Abstract
While intensification of therapy has improved event-free survival (EFS) and survival in newly diagnosed children with acute lymphoblastic leukemia (ALL), postrelapse outcomes remain poor. It might be expected that patients relapsing after inferior initial therapy would have a higher retrieval rate than after superior therapy. In the Children's Oncology Group Study CCG-1961, significantly superior EFS and survival were achieved with an augmented (stronger) versus standard intensity regimen of postinduction intensification (PII) for children with newly diagnosed high-risk ALL and rapid day 7 marrow response (EFS/survival 81.2%/88.7% vs 71.7%/83.4%, respectively). This provided an opportunity to evaluate postrelapse survival (PRS) in 272 relapsed patients who had received randomly allocated initial treatment with augmented or standard intensity PII. As expected, PRS was worse for early versus late relapse, marrow versus extramedullary site, adolescent versus younger age and T versus B lineage. However, no difference in 3-year PRS was detected for having received augmented versus standard intensity PII (36.4% ± 5.7% vs 39.2% ± 4.1%; log rank P = .72). Similar findings were noted within subanalyses by timing and site of relapse, age, and immunophenotype. These findings provide insight into mechanisms of relapse in ALL, and are consistent with emergence of a resistant subclone that has acquired spontaneous mutations largely independent of initial therapy. This study is registered at www.clinicaltrials.gov as NCT00002812.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy.1 Long-term survival has become the reality for the majority of children diagnosed with ALL, with approximately 85% surviving 5 years or longer after diagnosis.2-15 However, depending on certain risk factors, such as age at diagnosis, presenting white blood cell (WBC) count, hematopoietic lineage of the disease, and cytogenetic abnormalities, approximately 20% will experience relapse,2-15 most of whom are destined to die of their disease.16-22 Because of the relatively high prevalence of newly diagnosed ALL, relapsed ALL itself remains a common malignancy and a major cause of death among children.16 Thus, despite an increasing proportion of survivors who appear to be cured of ALL, the burden of relapsed disease for patients, families, and health care providers remains substantial and is the focus of considerable pediatric cancer research.
Intensification of antileukemia treatment regimens is thought to be a major contributor to the improvement in event-free survival (EFS) and survival documented by pediatric oncology cooperative study groups over the past 3 decades.2-15,23-26 As typified by these randomized clinical trials where statistically significant differences in outcomes were found between treatment regimens, improvement has generally been observed with intensified therapy, but the impact of treatment intensification has been more apparent on EFS than survival.
Unfortunately, good outcomes after leukemia relapse have proved more difficult to obtain. Intuitively, it might be expected that patients who relapse after receiving an inferior initial treatment regimen (resulting in a lower EFS) would have greater success in retrieval (resulting in greater postrelapse survival [PRS]) than patients who relapse after receiving a superior initial treatment regimen. Presumably, such an effect would occur because the leukemia clone at relapse is “less resistant” because it was treated with less effective (usually less intense) initial therapy. However, such an effect has been difficult to document. The Children's Oncology Group (COG) reported PRS among 9585 children with newly diagnosed ALL who had been enrolled on COG clinical trials from 1988 to 2002.17 A total of 1961 (2.5%) children and adolescents were reported to have relapsed at any site. Of these, 837 patients were alive at a median of 15.7 months after relapse. Time to relapse and site of relapse were the strongest predictors of PRS. Adjusting for those factors in multivariate analysis, older age, male sex, central nervous system (CNS) disease at diagnosis, and T-cell disease were significant predictors of inferior PRS. However, historical era of initial treatment, which was felt to serve as a marker for the increased treatment intensity of more recent trials, did not predict for PRS.17
PRS as a function of initial treatment intensity is best analyzed within the context of individual clinical trials that randomize subjects to treatment arms of different intensity. This opportunity was recently afforded by COG study CCG-1961, which found that “augmented” (greater intensity) compared with “standard” postinduction intensification (PII) produced statistically superior 5-year EFS and survival for children with newly diagnosed National Cancer Institute (NCI) high-risk ALL and a rapid early response during induction.27,28 The primary objective of the analysis reported here was to determine whether initial therapy on CCG-1961was predictive of PRS in patients who relapsed after receiving either augmented or standard PII for newly diagnosed ALL.
Methods
CCG-1961
The CCG-1961 protocol opened to patient entry in September 1996 and closed in May 2002. Eligibility for CCG-1961 included age 1-9 years with presenting WBC count > 50 000/μL or age 10-21 years with any WBC count. Diagnostic criteria and details of therapy have been published previously.28 All patients had a bone marrow aspiration performed on day 7 of induction. Patients who demonstrated 25% or fewer blasts on day 7 were considered rapid early responders (RER) and were randomized in a 2 × 2 factorial design to receive augmented versus standard-intensity PII and longer versus standard-duration PII. Patients randomized to augmented PII received additional vincristine (VCR) and pegylated asparaginase (PEG-asparaginase) courses during consolidation and delayed intensification (DI) phases, and VCR, intravenous methotrexate (MTX; without leucovorin rescue) and PEG-asparaginase during interim maintenance (IM) phases. Patients randomized to longer PII received 2 IM and DI phases rather than one. Patients who were CNS-3 and/or had Philadelphia chromosome–positive ALL were excluded from the RER randomization. The protocol was approved by the NCI and institutional review boards of the participating institutions. Informed consent was obtained from the patients, their parents, or both as deemed appropriate according to the US Department of Health and Human Services guidelines and the Declaration of Helsinki.
Current study
The subjects of this present study are randomized RER patients on CCG-1961 for whom postinduction relapse in any site has been reported. The occurrence of relapse, relapse site, and PRS status were ascertained for this study on the basis of institutional report. As part of CCG-1961, local centers were required to report first-relapse events and survival status on an annual basis, but were not required to report data regarding postrelapse therapy (including whether bone marrow transplantation [BMT] was performed). Causes of death were tabulated as reported by institutions on the standard COG Death Registration Form. The primary outcome measure for this study was PRS as a function of having received either augmented or standard-intensity PII as initial therapy for ALL on CCG-1961. In this article, the term “augmented” PII encompasses all patients treated on a stronger-intensity regimen (of either standard or increased duration), as described in detail in the primary CCG-1961 study report.28 Similarly, “standard” PII refers to all patients initially treated on a lesser-intensity regimen, regardless of treatment duration. No RER patients, relapsed or otherwise, were excluded from this analysis.
Statistical methods
Data received as of August 2009 are included in this report. The χ2 test for homogeneity of proportions was used to compare this study cohort of relapsed patients for similarities with all RER patients on CCG-1961. Life-table estimates for PRS were calculated by the Kaplan-Meier method and standard errors of the estimate were obtained by the method of Peto.29 The log-rank test was used to compare survival curves between groups. The Wilcoxon test was used to compare median times to relapse for the 2 initial regimens. Survival estimates at 3 years postrelapse are given in this report.
Results
Overall results of CCG-1961
As previously reported, the 5-year EFS for all subjects enrolled on CCG-1961 (n = 2078) and for RER subjects (n = 1299) was 71.3% ± 1.6% and 75.5% ± 1.8%, respectively.28 Compared with standard-intensity PII, the 5-year EFS after augmented PII increased from 71.7% to 81.2% (P < .0001) and survival from 83.4% to 88.7% (P = .003).
Patient characteristics
Two hundred seventy-two RER patients who had undergone randomization were reported to have relapsed in one or more anatomic site (ie, bone marrow, CNS, testicular, or other). Key presenting features, including age at diagnosis, sex, WBC count at diagnosis, and lineage of the leukemia are contrasted between the relapsed cohort (n = 272) and all randomized RER subjects (n = 1299) and are displayed in Table 1. There was no statistically significant difference between the groups on any of the characteristics compared. In addition, we compared key presenting features of our relapsed cohort with those RER patients who did not relapse (n = 1027). For age, sex, and lineage there was no difference (P = .69, .06, and .45, respectively). For WBC count, there was an overall difference between the 2 groups (P = .04); the percentage of relapsed versus nonrelapsed patients who had initial WBC < 50, 50-199, or ≥ 200 × 103/μL was 46% versus 52%, 40% versus 38%, and 14% versus 10%, respectively.
Comparison of relapsed cohort with all randomized RER subjects from CCG-1961
| Characteristic . | CCG-1961, n = 1299 (%) . | This cohort, n = 272 (%) . |
|---|---|---|
| Age at initial diagnosis, y | ||
| 1-9 | 478 (36.8) | 106 (39.0) |
| 10-15 | 658 (50.7) | 132 (48.5) |
| 16-20 | 163 (12.5) | 34 (12.5) |
| Sex | ||
| Male | 758 (58.4) | 173 (63.6) |
| Female | 541 (41.6) | 99 (36.3) |
| WBC at initial diagnosis, ×103/μL) | ||
| < 50 | 661 (50.9) | 124 (45.6) |
| 50-199 | 501 (38.6) | 110 (40.4) |
| ≥ 200 | 135 (10.4) | 38 (14.0) |
| Lineage | ||
| B | 880 (67.7) | 193 (71.0) |
| T | 235 (18.1) | 46 (16.9) |
| Characteristic . | CCG-1961, n = 1299 (%) . | This cohort, n = 272 (%) . |
|---|---|---|
| Age at initial diagnosis, y | ||
| 1-9 | 478 (36.8) | 106 (39.0) |
| 10-15 | 658 (50.7) | 132 (48.5) |
| 16-20 | 163 (12.5) | 34 (12.5) |
| Sex | ||
| Male | 758 (58.4) | 173 (63.6) |
| Female | 541 (41.6) | 99 (36.3) |
| WBC at initial diagnosis, ×103/μL) | ||
| < 50 | 661 (50.9) | 124 (45.6) |
| 50-199 | 501 (38.6) | 110 (40.4) |
| ≥ 200 | 135 (10.4) | 38 (14.0) |
| Lineage | ||
| B | 880 (67.7) | 193 (71.0) |
| T | 235 (18.1) | 46 (16.9) |
Difference not statistically significant (P > .05) for all comparisons.
Of the 272 subjects in the relapsed cohort, 109 had received initial treatment with augmented PII and 163 with standard PII. The median time to relapse for this cohort as a whole was 396 days. There were 190 patients who relapsed early (< 36 months from diagnosis) versus 82 who relapsed late. There were 186 patients with marrow relapse (with or without concurrent extramedullary disease [EMD]), 66 with isolated CNS relapse and 20 with isolated relapse in other sites of EMD. Two hundred thirty-eight were ≤ 15 years of age at diagnosis versus 34 who were 16-20 years old. One hundred ninety-three were classified as having precursor-B disease at diagnosis, whereas 46 had T-lineage disease.
Causes of death by initial treatment regimen
Of the relapsed cohort, a total of 162 died: 99 (60.7%) of 163 initially treated with standard PII and 63 (57.8%) of 109 treated with augmented PII. Table 2 summarizes the causes of death for these patients as reported by the treating institutions.
Reported causes of death among relapsed cohort who died (n = 162)
| Reported cause . | Standard PII, n = 99, n (%) . | Augmented PII, n = 63, n (%) . |
|---|---|---|
| Progressive disease | 55 (55.6) | 34 (54.0) |
| Infection | 20 (20.2) | 16 (25.4) |
| Toxicity | 4 (4.0) | 3 (4.8) |
| Graft-versus-host disease | 3 (3.0) | 4 (6.3) |
| Hemorrhage | 1 (1.0) | 1 (1.6) |
| Other | 15 (15.2) | 5 (7.9) |
| Acute respiratory distress syndrome | 6 | 1 |
| Cardiac arrest | 3 | |
| Multiorgan failure | 1 | 1 |
| Pulmonary embolism | 1 | 1 |
| Cardiac myolysis | 1 | |
| Gastrointestinal process | 1 | |
| Lymphoproliferative disease | 1 | |
| Renal failure | 1 | |
| Secondary medulloblastoma | 1 | |
| No details provided | 1 | |
| Unknown | 1 (1.0) |
| Reported cause . | Standard PII, n = 99, n (%) . | Augmented PII, n = 63, n (%) . |
|---|---|---|
| Progressive disease | 55 (55.6) | 34 (54.0) |
| Infection | 20 (20.2) | 16 (25.4) |
| Toxicity | 4 (4.0) | 3 (4.8) |
| Graft-versus-host disease | 3 (3.0) | 4 (6.3) |
| Hemorrhage | 1 (1.0) | 1 (1.6) |
| Other | 15 (15.2) | 5 (7.9) |
| Acute respiratory distress syndrome | 6 | 1 |
| Cardiac arrest | 3 | |
| Multiorgan failure | 1 | 1 |
| Pulmonary embolism | 1 | 1 |
| Cardiac myolysis | 1 | |
| Gastrointestinal process | 1 | |
| Lymphoproliferative disease | 1 | |
| Renal failure | 1 | |
| Secondary medulloblastoma | 1 | |
| No details provided | 1 | |
| Unknown | 1 (1.0) |
Summary data from COG Death Registration Forms submitted by treating institutions.
PRS of RER subjects
The primary objective of this study was to determine whether any patient-, disease- or treatment-related variables were predictive of PRS in the context of CCG-1961. As summarized in Table 3, several factors were associated with significantly inferior PRS, including earlier timing of relapse, older age at diagnosis, and relapse involving the marrow. PRS also appeared to be worse for subjects diagnosed with T-lineage ALL, although the difference did not reach the level of statistical significance.
PRS by factors other than initial treatment regimen (n = 272)
| Characteristic . | 3-year postrelapse survival ± SE % (n) . | Log-rank P . |
|---|---|---|
| Timing from diagnosis, mo | ||
| < 36 | 30.0 ± 3.7 (190) | < .0001 |
| ≥ 36 | 57.8 ± 6.4 (82) | |
| Site | ||
| All | 38.1 ± 3.3 (272) | |
| Marrow (±EMD) | 29.7 ± 3.7 (186) | < .0001 |
| Isolated CNS | 52.2 ± 7.4 (66) | |
| Other isolated EMD | 68.2 ± 11.6 (20) | |
| Sex | ||
| Male | 38.2 ± 4.2 (173) | .92 |
| Female | 37.9 ± 5.4 (99) | |
| Age at initial diagnosis, y | ||
| 1-9 | 48.6 ± 5.3 (106) | .001 |
| 10-15 | 35.4 ± 5.0 (132) | |
| 16-20 | 14.7 ± 6.8 (34) | |
| Lineage | ||
| B | 40.5 ± 3.9 (193) | .09 |
| T | 29.2 ± 8.2 (46) |
| Characteristic . | 3-year postrelapse survival ± SE % (n) . | Log-rank P . |
|---|---|---|
| Timing from diagnosis, mo | ||
| < 36 | 30.0 ± 3.7 (190) | < .0001 |
| ≥ 36 | 57.8 ± 6.4 (82) | |
| Site | ||
| All | 38.1 ± 3.3 (272) | |
| Marrow (±EMD) | 29.7 ± 3.7 (186) | < .0001 |
| Isolated CNS | 52.2 ± 7.4 (66) | |
| Other isolated EMD | 68.2 ± 11.6 (20) | |
| Sex | ||
| Male | 38.2 ± 4.2 (173) | .92 |
| Female | 37.9 ± 5.4 (99) | |
| Age at initial diagnosis, y | ||
| 1-9 | 48.6 ± 5.3 (106) | .001 |
| 10-15 | 35.4 ± 5.0 (132) | |
| 16-20 | 14.7 ± 6.8 (34) | |
| Lineage | ||
| B | 40.5 ± 3.9 (193) | .09 |
| T | 29.2 ± 8.2 (46) |
EMD indicates extramedullary disease; and CNS, central nervous system.
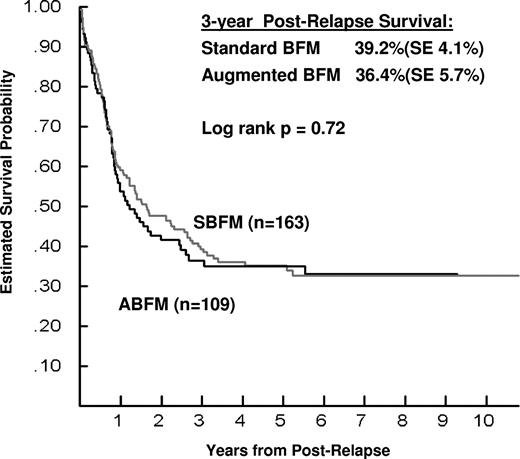
In contrast, the intensity of the initial treatment regimen was not associated with significant differences in PRS. For subjects initially treated with augmented (n = 109) versus standard-intensity (n = 163) PII, the 3-year PRS was 36.4% ± 5.7% versus 39.2% ± 4.1%, respectively (relative hazard ratio 1.06; log rank P = .72; Figure 1). There was no difference by initial regimen in the median time-to-death postrelapse, which was 10.5 months for augmented versus 16.2 months for standard-intensity PII (P = .27). Similarly, no difference in PRS was seen after adjusting for timing of relapse, site of relapse, age at diagnosis, and lineage of the leukemia (Table 4). Interestingly, for subjects aged 16-20 years who had received standard PII (n = 19), the 3-year PRS was 21.1% ± 8.4%, whereas none of those who had received augmented PII (n = 15) were alive 3 years after relapse, although this difference was not statistically significant (log rank, P = .38). Small numbers of subjects in these cells preclude additional analyses of potential interactions of variables.
PRS by initial RER treatment regimen on CCG-1961. Kaplan-Meier plots of survival for 272 children who relapsed after treatment for NCI high-risk ALL on CCG-1961. The terms “Standard BFM” and “Augmented BFM” refer to the intensity of the initial randomized regimen of PII. The figure shows no significant difference in 3-year PRS as a function of initial treatment intensity.
PRS by initial RER treatment regimen on CCG-1961. Kaplan-Meier plots of survival for 272 children who relapsed after treatment for NCI high-risk ALL on CCG-1961. The terms “Standard BFM” and “Augmented BFM” refer to the intensity of the initial randomized regimen of PII. The figure shows no significant difference in 3-year PRS as a function of initial treatment intensity.
PRS by initial treatment regimen (n = 272)
| Characteristic . | 3-year postrelapse survival ± SE % . | ||
|---|---|---|---|
| Standard PII, n = 163 (n) . | Augmented PII, n = 109 (n) . | Log-rank P . | |
| Timing from diagnosis, mo | |||
| < 18 | 25.5 ± 6.1 (56) | 21.6 ± 7.2 (45) | 0.97 |
| 18-35 | 40.0 ± 6.8 (59) | 31.5 ± 9.8 (30) | 0.47 |
| ≥ 36 | 55.0 ± 7.7 (48) | 62.2 ± 11.0 (34) | 0.68 |
| Site | |||
| All | 39.2 ± 4.1 (163) | 36.4 ± 5.7 (109) | 0.72 |
| Marrow | 33.0 ± 4.7 (112) | 24.1 ± 5.8 (74) | 0.33 |
| (±EMD) | |||
| Isolated CNS | 48.8 ± 9.7 (36) | 56.1 ± 10.7 (30) | 0.51 |
| Age at initial diagnosis, y | |||
| 1-9 | 51.1 ± 6.6 (67) | 44.3 ± 8.3 (39) | 0.46 |
| 10-15 | 33.6 ± 5.8 (77) | 39.2 ± 9.2 (55) | 0.50 |
| 16-20 | 21.1 ± 8.4 (19) | 0 (15) | 0.38 |
| Lineage | |||
| B | 41.9 ± 5.0 (112) | 38.5 ± 6.4 (81) | 0.79 |
| T | 28.2 ± 9.0 (28) | 31.8 ± 15.1 (18) | 0.63 |
| Characteristic . | 3-year postrelapse survival ± SE % . | ||
|---|---|---|---|
| Standard PII, n = 163 (n) . | Augmented PII, n = 109 (n) . | Log-rank P . | |
| Timing from diagnosis, mo | |||
| < 18 | 25.5 ± 6.1 (56) | 21.6 ± 7.2 (45) | 0.97 |
| 18-35 | 40.0 ± 6.8 (59) | 31.5 ± 9.8 (30) | 0.47 |
| ≥ 36 | 55.0 ± 7.7 (48) | 62.2 ± 11.0 (34) | 0.68 |
| Site | |||
| All | 39.2 ± 4.1 (163) | 36.4 ± 5.7 (109) | 0.72 |
| Marrow | 33.0 ± 4.7 (112) | 24.1 ± 5.8 (74) | 0.33 |
| (±EMD) | |||
| Isolated CNS | 48.8 ± 9.7 (36) | 56.1 ± 10.7 (30) | 0.51 |
| Age at initial diagnosis, y | |||
| 1-9 | 51.1 ± 6.6 (67) | 44.3 ± 8.3 (39) | 0.46 |
| 10-15 | 33.6 ± 5.8 (77) | 39.2 ± 9.2 (55) | 0.50 |
| 16-20 | 21.1 ± 8.4 (19) | 0 (15) | 0.38 |
| Lineage | |||
| B | 41.9 ± 5.0 (112) | 38.5 ± 6.4 (81) | 0.79 |
| T | 28.2 ± 9.0 (28) | 31.8 ± 15.1 (18) | 0.63 |
EMD indicates extramedullary disease; and CNS, central nervous system.
Discussion
Relapse after treatment for childhood ALL is a serious event, as the likelihood of long-term survival is relatively poor. Although recent studies demonstrate that achievement of second complete remission (CR2) is successful in more than 80% of patients,18-20 these and other studies show that long-term survival ranges from approximately 20% to 50% for relapsed patients as a whole.16-22,30 Relative to this range, certain patient subsets fare somewhat better or worse. In an effort to understand the phenomenon of leukemia relapse, several predictors of ultimate outcome have been identified in hopes of providing clues that may lead to more effective treatment. To date, the strongest and most consistent predictors have been duration of first remission (CR1) and anatomic site of relapse.16-22 Regarding duration of CR1, a recent retrospective analysis of COG experience involving nearly 2000 children found that the 5-year PRS estimate for those who relapsed within 18 months of diagnosis was only 21%.17 Regarding relapse site, survival at 3 to 6 years after relapse has been found to range from approximately 20% for isolated marrow relapse to 70% for isolated EMD (eg, CNS or testicle), with combined-site (ie, marrow plus EMD) relapses having an intermediate outcome.16-18,20-22 Other predictors from initial presentation that some recent studies have found to portend worse PRS include T-cell immunophenotype,17,18,20 male sex,17 initial WBC count,19 and certain unfavorable clonal cytogenetic abnormalities.19,20 A relapse score incorporating duration of CR1, relapse site, and immunophenotype developed by the Berlin-Frankfurt-Münster (BFM) cooperative group31,32 was recently found to discriminate 6-year PRS rate of 78%, 41% and 19% for patients classified as low-, intermediate-, and high-risk, respectively.19
Our study was developed to explore the possibility that, in addition to the aforementioned patient- and disease-related variables, type of initial therapy received might also be predictive of PRS. Specifically, our hypothesis was that relapsed patients who had received the more intensive of 2 initial treatment regimens for NCI high-risk ALL, which was shown to yield a significantly higher initial EFS and survival,28 would have lower PRS than those initially treated with the inferior regimen. However, our results indicate there was no such difference, with the 3-year survival being equally poor at 36.4% and 39.2% (log rank P = .72) for the augmented and standard-intensity regimens, respectively. In subset analysis, no statistically significant effect of timing of relapse (duration of CR1), relapse site, age at diagnosis, or immunophenotype was detected on this finding (Table 3). For age at diagnosis, an interesting (but statistically nonsignificant) trend in the direction of our hypothesis was noted for patients who were 16-20 years old at diagnosis, although very small patient numbers in these groups prevent further analysis for potential interaction of the covariates. No difference in cause of death according to initial treatment regimen was apparent in our results.
While very few studies have directly compared PRS as a function of initial treatment regimen, our findings are consistent with most that have. In a retrospective study by the COG of postrelapse outcomes in 347 subjects treated for NCI standard-risk ALL on CCG-1952, there was no difference noted after initial treatment with the 4 regimens that tested the efficacy of 6-mercapturine (6-MP) versus 6-thioguanine and CNS prophylaxis with intrathecal MTX alone versus triple intrathecal therapy (addition of cytarabine and hydrocortisone).22 A historical analysis of PRS after treatment on 2 multiarm studies for newly diagnosed ALL, CCG-105 (for intermediate-risk ALL) and CCG-123 (for ALL with lymphomatous features), found no difference as a function of initial treatment regimen received.16 Exploiting the stepwise intensification of antileukemia therapy that has taken place over an extended series of CCG/COG studies for newly diagnosed ALL, 2 comprehensive analyses of postrelapse outcomes involving several thousand children were unable to demonstrate any significant differences in survival as a function of treatment era (“early” vs “late”) spanning more than the past 20 years.16,17
In comparison with the above studies, ours offers the combined advantages of uniformity of patient and disease characteristics (because the sample was derived from a single clinical trial), the distinct difference in intensity between 2 randomized regimens delivered contemporaneously (which produced a significantly higher EFS), and a relatively long period of follow-up after relapse. Overall, the results of our study and those cited suggest that differing intensity of initial treatment, as reflected in either the cross-regimen setting of single studies (CCG-1952 and CCG-1961) or the trans-era context of sequential CCG/COG studies involving both standard- and high-risk patients, does not alter the generally poor outcome associated with relapsed childhood ALL of any initial risk category.
We are aware of only 2 published exceptions to this conclusion. First, the Austrian BFM Study Group recently reported somewhat higher postrelapse EFS (but not survival) for 203 children with relapsed ALL who received treatment on the more recent of their frontline studies conducted during the 1980s and 1990s.20 However, the report did not address the potential relationship of that finding with either the intensity of their initial treatment or systematic innovations in relapse therapy that may have occurred over the same interval. Second are the provocative results of Tolar and colleagues33 describing the survival of 179 children who relapsed after treatment for NCI standard-risk ALL on CCG-1922. In CCG-1922, subjects were randomized to receive either dexamethasone or prednisone as their steroid therapy in the induction and maintenance phases, and either oral or intravenous 6-MP during a 3-month consolidation phase after remission induction; all subjects received continuous oral 6-MP during a prolonged maintenance phase lasting 20 months for girls and 32 months for boys.34 While EFS but not overall survival was improved for subjects randomized to receive dexamethasone, overall survival was significantly lower for those randomized to receive intravenous 6-MP.34 The analysis of relapsed patients from CCG-1922 demonstrated a 4-year PRS of 67% versus 48% (log rank, P = .002) for those randomized to oral versus intravenous 6-MP, respectively, while no difference in PRS was noted as a function of initial steroid assignment.33 Thus, in the case of CCG-1922, relapsed patients were less salvageable if they had received intravenous 6-MP during their initial therapy. Against this, a subsequent analysis of patients initially treated for standard-risk ALL on a Pediatric Oncology Group (POG) trial that randomized subjects to receive early intensification with one of 3 regimens, 2 of which included intravenous 6-MP and a third that did not, found no significant difference in PRS as a function of initial regimen.26 The reasons for these disparate findings pertaining to intravenous 6-MP are unclear and have been the subject of considerable speculation.33,35,36 Similar to our study, those studies of relapsed CCG and POG patients were retrospective and not able to collect data on relapse-directed treatment, including whether BMT was performed.33,35 Overall, an impact of differing postrelapse treatment approaches on long-term survival has been difficult to document consistently, including the role of BMT.30 Key differences in relapse-directed therapy would be particularly important to ascertain when a disparity in PRS is detected as a function of initial treatment. In our study, where PRS was equally poor regardless of initial treatment intensity, systematic differences in relapse-directed therapy between the groups, if any, were ineffectual, underscoring the critical need for a more enlightened understanding of the fundamental biologic events underlying the development of leukemia relapse and its current lack of lasting responsiveness to retrieval therapy.
The results of the current study may offer insights into those events. Intensification of initial therapy for ALL does not seem simply to prevent “soft” or “marginal” relapses that would otherwise be salvaged with good retrieval therapy. Rather, the equivalent PRS observed in our study suggests that a residuum of malignant cells is behaving according to the Goldie-Coldman hypothesis.37-39 According to that model, malignant cells responsible for relapse are present at diagnosis and mutate to a resistant phenotype through the acquisition of spontaneous mutations that are dependent on intrinsic genomic instability rather than treatment exposures. In support of this model, 2 research groups have performed single nucleotide polymorphism array analyses of matched leukemia cell samples obtained at diagnosis and relapse, compared with germline, in children with ALL.40,41 Despite the gain or loss of somatic copy number alterations (CNAs) from diagnosis to relapse, both groups found evidence for a common clonal origin. Somatic alterations of genes involved in regulated B-cell development, such as CDKN2A/B, IKZF1, ETV6, and EBF1, were more frequent in relapse specimens. In the majority of the cases where acquired CNAs were present at relapse, the relapse clone was detected at low levels in diagnosis specimens by using lesion-specific backtracking genomic polymerase chain reaction assays.41 Together, those findings indicate that the diagnosis and relapse clones originated from a common ancestral clone and acquired distinct CNAs before emerging as the predominant clones at diagnosis or relapse. Thus, the genetic basis of relapse appears more complex than can be explained by undertreatment or simple drug resistance. Further analyses examining additional types of genetic alterations in this context will be important for identifying potential pathways for therapeutic intervention.
The corollary for treatment derived from the Goldie-Coldman hypothesis, in conjunction with the Norton-Simon hypothesis,42 is that eradication of all malignant or premalignant subclones present at diagnosis, including those otherwise destined to escape initial therapy and lead to relapse, must be accomplished through early and continuing treatment with multiple, non–cross-resistant chemotherapy drugs in a “dose-dense” fashion. Although statistical improvements in EFS have been observed after treatment intensification in recent COG studies,2 these are incremental compared with gains achieved in earlier eras. In addition, our results show that patients initially treated with the less intensive regimen of CCG-1961 are no more salvageable after relapse. Furthermore, it may not even be feasible to achieve further clinically meaningful treatment intensification, given the likelihood of escalating treatment-related morbidity and mortality in the process. These considerations suggest that with our current repertoire of antileukemia agents, most of what stands to be gained through intensification of initial treatment may have been achieved already. Mechanistically novel agents that can be integrated into existing front-line treatment regimens may be a more rational way forward. For example, encouraging preliminary results have been obtained with the active monoclonal antibody agent blinatumomab, a bispecific single-chain antibody construct designed to link B cells and T cells resulting in T-cell activation and a cytotoxic T-cell response against CD19-expressing cells. In vitro data indicate CD19+ lymphoma and leukemia cell lines to be extremely sensitive to blinatumomab-mediated cytotoxicity. Blinatumomab is well tolerated in adults and has shown activity in adults with non-Hodgkin lymphoma and minimal residual disease-positive B-cell precursor ALL.43,44 In addition, recent discovery of new genetic alterations associated with high rates of relapse, such as the rearrangement of CRLF2, IKZF1 deletions/mutations and JAK family mutations,45-47 offers the potential for identification of patients at diagnosis who should be treated more aggressively and with agents targeting those molecular lesions. Although the incidence of these specific mutations is not known in the current study sample, such questions need to be studied in relapsed populations and in the future the information may be incorporated in risk-stratification systems at diagnosis. Novel agents such as those mentioned are also needed for testing in biology-linked clinical trials for newly diagnosed patients who are designed to detect and monitor low-level persistence or reemergence of distinct leukemia subclones. Similarly, clinical trials for relapsed patients would not only allow prospective collection of valuable data on treatment given, tolerance of therapy and causes of death, but an opportunity to explore the biologic events underlying response and refractoriness to retrieval therapy itself.
The online version of this article contains a data supplement.
Presented in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants U10 CA98543, CA13539, and CA30969).
S.P.H. is the Ergen Family Chair in Pediatric Cancer.
National Institutes of Health
Authorship
Contribution: D.R.F. contributed to the study design and data analysis and wrote the manuscript; M.D. and M.L. performed the statistical analyses; M.D., M.L., W.L.C., P.S.G., S.P.H., and N.L.S. contributed to the study design and data analysis and edited the manuscript; and N.L.S. was Chair of the CCG-1961 study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Children's Oncology Group appears in the supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article.
Correspondence: David R. Freyer, DO, MS, Children's Center for Cancer and Blood Diseases, Children's Hospital Los Angeles, 4650 Sunset Blvd, Mailstop 54, Los Angeles, CA 90027-6016; e-mail: dfreyer@chla.usc.edu.