Abstract
Increased understanding of pediatric acute lymphoblastic leukemia (ALL) pathobiology has led to dramatic improvements in patient survival. However, there is still a need to develop targeted therapies to enable reduced chemotherapy intensity and to treat relapsed patients. The interleukin-7 receptor α (IL-7Rα) signaling pathways are prime therapeutic targets because these pathways harbor genetic aberrations in both T-cell ALL and B-cell precursor ALL. Therapeutic targeting of the IL-7Rα signaling pathways may lead to improved outcomes in a subset of patients.
Introduction
Treatment of pediatric acute lymphoblastic leukemia (ALL) is a major success of modern medicine. Fifty years ago, children diagnosed with ALL survived for a median of 2 months.1 Today, the 5-year overall survival rate is >90% in some series.2 However, survival rates are much lower in children who experience relapse, varying between 21% and 39%.3,4 In addition, although this overall improvement in outcome is staggering, it comes at a cost. To achieve remission, patients typically undergo 2 to 3 years of chemotherapy.1 Acute side effects include infections, allergic reactions, thrombosis, pancreatitis, and neurologic impairment. Chronic side effects include osteonecrosis, obesity, secondary malignancies, and neurocognitive deficits.1
As we characterize the pathophysiology of ALL, we must use our improved understanding to develop targeted therapies.5 Targeting molecular lesions in specific patients may enable reduced-intensity chemotherapy and may help to rescue children whom current protocols fail to cure. Although there are many potential targets for therapy, this spotlight will focus on genetic aberrations involving interleukin-7 receptor α (IL-7Rα) signaling pathways.
In health, the IL-7Rα chain forms 2 heterodimeric cytokine receptors (reviewed by Tal et al6 ). The receptor for IL-7 is formed by IL-7Rα pairing with γc, and the receptor for thymic stromal lymphopoietin (TSLP) is formed by IL-7Rα pairing with TSLP receptor (TSLPR) (encoded by the CRLF2 gene).6 IL-7 signaling is vital for normal T-cell development and survival of most mature T cells (reviewed by Mazzuchelli and Durum7 ). Conversely, TSLP signaling is involved in stimulation of growth and differentiation of B1 B-cell progenitors.8 In addition, TSLP is involved with CD4+ T-cell homeostasis, regulatory T-cell development, and dendritic cell activation (reviewed by Liu et al9 ). Finally, TSLP plays a prominent role in the pathogenesis of allergy and asthma (reviewed by Ziegler and Artis10 ). Binding of IL-7 or TSLP to their respective receptors causes activation of the JAK/STAT and PI3K/AKT/mTOR intracellular signaling cascades.6 The MAPK cascade could also be activated with normal TSLP signaling and with IL-7 signaling in T-cell ALL (T-ALL) and immature B and T cells.11-13 However, MAPK signaling is not required for the prosurvival and proliferation effects of IL-7 in T-ALL cells, and it does not seem to play a role in IL-7 signaling in normal T cells.12,14 Because the JAK/STAT and PI3K/AKT/mTOR intracellular signaling cascades seem to be the major players in IL-7Rα signaling, we will focus on them for the remainder of the review.
IL-7Rα signaling pathway perturbations in T-ALL
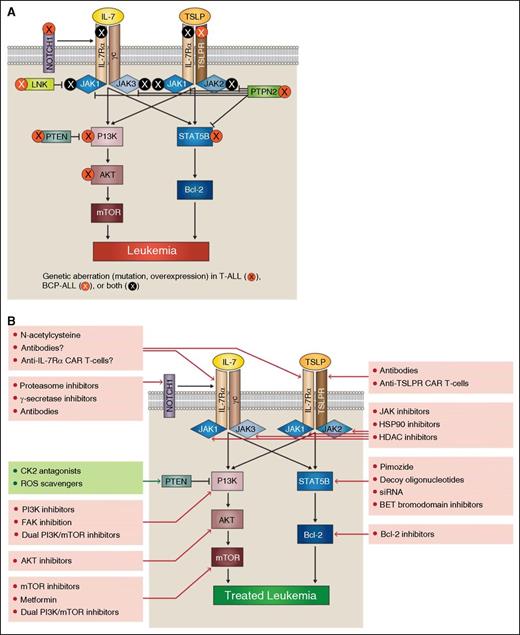
As might be expected for a receptor that is vital to T-cell development, mutations in IL-7Rα have recently been identified in a subset of pediatric T-ALL cases.15-17 These mutations generally occur in exon 6 of the gene and lead to insertion of multiple amino acids, usually including cysteine. The insertions induce IL-7Rα homodimerization and constitutively activate JAK1 in the absence of IL-7, γc, or JAK3.16 IL-7Rα mutations seem to be more prevalent in HOXA, TLX3, and early T-cell precursor (ETP-ALL) patient subgroups.16,17 The mutation does not seem to have prognostic implications.16
Although mutations in IL-7Rα occur in ∼10% of pediatric patients, mutations in an upstream regulator of IL-7Rα, NOTCH1, occur in >50% of T-ALL cases.18 NOTCH1 is the most commonly mutated gene in T-ALL and is known to regulate IL-7Rα transcription and expression. Increases in IL-7R signaling promoted by NOTCH1 mutations may play a role in NOTCH1 oncogenicity.19
Mutations downstream of IL-7R also occur in T-ALL. The JAK/STAT pathway can be activated by several genetic aberrations. Activating mutations in JAK1 and JAK3 have been reported, and a TEL-JAK2 fusion has also been described.17,20-26 Notably, JAK2 mutations generally seem to be more associated with B-cell precursor ALL (BCP-ALL) than T-ALL. Further downstream, T-ALL patients have also had mutations in STAT5B.27,28 In addition, a negative regulator of JAK-STAT signaling, PTPN2, is deleted in some cases of T-ALL.29
Activation of the PI3K/AKT/mTOR pathway also occurs in T-ALL secondary to genetic aberrations, and an estimated 47.7% of pediatric cases have deletion or mutation of PTEN, PI3K, or AKT.30,31 The PI3K/AKT/mTOR-negative regulator PTEN is mutated in an estimated 8.7% to 22% of T-ALL cases or deleted in 27.3%.30,31 PTEN activity can also be decreased by posttranslational effects such as casein kinase 2 (CK2) overexpression, high levels of reactive oxygen species (ROS), or miRNAs.32,33
Furthermore, T-ALL cells from patients proliferate in response to IL-7 signaling, suggesting that targeting this pathway may be useful for many T-ALL patients, not only those with IL-7Rα genetic aberrations.34
IL-7Rα signaling pathway perturbations in BCP-ALL
In BCP-ALL patients, IL-7Rα pathway perturbations are most commonly caused by genetic aberrations affecting the CRLF2 gene, though mutations in IL-7Rα also occur. In most cases, chromosomal translocations, rearrangements, or gene duplications caused overexpression of CRLF2. Translocation leads to IGH-CRLF2 fusion, whereas interstitial deletion causes P2RY8-CRLF2 fusion.35-37 P2RY8-CRLF2 fusions have been found at a much higher rate (53%) in patients with Down syndrome–associated ALL (DS-ALL).35 Other chromosomal abnormalities can also lead to CRLF2 deregulation.36,37 It appears that rearranged TSLPRs signal conventionally through JAK/STAT and PI3K/AKT/mTOR pathways.38 Less commonly, the CRLF2 gene can have a F232C mutation that enables constitutive activation of TSLPR in the absence of IL-7Rα.39,40 Mutant TSLPR may not signal through the same kinases as wild-type TSLPR, particularly with regards to MAPK, which is downregulated in response to mutant TSLPR/mutant JAK2 signaling.11
Notably, CRLF2 overexpression is not always attributable to structural rearrangement involving the CRLF2 gene.41,42 In all, ∼15% of patients with BCP-ALL have CRLF2 overexpression (excluding those with MLL, TCF3, TEL, and BCR/ABL rearrangements).37,40,43 Genetic aberrations of CRLF2 seem to confer a poorer prognosis in most studies.40,41,44,45 Fusion type may be associated with prognosis.45 However, not all studies support a poorer prognosis associated with CRLF2 aberration.37,46
Patients with CRLF2 aberrations often have additional genetic abnormalities including deletion/mutation of IKZF1 (encoding IKAROS) and mutation or translocation of JAK2. Of note, CRLF2 aberrations can also be paired with concurrent mutations in IL-7Rα as well as JAK1, JAK3, or SH2B3 (which encodes the JAK2-negative regulator LNK).40,41,43 Patients with combined CRLF2 and IKZF1 aberrations often have gene expression profiles similar to patients with the BCR-ABL fusion protein, leading them to be considered “Philadelphia chromosome–like” (along with other genetic lesions inducing similar gene expression profiles). Patients with a Ph-like gene expression profile have a poor prognosis.40,47 Mutation or translocation of JAK2 also occurs independently of CRLF2 mutations in BCP-ALL.47-49
Therapeutic targeting of IL7Rα signaling pathways
Receptor targeting
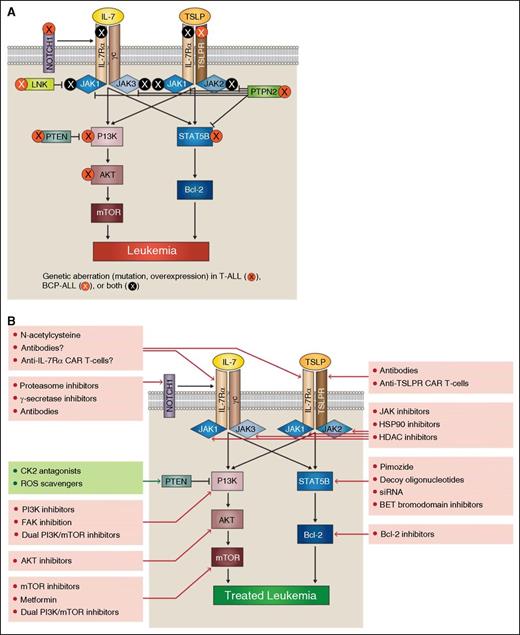
The mutant IL-7Rα homodimers found in T-ALL depend on formation of disulfide bonds for stability. These bonds could potentially be targeted therapeutically using the reducing agent N-acetylcysteine (NAC). In T-ALL cell lines, NAC disrupts homodimerization, and it slows progression of disease in a xenograft model. Although NAC is inexpensive, it requires frequent dosing.50 In addition to using NAC, development of antibodies or T cells engineered to express chimeric antigen receptors (CAR-T-cells) against IL-7Rα and/ or against mutant homodimers would be potentially therapeutic.6
Although antibodies and CAR-T-cells against normal IL-7Rα might be expected to target normal T cells as well as leukemic cells, promising results have been shown with anti-CD19 CAR-T-cells in the treatment of B-ALL. An anti–IL-7Rα CAR-T-cell may have similar effects. Anti-CD19 CAR-T-cell therapy induces severe, temporary B-cell lymphopenia, but B-cell populations rebound after therapy. Similar rebound of T-cell populations might be expected after anti–IL-7Rα CAR-T-cell therapy, given the active thymic function in these patients. In anti-CD19 CAR-T-cell therapy, treatment induces minimal residual disease-negative complete response in 60% of relapsed and refractory B-ALL patients. Therapy serves as an effective bridge to hematopoietic stem cell transplantation, resulting in disease-free survival.51 Perhaps anti–IL-7Rα CAR-T-cells or antibodies could also be used as a “bridge to transplant.” In addition, development of a mutant-specific antibody or CAR-T-cell may reduce potential off-target effects on normal T cells.
NOTCH1 targeting
JAK/STAT targeting
Efforts investigating JAK inhibitors to treat neoplasia have focused mainly on the effects of blocking JAK2 in myeloproliferative neoplasms (MPN), polycythemia vera, and myelofibrosis (reviewed by Santos and Verstovsek57 ). In ALL, most studies of JAK inhibition have explored its effects on BCP-ALL. These studies have used primary patient samples or cell lines, including CRLF2-overexpressing cells and Ph-like cells with IL7Rα-activating mutation and SH2B3 mutation. Results from these in vitro and in vivo experiments suggest JAK inhibition may have some efficacy against BCP-ALL.38,40,43,48,58,59 A recent phase 1 clinical trial of the JAK1/2 inhibitor ruxolitinib suggested that the drug is well tolerated in children.60 Fewer experimental studies have focused on JAK inhibition in experimental models of T-ALL and ETP-ALL, though results from these studies are promising.61
Experience with MPN suggests that resistance to JAK inhibition may develop, fueled by additional mutations in JAK proteins, increased JAK2 expression, or shifting transphosphorylation partners; resistance has been identified in both MPN and T-ALL.62,63 There are several potential approaches to overcoming resistance to JAK inhibition. New generations of JAK inhibitors are being developed to bind inactive forms of JAK to overcome resistance.64 Alternatively, JAK inhibitors may be combined with histone deacetylase (HDAC) inhibitors or heat shock protein 90 (HSP90) inhibitors.65,66 HDAC inhibitors induce hyperacetylation of HSP90, block its chaperone function, and promote JAK2 degradation.66 Inhibition of HSP90 increases degradation of both wild-type and mutant JAK2 and shows some efficacy against xenograft models of CRLF2-overexpressing BCP-ALL.65 However, both HSP90 inhibitors and HDAC inhibitors have multiple side effects (reviewed by Hong et al67 ). Phase 1 clinical trials of the HDAC inhibitor panobinostat included patients with myeloid and lymphoid malignancies, though none with ALL.68
STAT5B targeting is less advanced than JAK targeting (reviewed by Dorritie et al69 ). The drug pimozide targets STAT5B and induces apoptosis in cultures of chronic myelogenous leukemia cells. Alternative efforts to target STAT5B include the use of decoy oligonucleotides in chronic myeloid leukemia and potentially the use of siRNA.70,71 In an experimental model of BCP-ALL, STAT5B gene expression was targeted by epigenetics-based therapy using BET bromodomain inhibitors. The study indicated that bromodomain inhibition induces apoptosis in vitro, improves survival in a xenograft model, and promotes downregulation of IL-7Rα.72 In addition, use of a pan–BCL-2 inhibitor, navitoclax, has demonstrated efficacy against STAT5B-mutated cells in vitro.27
PI3K/AKT/mTOR targeting
The PI3K/AKT/mTOR pathway can be targeted by inhibitors of PI3K, AKT, and mTOR, dual inhibitors targeting both PI3K and mTOR, or inhibition of other components of the pathway including eukaryotic translation initiation factor 4E and phosphoinositide-dependent protein kinase I (reviewed by Rodon et al73 ).
Studies of PI3K and AKT inhibition have focused on effects in experimental models of T-ALL. PI3K inhibitor NVP-BKM120 induces apoptosis in cell lines and primary T-ALL cells and synergizes with vincristine and doxorubicin in vitro and in vivo.74 Conversely, although the PI3K inhibitor AS605240 synergizes with glucocorticoids, it antagonizes methotrexate and daunorubicin activity in cell lines, primary T-ALL cells, and murine xenograft models.75 Dual PI3K and mTOR inhibitors are also effective against T-ALL cell lines and patient samples in vitro and are more effective than individual inhibition of PI3K, AKT, or mTOR.76 Dual inhibitor BEZ235 synergizes with dexamethasone in vitro and in vivo, suggesting that targeting the PI3K/AKT/mTOR pathway could modulate glucocorticoid resistance in T-ALL.77 However, dual inhibitor PI-103 has been shown to cause upregulation of NOTCH1 and c-myc; combining PI-103 with c-myc or short-term NOTCH1 inhibition is synergistic and leads to increased death of T-ALL cell lines.78 In addition, inhibition of the PI3K pathway can lead to activation of the FAK/NF-κB/Bcl-2 pathway, suggesting that inhibition of PI3K may be paired with FAK inhibition for greater efficacy.79
AKT inhibition by GSK690693 is effective against both T-ALL and BCP-ALL cell lines in vitro.80 AKT inhibitor MK-2206 decreases T-ALL cell viability and induces apoptosis in leukemia-initiating cell populations.81 Combining 3 AKT inhibitors with different mechanisms of action leads to synergistic effects against T-ALL cell lines.82 AKT inhibition can also lead to reversal of T-ALL glucocorticoid resistance, as demonstrated in cell lines and primografts in vitro as well as in several mouse models.83
Inhibition of mTOR has included studies in both T-ALL and BCP-ALL. Rapamycin, the first mTOR inhibitor, is an allosteric inhibitor that does not fully inhibit the mTORC1 and mTORC2 enzymes.84 Rapamycin shows efficacy against xenografted BCP-ALL with mutated IL-7Rα and altered CRLF2 expression, as well as Ph-like BCP-ALL and a transgenic model of BCP-ALL.58,85 However, treatment with rapamycin alone is not considered highly effective against T-ALL.76
To improve on the efficacy of rapamycin, several other drug types have been developed to target mTOR. Drugs targeting the active site of mTOR are effective against both T-ALL and BCP-ALL cell lines.86,87 More specific targeting of mTORC1 also induces apoptosis and autophagy in BCP-ALL cell lines and synergizes with AKT inhibitor MK-2206.87,88 Alternatively, mTORC1 activity can be targeted with metformin, an activator of LKB1/AMPK, which down-modulates mTORC1 activity. Metformin is effective in vitro against T-ALL cell lines, primary cells, and leukemia-initiating cells. There is currently a clinical trial recruiting pediatric patients with relapsed ALL for treatment with a combination of metformin and chemotherapy (NCT01324180).89 In both T-ALL and BCP-ALL cell lines, inhibition of mTOR synergizes with methotrexate and vincristine, potentially targeting leukemia-initiating cells.86,90 In addition, inhibition of mTOR may synergize with NOTCH1 or Bcl-2 inhibition, as demonstrated in murine xenografts and T-ALL cell lines and primary samples, respectively.91,92 Recently, inhibition of the PI3K/AKT/mTOR pathway has been shown to synergize with MEK inhibition in human T-ALL primary cells and BaF3 cells transduced with mutated IL-7Rα signaling pathway members.93
Conclusion
In the search for targeted ALL therapies, genetic aberrations in the IL-7Rα signaling pathways offer many potential opportunities for therapeutic intervention (Figure 1). Because drugs targeting IL-7Rα signaling pathways have the potential to reduce cellular proliferation and survival, we believe these drugs could currently be used as adjunctive therapies for many leukemia patients, even those without genetic aberrations involving the IL-7Rα signaling pathways. However, the real promise of these therapies lies in the (it is hoped) near future, when each new case of pediatric leukemia will be sequenced. This will enable clinicians to target an individual leukemia’s specific genetic lesions with specific IL-7Rα pathway–directed therapies. The appropriate therapy or therapies will vary depending on the location of the genetic lesion within the signaling pathway. For example, a BCP-ALL patient with overexpressed CRLF2 might be treated by targeting multiple levels of the signaling pathway, including use of anti-TSLPR CAR-T-cells, monoclonal antibodies, JAK inhibitors, and/or dual PI3K/mTOR inhibitors. A patient with AKT mutation might benefit from AKT or mTOR inhibition. As we move forward, we will need to determine the best use of these therapies to minimize side effects and maximize patient benefit. Perhaps someday, targeted therapy will enable patients with IL-7Rα signaling pathway mutations to be treated with less intensive chemotherapy, and relapsed patients or those patients whom current therapies fail will be successfully treated.
IL-7Rα pathway mutations provide potential targets for acute lymphoblastic leukemia therapy. In both T-ALL and BCP-ALL, mutations can occur at many points within the IL-7Rα signaling pathways (A). Aberrant signaling through these pathways offers multiple potential therapeutic targets (B).
IL-7Rα pathway mutations provide potential targets for acute lymphoblastic leukemia therapy. In both T-ALL and BCP-ALL, mutations can occur at many points within the IL-7Rα signaling pathways (A). Aberrant signaling through these pathways offers multiple potential therapeutic targets (B).
Acknowledgments
The authors are grateful to Wenqing Li, Julie Hixon, and Emilee Senkevitch for their extensive review of the manuscript.
This work was supported by grants from the Children’s Cancer Foundation, Inc.; the National Multiple Sclerosis Society (PP1882) (S.K.D.), the Comparative Biomedical Scientist Training Program (Z01 BC 010931), National Institutes of Health (S.D.C.); and the Intramural Research Program of the National Cancer Institute, National Institutes of Health (ZIA SC 010378 [P.D.A.] and ZIA BC 009287 [S.K.D.]).
Authorship
Contribution: S.D.C. researched and wrote the initial manuscript; and P.D.A. and S.K.D. contributed expertise and guidance and edited the manuscript.
Conflict-of-interest disclosure: S.K.D. has applied for a patent titled “IL-7Rα specific antibodies for treating acute lymphoblastic leukemia” (US patent application #62/238 612). The remaining authors declare no competing financial interests.
Correspondence: Scott K. Durum, Center for Cancer Research, National Cancer Institute, Building 560, Room 31-71, Frederick, MD 21702-1201; e-mail: durums@mail.nih.gov.