cells are shown as mean ± SD determined for 3 mice of each group. Scale bars, 50 µm. (E) Representative PCR-based genotyping of sorted GFP+ cells from affected lymph nodes or BM of individual recipient mice. NC, negative control consisting of genomic DNA from BM cells of Mx1-Cre(−);Fzr1f/f mice; PC, positive control, consisting of genomic DNA from BM cells of Mx1-Cre(+);Fzr1f/f mice after pIpC injection.
Fzr1-intact and Fzr1-deficient B-ALL share similar pathological characteristics. (A) Experimental design for retrovirus-mediated introduction of Mycn into Fzr1-intact (Fzr1f/f) or Fzr1-deficient (Fzr1∆/∆) BM-MNCs and transplantation of the infected cells into WT recipient mice (see Materials and methods for experimental details). 5FU, 5-fluorouracil. (B) Flow cytometry–based determination of the proportion of GFP+ cells in the PB of recipient mice immediately and 9 weeks after transplantation of Mycn-overexpressing Fzr1-intact or Fzr1-deficient BM-MNCs. The data are presented as mean ± standard deviation (SD) (n = 3 mice per group). NS, not significant; P = .44. (C) Gross appearance of enlarged lymph nodes (left) as well as hematoxylin and eosin staining of affected LNs (middle) and BM (right) in mice given Fzr1-intact (top) or Fzr1-deficient (bottom) cells when they became moribund. Insets show higher-magnification views. Scale bars, 50 µm (low power) or 20 µm (high power). (D) Immunohistochemical staining of Ki67 in affected lymph nodes as in panel C. The percentages of Ki67+ cells are shown as mean ± SD determined for 3 mice of each group. Scale bars, 50 µm. (E) Representative PCR-based genotyping of sorted GFP+ cells from affected lymph nodes or BM of individual recipient mice. NC, negative control consisting of genomic DNA from BM cells of Mx1-Cre(−);Fzr1f/f mice; PC, positive control, consisting of genomic DNA from BM cells of Mx1-Cre(+);Fzr1f/f mice after pIpC injection.
To confirm the GT for Fzr1 in Fzr1-deficient B-ALL cells, we performed genomic polymerase chain reaction (PCR) analysis of sorted GFP+ cells from BM or lymph nodes of both recipient groups (Figure 1E; supplemental Figure 1B). All of the examined Fzr1-deficient lesions were positive for the smaller recombined PCR products and negative for the larger ones derived from the Fzr1 complementary DNA site, indicating that tumor cells indeed uniformly lack this site in the recipients of Fzr1-deficient BM-MNCs.
Increased survival time of mice with Fzr1-deficient B-ALL is associated with increased B-ALL cell death
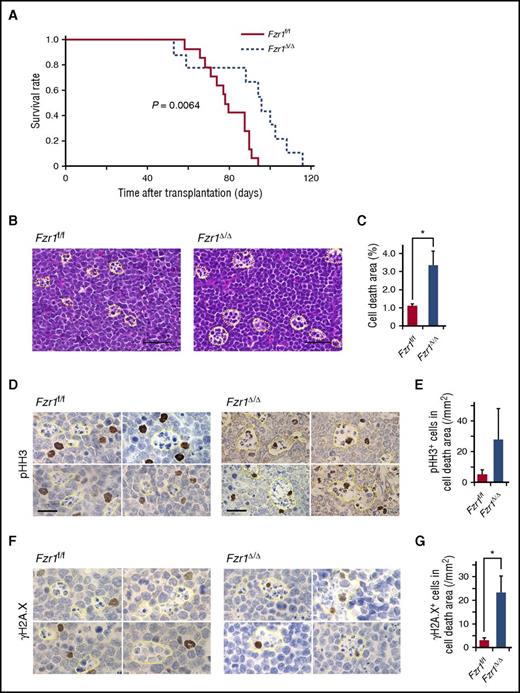
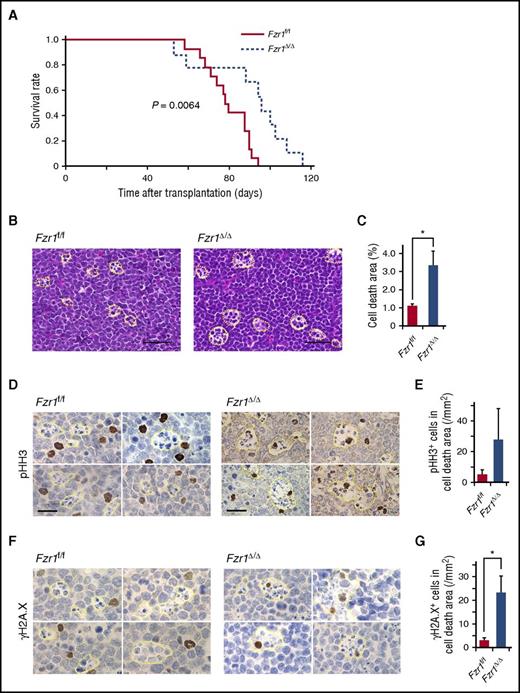
Despite the similar pathological phenotypes of the 2 groups of recipient mice, the median survival time of the Fzr1-deficient group was significantly longer than that of the Fzr1-intact group (95 vs 78 days) (Figure 2A). We obtained similar results with recipients of BM cells from Fzr1 heterozygous GT vs WT mice in the same B-ALL model (supplemental Figure 2A), demonstrating that longer survival of B-ALL mice is also achieved with Fzr1 haploinsufficiency.10 Given that the proportion of Ki67+ cells did not differ between the Fzr1-intact and Fzr1-deficient groups (Figure 1D), we speculated that reduced viability of Fzr1-deficient B-ALL cells might be responsible for the survival advantage of mice harboring these cells. Consistent with this, affected LNs in the Fzr1-deficient group manifested a more prominent “starry-sky” histopathology (indicating focal areas of cell death) than those in the Fzr1-intact group (Figure 2B-C). We confirmed that most cells in the starry-sky regions were apoptotic (supplemental Figure 2B). The starry-sky pattern is a result of high tumor proliferation rate and subsequent apoptosis. It clinically assessed on the basis of Ki67 positivity and indicates progressive lymphoid malignancy. However, given the similar levels of Ki67 positivity in our 2 experimental groups of recipient mice (Figure 1D), we concluded that the lesions in the Fzr1-deficient animals reflect increased susceptibility to cell death, in addition to a high proliferation rate.
Longer survival in mice with Fzr1-deficient B-ALL than in those with Fzr1-intact B-ALL is associated with increased B-ALL cell fragility induced by DNA DSBs. (A) Survival curves for mice with B-ALL originating from Fzr1-intact (n = 14 mice) or Fzr1-deficient (n = 9 mice) BM cells. The P value was determined with the log-rank test. (B) Areas of cell death (outlined with yellow dotted lines) in hematoxylin and eosin–stained sections of B-ALL–affected lymph nodes from moribund recipient mice. Scale bars, 50 µm. (C) Proportion of lymph nodes occupied by areas of cell death as determined for sections in panel B. The data are presented as the mean ± SD (n = 3 mice per group). *P = .049. (D and F) Immunohistochemical staining for pHH3 (D) and γH2A.X (F) in affected lymph nodes from mice with Fzr1-intact or Fzr1-deficient B-ALL. Scale bars, 20 µm. (E and G) Density of cells positive for pHH3 (E) or γH2A.X (G) in areas of cell death as determined for sections similar to those in panels D and F, respectively. The data are presented as mean ± SD (n = 3 mice per group). P = .191 for pHH3; *P = .0077 for γH2A.X.
Longer survival in mice with Fzr1-deficient B-ALL than in those with Fzr1-intact B-ALL is associated with increased B-ALL cell fragility induced by DNA DSBs. (A) Survival curves for mice with B-ALL originating from Fzr1-intact (n = 14 mice) or Fzr1-deficient (n = 9 mice) BM cells. The P value was determined with the log-rank test. (B) Areas of cell death (outlined with yellow dotted lines) in hematoxylin and eosin–stained sections of B-ALL–affected lymph nodes from moribund recipient mice. Scale bars, 50 µm. (C) Proportion of lymph nodes occupied by areas of cell death as determined for sections in panel B. The data are presented as the mean ± SD (n = 3 mice per group). *P = .049. (D and F) Immunohistochemical staining for pHH3 (D) and γH2A.X (F) in affected lymph nodes from mice with Fzr1-intact or Fzr1-deficient B-ALL. Scale bars, 20 µm. (E and G) Density of cells positive for pHH3 (E) or γH2A.X (G) in areas of cell death as determined for sections similar to those in panels D and F, respectively. The data are presented as mean ± SD (n = 3 mice per group). P = .191 for pHH3; *P = .0077 for γH2A.X.
Fzr1 loss increases mitotic cell death due to DNA damage in B-ALL cells in vivo
To determine why Fzr1-deficient B-ALL cells are more prone to cell death than Fzr1-intact cells, we performed gene expression profiling for GFP+ BM cells isolated from the 2 groups of recipients (n = 3 per group). GSEA revealed that 3 gene sets related to mitosis or the DNA damage response (DDR) were significantly enriched in the Fzr1-deficient cells (Table 1; supplemental Figure 3), suggesting greater activation of DDR at the G2-M checkpoint with Fzr1 deficiency. We therefore hypothesized that increased susceptibility to cell death in Fzr1-deficient B-ALL cells results from accumulation of DNA damage in G2-M phase and that apparent activation of the G2-M checkpoint gene signature reflects insufficient ability to overcome checkpoint dysfunction due to Fzr1 loss.
To examine the relationship between mitosis or DNA damage and cell death, we stained B-ALL–affected LNs obtained from mice in the Fzr1-deficient and Fzr1-intact groups for phosphorylated histone H3 (pHH3) and histone γH2A.X. The number of pHH3+ cells in the starry-sky lesions tended to be higher in the Fzr1-deficient group than in the Fzr1-intact group (Figure 2D-E), suggesting that Fzr1 loss might increase the rate of mitotic cell death. Similarly, the number of γH2A.X+ cells in the areas of cell death was significantly higher in the Fzr1-deficient group (Figure 2F-G), indicating that loss of Fzr1 impairs the ability of B-ALL cells to overcome cell death signaling triggered by DNA DSBs. These results thus suggested that a normal G2-M checkpoint via intact Fzr1 protects B-ALL cells from DNA DSBs by repairing DNA damage properly in vivo.
Conditional knockdown of FZR1 in human B-ALL cell lines has antileukemia effects and sensitizes cells to DNA damage–induced apoptosis and mitototic catastrophe
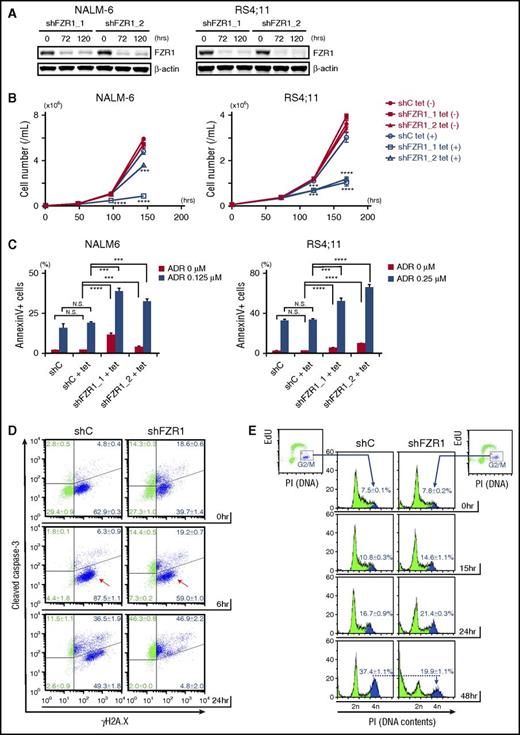
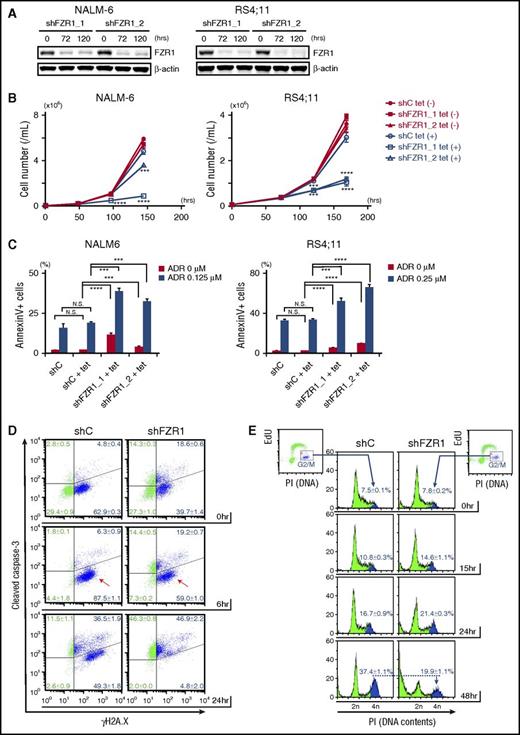
To determine the role of FZR1 in leukemia progression and in DNA damage–induced cell death, we generated human B-ALL cell lines with inducible knockdown of FZR1 using a tetracycline-inducible short hairpin RNA (shRNA) system. Among 7 B-ALL cell lines, we chose NALM-6, RS4;11, and Tanoue cells, which had the highest expression of both FZR1 and MYC proteins (supplemental Figure 4A). We transfected them with lentivirus carrying tetracycline-inducible shRNAs targeting FZR1 (shFZR1) or scramble control. Decreased FZR1 (75% to 90% reduction in protein expression) was confirmed by immunoblotting analysis (Figure 3A; supplemental Figure 4B). FZR1 knockdown showed antiproliferative effects with minimally induced apoptosis (Figure 3B-C; supplemental Figure 4C). This was consistent with our findings in vivo that mice bearing Fzr1-deficient B-ALL, and no additional perturbations, had slower progression and increased apoptosis as evidenced by enlarged starry-sky LN patterns (Figure 2A-B). Next, NALM-6 and RS4;11 cells with FZR1 knockdown were treated with doxorubicin, a DNA damage inducer and a key drug in chemotherapy for B-ALL patients. We found that FZR1 knockdown sensitized cells to doxorubicin-induced apoptosis (Figure 3C). We costained γH2A.X and cleaved caspase-3 and found that γH2A.X became positive in >90% of cells in both FZR1 knockdown and FZR1-intact NALM-6 cells by 6 hours of doxorubicin treatment without a concomitant increase in cleaved caspase-3 (Figure 3D). Of note, γH2A.X was positive in 50% to 60% of NALM6 cells before doxorubicin treatment, consistent with the fact that cells in S phase are detected as positive for γH2A.X from DNA DSBs during DNA replication.24 Cleaved caspase-3 was increased later by 24 hours of doxorubicin treatment (Figure 3D), demonstrating that the increased positivity of γH2A.X by 6 hours was not due to DNA fragments induced by an apoptotic process. The results demonstrated that FZR1 knockdown did not affect the extent of doxorubicin-induced DNA damage but instead increased cell susceptibility to DNA damage–induced apoptosis. We further analyzed 5-ethynyl-2′-deoxyuridine incorporation combined with DNA contents analysis by propidium iodide staining in NALM-6 cells with FZR1 knockdown. Accumulation of cells in G2-M phase, as normally induced by doxorubicin-induced DNA damage, was significantly reduced by FZR1 knockdown (the percentage of cells in G2-M phase was 19.9% ± 1.1% in FZR1 knockdown vs 37.4% ± 1.1% in FZR1 intact cells; P < .0001) with increased cells noted in sub-G1 phase (Figure 3E; supplemental Figure 4D). This strongly suggested that mitotic catastrophe is involved in increased apoptosis, possibly due to G2-M checkpoint dysfunction caused by FZR1 knockdown. Reduction of cells in G1 phase was also observed, suggesting that FZR1 depletion may also promote DNA damage-induced apoptosis in G1 phase.
FZR1 knockdown sensitizes human B-ALL cells to DNA damage–induced cell death and mitotic catastrophe. (A) Immunoblot of FZR1 in NALM-6 and RS4;11 cells transduced with lentivirus expressing tetracycline-inducible control (C) shRNA or shRNA specific to FZR1 (shFZR1_1 and shFZR1_2) after 72-hour and 120-hour treatment with 1 μg/mL tetracycline. (B) Time-course cell proliferation assay of NALM-6 and RS4;11 cells with tetracycline-induced shC or shFZR1 treated with tetracycline (0.25 μg/mL for NALM-6 cells and 0.10 μg/mL for RS4;11). Trypan blue–negative cells were counted at described time points. P values were calculated by comparing with untreated controls. (C) Apoptosis analysis of NALM-6 and RS4;11 cells with tetracycline-induced shC or shFZR1 after treatment with tetracycline and doxorubicin. Cells were treated with tetracycline (0.125 μg/mL for NALM-6 and 0.10 μg/mL for RS4;11) for 5 days and then treated with doxorubicin (0.125 μM for NALM-6 and 0.25 μM for RS4;11) for 36 hours. Annexin V–positive cells were counted by flow cytometry. (D) Costaining of γH2A.X and cleaved caspase-3 in NALM-6 cells with tetracycline-inducible shC or shFZR1 (shFZR1_1). Cells were treated with 0.125 μg/mL tetracycline for 5 days and then treated with 0.5 μM doxorubicin. (E) Flow cytometric analysis of 5-ethynyl-2′-deoxyuridine (EdU) incorporation combined with DNA content analysis with propidium iodide (PI) staining in NALM-6 cells with tetracycline-inducible shC or shFZR1 (shFZR1_1). Cells were treated with 0.125 μg/mL tetracycline for 5 days and then treated with 0.125 μM doxorubicin. Graphs in panels D and E are representative examples from 3 experiments. Except for panel A, results are expressed as mean ± SD (n = 3). *P < .05, ** P < .01, ***P < .001, ****P < .0001.
FZR1 knockdown sensitizes human B-ALL cells to DNA damage–induced cell death and mitotic catastrophe. (A) Immunoblot of FZR1 in NALM-6 and RS4;11 cells transduced with lentivirus expressing tetracycline-inducible control (C) shRNA or shRNA specific to FZR1 (shFZR1_1 and shFZR1_2) after 72-hour and 120-hour treatment with 1 μg/mL tetracycline. (B) Time-course cell proliferation assay of NALM-6 and RS4;11 cells with tetracycline-induced shC or shFZR1 treated with tetracycline (0.25 μg/mL for NALM-6 cells and 0.10 μg/mL for RS4;11). Trypan blue–negative cells were counted at described time points. P values were calculated by comparing with untreated controls. (C) Apoptosis analysis of NALM-6 and RS4;11 cells with tetracycline-induced shC or shFZR1 after treatment with tetracycline and doxorubicin. Cells were treated with tetracycline (0.125 μg/mL for NALM-6 and 0.10 μg/mL for RS4;11) for 5 days and then treated with doxorubicin (0.125 μM for NALM-6 and 0.25 μM for RS4;11) for 36 hours. Annexin V–positive cells were counted by flow cytometry. (D) Costaining of γH2A.X and cleaved caspase-3 in NALM-6 cells with tetracycline-inducible shC or shFZR1 (shFZR1_1). Cells were treated with 0.125 μg/mL tetracycline for 5 days and then treated with 0.5 μM doxorubicin. (E) Flow cytometric analysis of 5-ethynyl-2′-deoxyuridine (EdU) incorporation combined with DNA content analysis with propidium iodide (PI) staining in NALM-6 cells with tetracycline-inducible shC or shFZR1 (shFZR1_1). Cells were treated with 0.125 μg/mL tetracycline for 5 days and then treated with 0.125 μM doxorubicin. Graphs in panels D and E are representative examples from 3 experiments. Except for panel A, results are expressed as mean ± SD (n = 3). *P < .05, ** P < .01, ***P < .001, ****P < .0001.
Low FZR1 expression is associated with a better clinical outcome in newly diagnosed, but not relapsed, B-ALL patients
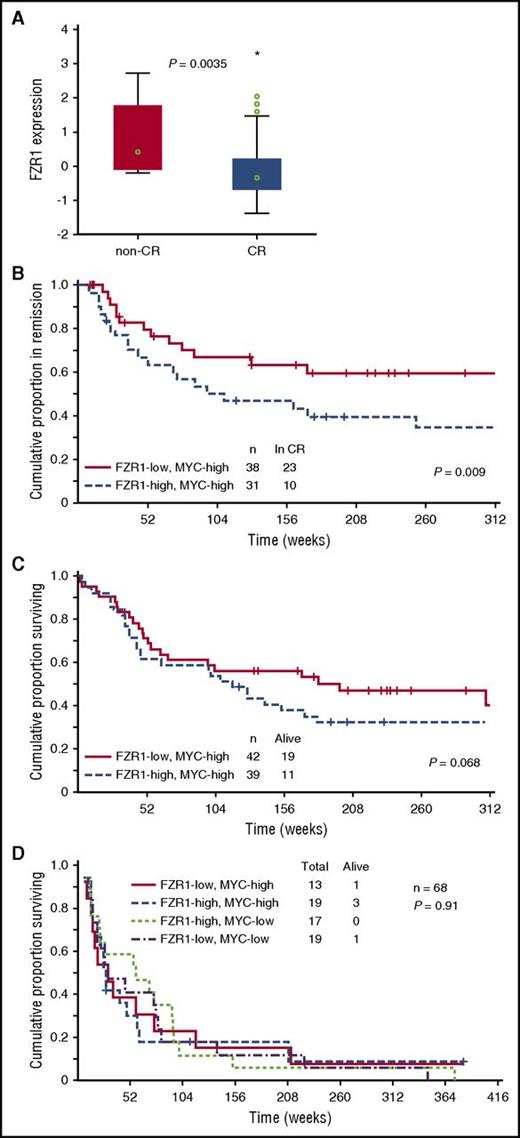
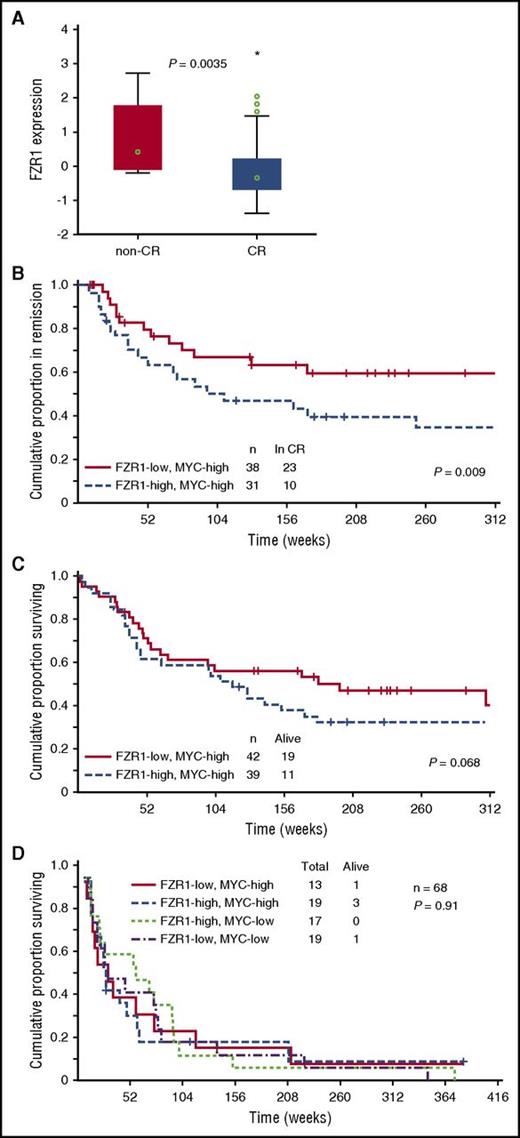
To examine the clinical relevance of FZR1 expression in human B-ALL, we determined FZR1 abundance at the protein level with RPPA analysis of B-ALL cells derived from 224 patients. The level of FZR1 expression in specimens of newly diagnosed B-ALL was significantly higher than that in normal CD34+ BM cells (P = 6.02 × e-9; supplemental Figure 5), ranging from the same level as that in normal CD34+ cells (48.3%) to levels that were statistically significantly higher than the normal CD34+ controls (51.7%). Given that B-ALL in our mouse model is induced by MYC overexpression, among the 172 clinically annotated specimens, we focused on 81 expressing high levels of MYC by RPPA relative to normal CD34+ cells. The expression level of FZR1 was significantly lower in B-ALL from patients who achieved complete remission compared with those who did not achieve remission (Figure 4A), consistent with the increased susceptibility to cell death of murine Fzr1-deficient B-ALL cells. We next divided these 81 patients into 2 groups based on high vs low FZR1 expression. Remission duration was significantly increased for patients with a low level of FZR1 expression (Figure 4B). A proportional hazard (Cox) regression model was used to identify factors that are independent predictors of remission duration. Starting with 8 factors that were determined to be significant predictors in univariate analysis, a stepwise analysis was performed until only significant (P < .05) variables remained. The final model contained 5 variables: percent BM blasts, white blood cell count, CD10 positivity, IKZF1 deletion, and FZR1 protein expression (Table 2). In addition, rate of relapse in patients with low FZR1 expression was significantly decreased compared with those with high FZR1 expression (39.5% vs 67.7%, P = .019). Despite the longer remission duration and lower relapse rate of patients with a low level of FZR1 expression, no significant difference in overall survival was apparent; however, the former patients showed a tendency toward longer survival (Figure 4C). We next selected patients who relapsed after complete remission from among the 172 clinically annotated patients with newly diagnosed B-ALL (n = 68). The survival time after relapse for these patients was short irrespective of FZR1 and MYC expression at initial diagnosis (Figure 4D), indicating that the clinical benefit associated with lower FZR1 expression may be transient and no longer apparent after relapse.
Relation of clinical characteristics to FZR1 and MYC expression levels in 172 B-ALL patients with clinically annotated specimens. (A) Comparison of FZR1 expression levels in B-ALL specimens obtained from patients with newly diagnosed disease who had high levels of MYC expression and either complete remission (CR; n = 69) or no remission (n = 12). The relation between FZR1 expression level and clinical response was evaluated with the Kruskal-Wallis test. (B) Remission duration stratified according to FZR1 expression level in the highly MYC-expressing (MYC-high) B-ALL specimens for the 69 patients in panel A who had complete remission. (C) Overall survival stratified according to FZR1 expression level in the MYC-high B-ALL specimens for the 81 patients in panel A. (D) Survival curves after relapse of B-ALL (68 of the 172 patients) stratified according to the levels of FZR1 and MYC expression in B-ALL specimens obtained at diagnosis.
Relation of clinical characteristics to FZR1 and MYC expression levels in 172 B-ALL patients with clinically annotated specimens. (A) Comparison of FZR1 expression levels in B-ALL specimens obtained from patients with newly diagnosed disease who had high levels of MYC expression and either complete remission (CR; n = 69) or no remission (n = 12). The relation between FZR1 expression level and clinical response was evaluated with the Kruskal-Wallis test. (B) Remission duration stratified according to FZR1 expression level in the highly MYC-expressing (MYC-high) B-ALL specimens for the 69 patients in panel A who had complete remission. (C) Overall survival stratified according to FZR1 expression level in the MYC-high B-ALL specimens for the 81 patients in panel A. (D) Survival curves after relapse of B-ALL (68 of the 172 patients) stratified according to the levels of FZR1 and MYC expression in B-ALL specimens obtained at diagnosis.
Serial transplantation of Fzr1-deficient B-ALL cells gives rise to progressive and resistant disease
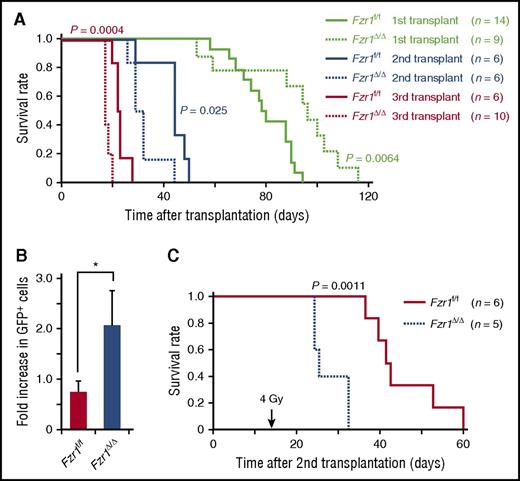
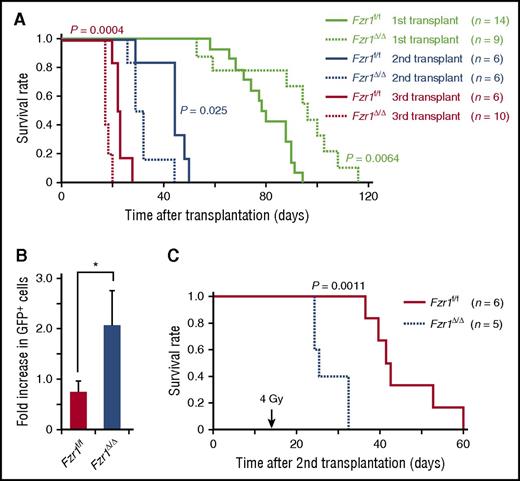
To determine long-term in vivo outcomes of Fzr1 deficiency in B-ALL, we performed secondary and tertiary transplantation of Fzr1-intact and Fzr1-deficient B-ALL cells in mice. Remarkably, the survival of comparison groups was the opposite of that predicted based on primary transplantation, where the Fzr1-deficient group had significantly shorter survival time than the Fzr1-intact group for both secondary and tertiary transplantation (Figure 5A). This finding suggested that increased susceptibility to cell death, thought to account for increased survival of mice undergoing primary transplant with Fzr1-deficient B-ALL cells, was less a factor in secondary or tertiary transplants. To assess the resistance of B-ALL cells to DNA damage, we irradiated mice 14 days after secondary transplantation of Fzr1-intact or Fzr1-deficient B-ALL cells. The increase in the number of GFP+ cells in PB from the day of irradiation to 3 days afterward was significantly higher in the Fzr1-deficient group (Figure 5B). In addition, the survival time was significantly shorter in the irradiated Fzr1-deficient group (Figure 5C). Together, these results indicated that Fzr1-deficient B-ALL cells became more resistant to irradiation stress after serial transplantation.
Serial transplantation of Fzr1-deficient B-ALL cells generates more progressive and resistant disease. (A) Survival curves for the indicated numbers of mice receiving primary, secondary, or tertiary transplants of Fzr1-intact or Fzr1-deficient B-ALL cells. P values for comparisons between the 2 types of B-ALL cells for each transplantation were determined with the log-rank test. The data for the primary transplant recipients are the same as those in Figure 2A. (B) Increase in the percentage of GFP+ cells in PB between the day of and 3 days after irradiation for secondary transplant recipients that were irradiated 14 days after transplantation. Data are presented as mean ± SD (n = 3 mice per group); *P = .0043. (C) Survival curves for the secondary transplant recipient mice as in (B). The P value for a comparison between the 2 groups was determined with the log-rank test (n = 6 mice for Fzr1-intact and 5 mice for Fzr1-deficient group).
Serial transplantation of Fzr1-deficient B-ALL cells generates more progressive and resistant disease. (A) Survival curves for the indicated numbers of mice receiving primary, secondary, or tertiary transplants of Fzr1-intact or Fzr1-deficient B-ALL cells. P values for comparisons between the 2 types of B-ALL cells for each transplantation were determined with the log-rank test. The data for the primary transplant recipients are the same as those in Figure 2A. (B) Increase in the percentage of GFP+ cells in PB between the day of and 3 days after irradiation for secondary transplant recipients that were irradiated 14 days after transplantation. Data are presented as mean ± SD (n = 3 mice per group); *P = .0043. (C) Survival curves for the secondary transplant recipient mice as in (B). The P value for a comparison between the 2 groups was determined with the log-rank test (n = 6 mice for Fzr1-intact and 5 mice for Fzr1-deficient group).
Discussion
We have shown that loss of Fzr1 results in two opposite, sequential effects on the survival of B-ALL cells in a mouse model. Fzr1 deficiency initially confers sensitivity to genotoxic stress, however it also promotes expansion of increased robustness. The biphasic nature of Fzr1 deficiency on leukemia cells over time is critical knowledge when considering therapies targeting FZR1 or affecting DDR.
Given that the antitumor effects on murine and human B-ALL cells were induced by Fzr1 loss in the absence of any external perturbations (such as chemotherapy and irradiation), our findings support the notion that FZR1 may be a candidate therapeutic target in B-ALL in context and that specific molecularly targeted therapy alone may be effective. Similarly, a therapeutic strategy for cancers based on targeting mitotic exit through inhibition of APC/C has been supported by several recent studies.16,25,26 Furthermore, increased sensitivity to DNA damage–induced apoptosis by FZR1 knockdown provides a rationale for targeting FZR1 in combination with standard genotoxic chemotherapy. Indeed, adult patients with a low level of FZR1 expression in B-ALL cells showed longer remission times and a lower risk of relapse than those exhibiting a high level of FZR1 expression. These results may be explained by the depth of complete remission as measured by the presence and quantity of minimal residual disease, which is an important predictor of remission duration and relapse for both childhood and adult ALL.27-29 A low level of FZR1 expression in B-ALL cells may result in very deep complete remission and a lower relapse risk as a consequence of increased chemosensitivity of the cells.
Clonal heterogeneity and genetic variegation in tumorigenesis must be carefully taken into consideration in our mouse models, based on previous reports.30-32 Even if leukemogenesis starts with clonal evolution from a preleukemic cell, secondary genetic changes can generate multiple subclones within a developed disease. Also, in our B-ALL mouse model, there was a latency period of several weeks (presumably a preleukemic stage) from primary transplantation to leukemia development, and it is likely that the secondary mutations and other genetic alterations were accumulated during this period. Therefore, we may not be able to assume that Fzr1-deficient and Fzr1-intact groups shared the exact same genetic subclones. Acknowledging this possible complexity in genetic heterogeneity, it is still reasonably possible that the overall difference in survival between 2 primary transplant groups was the direct result of the difference in Fzr1 expression levels. This is because secondary genetic changes should have occurred stochastically in individual mice and provided the same extent of polyclonal conditions, and because we randomly selected 2 donor mice from individual groups at each transplantation to reduce the possibility of batch effects on genetic events. However, we cannot exclude the possibility that the difference in survival at primary transplant groups was actually due to the difference in secondary genetic changes that were unrelated to Fzr1 deficiency. Additionally, the transition over time from a vulnerable state (primary transplant) to a robust state (secondary and tertiary transplant) of Fzr1-deficient B-ALL cells may be explained by a difference in secondary changes in the mechanistic balance between apoptosis signaling and cell survival. The cell survival benefit may well be conferred by concomitant genetic instability directly caused by DDR dysfunction due to Fzr1 loss. More specifically, Fzr1 deficiency promotes aberrant G2-M checkpoint signaling that, over the long-term, allows accumulation of gene mutations that result in clonal selection of cells resistant to DNA damage. Mutation analyses using next-generation sequencing and other clonality assays will be useful for further assessments on secondary genetic changes and clonal heterogeneity.
Robustness of tumor cells is defined as the ability to sustain cell survival even in the face of perturbations such as chemotherapy, irradiation, or cell stress as experienced in hypoxia or oxidative states. In addition to the need for comprehensive understanding of the mechanisms underlying robustness, identification of its vulnerabilities presents an opportunity for the development of new therapeutic approaches.33 In the present study, we demonstrated the proof of concept that targeting of FZR1 as a key regulator of the DDR has the potential to undermine cancer cell robustness. However, persistent loss of FZR1 (and perhaps that of DDR regulators in general) ultimately confers a secondary state of robustness. While induction of synthetic lethality by targeting DDR remains a promising strategy for cancer therapy,34-36 the transient vs tonic nature of inhibiting DDR regulatory molecules must now be carefully considered.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank K. Yamamura and K. Araki (Kumamoto University, Japan) for kindly providing Fzr1 transgenic mice; M. Nakanishi, K. Nagata, S. Suzuki, I. Ishimatsu, Y. Ito, and S. Hayashi (Keio University) for technical assistance; O. Nagano, T. Shimizu, K. Kai, N. Onishi, and M. Tamada (Keio University) for technical advice; and K. Arai (Keio University, Japan) and D. Norwood (MD Anderson Cancer Center) for help with manuscript preparation.
This study was supported in part by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad Award (J.I.); the National Institutes of Health, National Cancer Institute (grants CA49639, CA100632, CA136411, and CA16672) and the Paul and Mary Haas Chair in Genetics (M.A.); the Leukemia & Lymphoma Society (grant 6089) (S.M.K.); and a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.S.). This study was also supported in part by research funding from Daiichi Sankyo Inc. and Eisai Co. Ltd. (H.S.).
Authorship
Contribution: J.I., E.S., S.K., and H.S conceived and designed the study; J.I., E.S., D.C., R.O.J., R.Z., Y.Q., and S.M.K. acquired data; J.I., E.S., K.M., K.K., C.B.B., S.Y.Y., S.M.K., and H.S. analyzed and interpreted data; J.I., E.S., C.B.B., S.M.K., and H.S. wrote, reviewed, or revised the manuscript; and N.H., S.O., and M.A. provided administrative, technical, or material support.
Conflict-of-interest disclosure: H.S. received research grants from Daiichi Sankyo Inc. and Eisai Co. Ltd. The remaining authors declare no competing financial interests.
Correspondence: Hideyuki Saya, Division of Gene Regulation, Institute for Advanced Medical Research, Keio University School of Medicine, Tokyo 160-8582, Japan; e-mail: hsaya@a5.keio.jp; and Steven M. Kornblau, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX 77030; e-mail: skornblau@mdanderson.org.