Abstract
Optimal management of patients with relapsed/refractory chronic lymphocytic leukemia (CLL) is dictated by patient characteristics, prior therapy, and response to prior therapy. We report the final analysis of combined fludarabine, cyclophosphamide, and rituximab (FCR) for previously treated patients with CLL and identify patients who benefit most from this therapy. We explore efficacy of FCR in patients beyond first relapse, patients with prior exposure to fludarabine and alkylating agent combinations, and patients with prior exposure to rituximab. The FCR regimen was administered to 284 previously treated patients with CLL. Patients were assessed for response and progression by 1996 National Cancer Institute–Working Group (NCI-WG) criteria for CLL and followed for survival. The overall response rate was 74%, with 30% complete remission. The estimated median overall survival was 47 months and median progression-free survival for all patients was 21 months. Subgroup analyses indicated that the following patients were most suitable for FCR treatment: patients with up to 3 prior treatments, fludarabine-sensitive patients irrespective of prior rituximab exposure, and patients without chromosome 17 abnormalities. FCR is an active and well-tolerated therapy for patients with relapsed CLL. The addition of rituximab to FC improved quality and durability of response in this patient population.
Introduction
Chronic lymphocytic leukemia (CLL) is a B-cell malignancy with significant variability in clinical course depending on patients' disease characteristics, treatment, and response to prior treatment. Despite highly active treatment agents and combinations, no curative standard treatment is available. Stem cell transplantation is promising for long-term disease control and potential for cure; however, it is not a treatment modality available to most patients and has significant associated toxicities and morbidity.1,2 Most patients receive intermittent treatment with periods of remission or stable disease that are typically shorter with each intervention and many patients acquire treatment resistance with low response rates and short response duration and survival.3-6 Identifying therapeutic interventions for relapsed and refractory patients that result in long-term remission is a challenging aspect in the management of CLL.7
A purine analog combined with an alkylating agent improves the quality of response over single-agent therapy and is associated with longer progression-free survival (PFS) in previously treated and untreated patients with CLL.8-10 Although standard-dose rituximab monotherapy has only modest efficacy in CLL, when combined with fludarabine (F) there is synergism based on modulated levels of complement-resistance proteins and of antiapoptotic factors, such as Bcl-2.11,12 Monoclonal antibody–containing chemoimmunotherapy regimens including rituximab improve quality and duration of responses in CLL.13-15 The chemoimmunotherapy combination of fludarabine, cyclophosphamide, and rituximab (FCR) has become a standard treatment for CLL based on the German CLL Study Group (GCLLSG) Frontline CLL8 trial and the International REACH trial for patients in first relapse.13,15 However, the REACH trial excluded patients in second or subsequent relapse and those previously treated with rituximab or fludarabine and cyclophosphamide (FC) combination; therefore, there is limited understanding of the efficacy of the FCR regimen in such patient populations.
We previously reported results of FCR chemoimmunotherapy for relapsed and refractory patients with CLL.16 This regimen had a high response rate in relapsed patients and was a significant advance compared with that seen in historic patients treated with FC or F.9 We report a final analysis of this phase 2 trial, and present responses, response duration, and survival for 284 relapsed patients treated with FCR. The prolonged follow-up enables us to determine patient pretreatment characteristics associated with superior outcomes after therapy to identify relapsed patients most appropriate for this regimen.
Methods
The M. D. Anderson Cancer Center (MDACC) Institutional Review Board approved this study; patients provided informed consent per institutional guidelines. This study was conducted in accordance with the Declaration of Helsinki. For detailed information regarding patients and methods, refer to the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Synopsis of study design and treatment plan
Briefly, 288 patients were enrolled in this open-label, phase 2 trial from December 1999 through April 2008. Four patients were excluded as they did not have a diagnosis of CLL leaving 284 previously treated patients with CLL (supplemental Figure 1). All patients had active, progressive CLL with an indication for treatment by NCI-WG criteria.17 Patients were required to have adequate performance status (WHO/Eastern Cooperative Oncology Group [ECOG] performance status ≤ 3) and organ function (serum creatinine < 2 mg/dL and total bilirubin < 2 mg/dL). Eligibility was not restricted to number or type of prior treatment regimens or prior refractoriness to fludarabine or alkylating agents. The final analysis included 280 patients evaluable for response and 284 patients evaluable for PFS and overall survival (OS) by intent to treat. Results for 177 of these patients were previously reported in an interim analysis of the study and we present the final results of the completed study in this manuscript. Pretreatment evaluation included physical examination and peripheral blood examination (previously described).16 Patients were evaluated with pretreatment bone marrow aspiration and biopsy, including immunophenotype and CD38 expression. Standard metaphase cytogenetic analysis was performed on bone marrow in the majority of patients. After 2003, patients were routinely assessed for newer prognostic factors, including immunoglobulin heavy chain variable gene (IGHV) mutation status (CLL Research Consortium),18 and ZAP70 expression in B cells determined by either immunohistochemistry or flow cytometry (CLL Research Consortium).19 Refractoriness to fludarabine and alkylating agent was defined as failure to achieve at least a partial remission (PR) with the last fludarabine- and alkylating agent-based treatment, respectively, or progression within 6 months of treatment.
Treatment
Details of study and treatment plans were previously reported.16 Patients received fludarabine (F) 25 mg/m2 and cyclophosphamide (C) 250 mg/m2 on days 2-4 for course 1 and days 1-3 for courses 2-6. Rituximab (R) was administered on day 1 at 375 mg/m2 for course 1 and 500 mg/m2 for courses 2-6. Oral acetaminophen (650 mg) and diphenhydramine (25-50 mg) were intravenously administered before rituximab for each course. Ondansetron (24 mg) was intravenously administered before chemotherapy each day of treatment. Myeloid growth factors were not routinely administered but patients may have received granulocyte–colony-stimulating factor as secondary prophylaxis for neutropenia. Courses were 4 weeks or longer depending on recovery of neutrophil or platelet counts. Dose reductions for FC, but not rituximab, were made if patients experienced prolonged grade 3 or 4 hematologic toxicity or infections. Prophylaxis against tumor lysis syndrome for course 1, herpes virus reactivation, and Pneumocystis jiroveci pneumonia were recommended but not mandated. Patients who achieved stable PR or better after course 3 were given up to 3 additional courses of FCR.
Response criteria
Patients were evaluated for response according to 1996 NCI-WG response criteria at least 2 months after their last course.17 Computerized tomography (CT) scans were not routinely performed for response assessment. Flow cytometric evaluation (flow) of bone marrow aspirate was performed to estimate minimal residual disease (MRD) by evaluating for CD5+/CD19+ lymphocytes with light-chain restriction. Flow MRD negativity was defined as less than 1% of CD5+/CD19+ coexpressing cells with normal κ-λ ratio. Molecular monitoring for MRD was also performed using a polymerase chain reaction (PCR)–based ligase assay for patient-specific IGHV gene.20 The ratio of IGHV to RAS oncogene (PCR ratio) was calculated and used to quantify the levels of residual disease. A ratio of 0.001 to 0.10 was considered to be low positive, higher ratios were considered to be positive, and lower ratios were considered negative. Adverse events (AEs) and serious adverse events (SAEs) were documented according to the Common Toxicity Criteria (CTC) Version 2.0 (National Cancer Institute).
Statistical considerations
The primary objective was to improve the complete remission (CR) rate compared with that seen with historic regimens of FC and F. As pretreatment characteristics such as cytogenetic analysis were likely to be important in determining treatment outcomes, the original study design was extended to allow accrual of up to 300 patients to allow for subset analysis. OS, PFS, and time-to-progression (TTP) were secondary outcomes calculated from the first day of therapy. Actuarial survival, PFS, and TTP were estimated using the methods of Kaplan and Meier and survival estimates were compared among subgroups of patients using the log-rank test. The Fisher exact test (2-tailed) was used to analyze differences in response outcomes by pretreatment characteristics. Associations between CR and overall response (OR) and patient pretreatment characteristics were evaluated using univariable and multivariable logistic regression models. Associations between patient pretreatment characteristics and time-to-event endpoints were analyzed using Cox proportional hazards regression models. There were 24 pretreatment variables analyzed and therefore a negative stepwise analysis was performed with a P value cutoff of P < .05. All computations were carried out using SAS, S-plus, and Statistica (Stat-Soft).
FC comparison group
Patients enrolled at MDACC on clinical trials of FC as a salvage therapy for CLL were included as a historical comparison group (n = 114). A multivariate Cox regression analysis was used to adjust for differences in pretreatment characteristics and define the impact of therapy on PFS and OS.
Results
Patients
There were 284 relapsed or refractory patients with CLL entered into this study; the median age was 60 years and median number of prior treatments was 2 (1-10). Other pretreatment characteristics are shown in Tables 1 and 2.
Outcomes after FCR by patient pretreatment characteristics
| Pretreatment characteristics . | No. of patients . | NCI-WG response, % . | Median . | |||
|---|---|---|---|---|---|---|
| CR . | nPR . | OR . | PFS, mo . | OS, mo . | ||
| All patients | 284 | 30 | 14 | 74 | 21 | 46.5 |
| Age, y* | ||||||
| 0-60 | 154 | 39 | 12 | 74 | 28 | 60 |
| 61-70 | 90 | 23‡ | 19 | 78 | 22 | 48 |
| > 70 | 40 | 13‡ | 13 | 68 | 13‡ | 22§ |
| Rai stage* | ||||||
| 0-II | 154 | 40 | 18 | 84 | 29 | 67 |
| III | 34 | 26§ | 12 | 76 | 21‡ | 48§ |
| IV | 96 | 16§ | 10 | 57§ | 7§ | 31§ |
| Bulky disease† | ||||||
| No | 260 | 32 | 15 | 76 | 23 | 48 |
| Yes | 20 | 10‡ | 6 | 55 | 8 | 20 |
| β2-microglobulin, mg/L* | ||||||
| < 4 | 111 | 45 | 18 | 86 | 33 | 67 |
| 4-5.9 | 92 | 30‡ | 13 | 75 | 20 | 50‡ |
| ≥ 6 | 74 | 7§ | 12 | 57§ | 7‡ | 22§ |
| Karyotype (nd = 102)* | ||||||
| Diploid or 13q deletion | 97 | 34‡ | 18 | 82§ | 27§ | 54§ |
| Trisomy 12 | 16 | 38 | 13 | 69 | 20 | 78 |
| 11q deletion | 13 | 15 | 15 | 69 | 12‡ | 33 |
| Complex | 22 | 9 | 27 | 64 | 9‡ | 26‡ |
| Abnormal chromosome 17 | 20 | 0§ | 5 | 35§ | 5§ | 10.5§ |
| Others | 14 | 29 | 7 | 71 | 27 | 48 |
| No. of prior treatment regimens* | ||||||
| 1 | 116 | 44 | 14 | 82 | 30 | 58 |
| 2 | 80 | 33 | 15 | 75 | 28 | 54 |
| 3 | 46 | 17‡ | 26 | 70 | 20 | 38‡ |
| ≥ 4 | 42 | 2§ | 2 | 57‡ | 9§ | 25§ |
| Fludarabine refractory | ||||||
| No | 230 | 36 | 16 | 79 | 28 | 52 |
| Yes | 54 | 7§ | 9 | 56§ | 8§ | 38‡ |
| Alkylating agent refractory | ||||||
| No | 217 | 36 | 15 | 77 | 27 | 53 |
| Yes | 67 | 10§ | 12 | 66 | 9§ | 39‡ |
| Prior rituximab | ||||||
| No | 186 | 30 | 16 | 76 | 21 | 44 |
| Yes | 98 | 32 | 11 | 71 | 20 | 48 |
| CD38 expression, BM (nd = 35) | ||||||
| < 30% | 175 | 34 | 17 | 77 | 26 | 48 |
| ≥ 30% | 74 | 27 | 11 | 68 | 16‡ | 45 |
| ZAP70 expression, flow (nd = 215) | ||||||
| < 20% | 26 | 31 | 8 | 69 | 24 | 51 |
| ≥ 20% | 43 | 23 | 21 | 72 | 24 | 41 |
| IGHV mutation status (nd = 198) | ||||||
| Mutated | 27 | 33 | 11 | 78 | 44 | NR |
| Unmutated | 59 | 37 | 12 | 86 | 28‡ | 50‡ |
| Pretreatment characteristics . | No. of patients . | NCI-WG response, % . | Median . | |||
|---|---|---|---|---|---|---|
| CR . | nPR . | OR . | PFS, mo . | OS, mo . | ||
| All patients | 284 | 30 | 14 | 74 | 21 | 46.5 |
| Age, y* | ||||||
| 0-60 | 154 | 39 | 12 | 74 | 28 | 60 |
| 61-70 | 90 | 23‡ | 19 | 78 | 22 | 48 |
| > 70 | 40 | 13‡ | 13 | 68 | 13‡ | 22§ |
| Rai stage* | ||||||
| 0-II | 154 | 40 | 18 | 84 | 29 | 67 |
| III | 34 | 26§ | 12 | 76 | 21‡ | 48§ |
| IV | 96 | 16§ | 10 | 57§ | 7§ | 31§ |
| Bulky disease† | ||||||
| No | 260 | 32 | 15 | 76 | 23 | 48 |
| Yes | 20 | 10‡ | 6 | 55 | 8 | 20 |
| β2-microglobulin, mg/L* | ||||||
| < 4 | 111 | 45 | 18 | 86 | 33 | 67 |
| 4-5.9 | 92 | 30‡ | 13 | 75 | 20 | 50‡ |
| ≥ 6 | 74 | 7§ | 12 | 57§ | 7‡ | 22§ |
| Karyotype (nd = 102)* | ||||||
| Diploid or 13q deletion | 97 | 34‡ | 18 | 82§ | 27§ | 54§ |
| Trisomy 12 | 16 | 38 | 13 | 69 | 20 | 78 |
| 11q deletion | 13 | 15 | 15 | 69 | 12‡ | 33 |
| Complex | 22 | 9 | 27 | 64 | 9‡ | 26‡ |
| Abnormal chromosome 17 | 20 | 0§ | 5 | 35§ | 5§ | 10.5§ |
| Others | 14 | 29 | 7 | 71 | 27 | 48 |
| No. of prior treatment regimens* | ||||||
| 1 | 116 | 44 | 14 | 82 | 30 | 58 |
| 2 | 80 | 33 | 15 | 75 | 28 | 54 |
| 3 | 46 | 17‡ | 26 | 70 | 20 | 38‡ |
| ≥ 4 | 42 | 2§ | 2 | 57‡ | 9§ | 25§ |
| Fludarabine refractory | ||||||
| No | 230 | 36 | 16 | 79 | 28 | 52 |
| Yes | 54 | 7§ | 9 | 56§ | 8§ | 38‡ |
| Alkylating agent refractory | ||||||
| No | 217 | 36 | 15 | 77 | 27 | 53 |
| Yes | 67 | 10§ | 12 | 66 | 9§ | 39‡ |
| Prior rituximab | ||||||
| No | 186 | 30 | 16 | 76 | 21 | 44 |
| Yes | 98 | 32 | 11 | 71 | 20 | 48 |
| CD38 expression, BM (nd = 35) | ||||||
| < 30% | 175 | 34 | 17 | 77 | 26 | 48 |
| ≥ 30% | 74 | 27 | 11 | 68 | 16‡ | 45 |
| ZAP70 expression, flow (nd = 215) | ||||||
| < 20% | 26 | 31 | 8 | 69 | 24 | 51 |
| ≥ 20% | 43 | 23 | 21 | 72 | 24 | 41 |
| IGHV mutation status (nd = 198) | ||||||
| Mutated | 27 | 33 | 11 | 78 | 44 | NR |
| Unmutated | 59 | 37 | 12 | 86 | 28‡ | 50‡ |
NCI-WG indicates National Cancer Institute-Working Group; CR, complete remission; nPR, nodular partial remission; OR, overall response; PFS, progression-free survival; OS, overall survival; BM, bone marrow; IGHV, immunoglobulin heavy chain variable gene; LN, lymph node; and nd, not done.
Age, Rai stage, karyotype, β-2 microglobulin and number of prior treatment analysis performed in a hierarchical manner with each group compared to lower-ranked risk groups only (eg, Rai stage III compared to Rai stage 0 to II only). In karyotype analysis, diploid/deletion 13q also compared to all other cytogenetic abnormalities.
Bulky disease, by physical examination lymph nodes greater than 5 cm in maximum diameter.
P < .05,
P < .001 (2-tailed Fisher exact test).
Outcome after FCR by most-intensive prior therapy
| Prior therapy hierarchy . | No. of patients . | Median no. of prior . | F/Alk refractory, % . | NCI-WG Response (%) . | Median, mo . | ||
|---|---|---|---|---|---|---|---|
| CR + nPR . | OR . | PFS . | OS . | ||||
| Antibody only | 25 | 1 (1-2) | −/− | 64 | 92 | 49 | 69 |
| Fludarabine only | 61 | 1 (1-5) | 11/− | 61 | 80 | 39 | 88 |
| Alkylating agent only | 36 | 1 (1-4) | −/47 | 39 | 78 | 20 | 48 |
| F and alkylating agent, nonconcurrent | 57 | 3 (2-9) | 35/51 | 43 | 74 | 17 | 44 |
| FC, FCR, FCM, FND | 78 | 2 (1-10) | 13/9 | 42 | 73 | 19 | 41 |
| Multiagent (CHOP, ESHAP, DHAP) or SCT | 27 | 3 (1-10) | 63/52 | 11 | 44 | 6 | 20 |
| Prior therapy hierarchy . | No. of patients . | Median no. of prior . | F/Alk refractory, % . | NCI-WG Response (%) . | Median, mo . | ||
|---|---|---|---|---|---|---|---|
| CR + nPR . | OR . | PFS . | OS . | ||||
| Antibody only | 25 | 1 (1-2) | −/− | 64 | 92 | 49 | 69 |
| Fludarabine only | 61 | 1 (1-5) | 11/− | 61 | 80 | 39 | 88 |
| Alkylating agent only | 36 | 1 (1-4) | −/47 | 39 | 78 | 20 | 48 |
| F and alkylating agent, nonconcurrent | 57 | 3 (2-9) | 35/51 | 43 | 74 | 17 | 44 |
| FC, FCR, FCM, FND | 78 | 2 (1-10) | 13/9 | 42 | 73 | 19 | 41 |
| Multiagent (CHOP, ESHAP, DHAP) or SCT | 27 | 3 (1-10) | 63/52 | 11 | 44 | 6 | 20 |
NCI-WG indicates National Cancer Institute-Working Group; CR, complete remission; nPR, nodular partial remission; OR, overall response; PFS, progression-free survival; OS, overall survival; F, fludarabine; Alk, alkylating agent; FC, fludarabine and cyclophosphamide; FCR, fludarabine, cyclophosphamide, and rituximab; FCM, fludarabine, cyclophosphamide, and mitoxantrone; FND, fludarabine, mitoxantrone, and dexamethasone; CHOP, cyclophosphamide, adriamycin, vincristine, prednisone; ESHAP, etoposide, methylprednisone, cytarabine, cisplatin; DHAP, dexamethasone, cytarabine, cisplatin; and SCT, allogeneic or autologous stem cell transplantation.
Response to therapy
There were 280 of 284 patients evaluable for response to FCR: 86 (30%) achieved CR, 41 (14%) achieved nodular partial remission (nPR), and 84 (30%) achieved partial remission (PR), for an OR rate (ORR) of 74%. Responses were evaluated at least 2 months after completion of therapy to allow for recovery of peripheral blood counts if in CR with incomplete hematologic recovery (CRi). Responses revised by 2008 International Workshop on CLL (IWCLL) criteria at 2, 6, and 12 months after last course of therapy are shown in Table 3. Responses by pretreatment characteristics are shown in Table 1.21
Responses according to 2008 IWCLL criteria
| Response . | Time after last course (2008 IWCLL) . | 1996 CLL-WG overall . | ||
|---|---|---|---|---|
| 2 mo . | 6 mo . | 12 mo . | ||
| CR, % | 18 | 22 | 28 | 30 |
| CRi, % | 14 | 7 | 1* | |
| nPR, % | 9 | 10 | 11 | 11 |
| Response . | Time after last course (2008 IWCLL) . | 1996 CLL-WG overall . | ||
|---|---|---|---|---|
| 2 mo . | 6 mo . | 12 mo . | ||
| CR, % | 18 | 22 | 28 | 30 |
| CRi, % | 14 | 7 | 1* | |
| nPR, % | 9 | 10 | 11 | 11 |
Some patients (1%) are in CRi at 12 months due to late-onset neutropenia or thrombocytopenia having achieved CR previously by 1996 CLL-WG criteria.
When analyzed by prior therapy, patients with 3 or fewer prior therapies had significantly higher CR or nPR rates compared with those who received 4 or more prior regimens (CR/nPR = 52% vs. 4%, P < .0001). Patients were hierarchically categorized according to their most intensive prior treatment regimen, irrespective of number of prior treatments, as follows: multiagent chemotherapy (eg, cyclophosphamide, adriamycin, vincristine and prednisone (CHOP)) or stem cell transplantation; purine analog with alkylating agent or mitoxantrone combinations; sequential alkylating agent and purine analog exposure; alkylating agent or fludarabine monotherapy; and immunotherapy or experimental therapy without exposure to chemotherapy (Table 2).
Patients who received prior monoclonal antibody, including rituximab, or purine analog, without exposure to an alkylating agent demonstrated excellent response rates (CR/nPR 62% and OR 84%; Table 2). Patients previously exposed to combined fludarabine with alkylating agent had intermediate response rates (CR/nPR 42% and OR 73%; Table 2); however, among these patients, fludarabine refractoriness was associated with significantly lower CR and nPR rates compared with patients not refractory to fludarabine (CR/nPR = 8% vs 46%, P = .023). Prior exposure to rituximab did not negatively impact outcome with FCR treatment (Table 1).
Four patients received prior FCR after 20, 30, 50, and 55 months from prior FCR. The responses included 1 CR, 1 nPR, and 2 patients with no objective response; 3 of 4 patients have progressed or died at 4, 16, and 31 months. One patient was treated for minimal residual disease with lenalidomide after 9 months and remains alive and progression-free 22 months after FCR therapy.
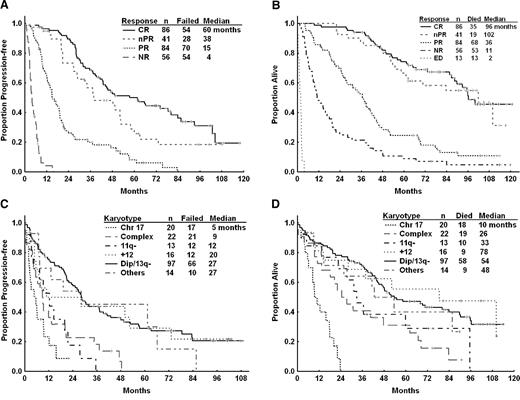
Patients were ranked according to karyotype risk group using a similar hierarchical classification system used by Dohner et al22 such that patients with abnormality of chromosome 17 were ranked in the highest risk group and patients with diploid karyotype or 13q deletion were in the lowest-risk group. Patients with more than 2 cytogenetic abnormalities other than an abnormality in chromosome 17 were classified as complex, and patients with karyotypic abnormalities not included in the Dohner classification were grouped as “others” (Table 1). Patients with diploid karyotype or 13q deletion as a sole abnormality by metaphase karyotype had the highest likelihood for response with an OR of 82% and 34% of patients achieving CR. In contrast, patients with an abnormal chromosome 17 by metaphase karyotype (n = 20) demonstrated particularly poor responses to therapy with an ORR of 35% and only 1 patient (5%) who achieved nPR (Table 1).
Superior outcomes for time-to-event endpoints were observed for patients who achieved CR or nPR. Therefore, we analyzed characteristics associated with a higher likelihood of CR or nPR by univariable analyses, as shown in supplemental Table 1. In a multivariable model, pretreatment characteristics independently associated with a higher likelihood of achieving CR or nPR were younger age, lower β-2 microglobulin (β2M), higher hemoglobin, no fludarabine refractoriness, lower number of prior treatments, and higher platelet count (supplemental Table 3). Pretreatment characteristics associated with a higher likelihood of OR by univariable analysis are shown in supplemental Table 2. In a multivariable model, patients not resistant to fludarabine, with higher hemoglobin and platelet count, lower serum creatinine and absence of chromosome 17 or complex cytogenetic abnormalities had a higher probability of achieving an overall response (supplemental Table 3).
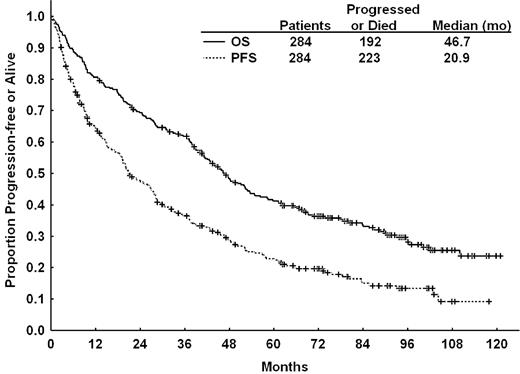
Progression-free survival
The estimated median PFS was 20.9 months (95% confidence interval [CI], 18.8-27.6 months) for the whole cohort of patients (n = 284). At the time of analysis, 223 patients (79%) experienced disease progression or died (Figure 1). The estimated median PFS for patients achieving CR was 60 months compared with 38 months for patients achieving nPR (P = .076) and 15 months for those achieving PR (P < .001; Figure 2A). Pretreatment characteristics that correlated with longer PFS by univariable analysis are displayed in supplemental Table 4. In multivariable analysis, pretreatment patient characteristics independently associated with lower risk of disease progression or death included lower serum creatinine, lower lactate dehydrogenase (LDH), higher platelet count, higher serum albumin, lower number of prior treatments and fludarabine sensitivity (Table 4). The presence of unfavorable chromosome abnormalities (11q deletion, chromosome 17, or complex abnormalities) was also associated with shorter PFS (Figure 2C). After including karyotype in the regression analysis (n = 177), the following were independently associated with longer PFS: lack of unfavorable karyotype, lower serum creatinine, higher platelets, lower number of prior treatments, and no prior refractoriness to fludarabine (Table 4).
FCR overall and progression-free survival. Overall survival (OS) and progression-free survival (PFS) for all relapsed/refractory patients treated with fludarabine, cyclophosphamide, and rituximab.
FCR overall and progression-free survival. Overall survival (OS) and progression-free survival (PFS) for all relapsed/refractory patients treated with fludarabine, cyclophosphamide, and rituximab.
Overall and progression-free survival by subgroups. (A) Progression free survival (PFS) by NCI-WG response for relapsed/refractory patients treated with FCR (n = 267). Early deaths (n = 13) shown in panel B. (B) Overall survival (OS) by NCI-WG response for relapsed and refractory patients treated with FCR (n = 280). Four patients were not evaluable for response. (C) PFS by karyotype for relapsed/refractory patients treated with FCR (n = 182). (D) OS by karyotype for relapsed/refractory patients treated with FCR (n = 182). CR indicates complete remission; nPR, nodular PR; PR, partial remission; NR, no objective response; ED, early death; Chr 17, chromosome 17 abnormalities; 11q−, 11q deletion; +12, trisomy 12; and Dip/13q−, diploid or 13q deletion.
Overall and progression-free survival by subgroups. (A) Progression free survival (PFS) by NCI-WG response for relapsed/refractory patients treated with FCR (n = 267). Early deaths (n = 13) shown in panel B. (B) Overall survival (OS) by NCI-WG response for relapsed and refractory patients treated with FCR (n = 280). Four patients were not evaluable for response. (C) PFS by karyotype for relapsed/refractory patients treated with FCR (n = 182). (D) OS by karyotype for relapsed/refractory patients treated with FCR (n = 182). CR indicates complete remission; nPR, nodular PR; PR, partial remission; NR, no objective response; ED, early death; Chr 17, chromosome 17 abnormalities; 11q−, 11q deletion; +12, trisomy 12; and Dip/13q−, diploid or 13q deletion.
Multivariate Cox proportional hazards models for OS and PFS
| Pretreatment characteristic . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| PFS (n = 278) | |||
| Serum creatinine, mg/L | 1.5 | 1.1-2.0 | .0034 |
| Ln(platelets, ×109/L) | 0.62 | 0.49-0.80 | < .001 |
| Ln(LDH, IU/L) | 1.7 | 1.2-2.5 | .0040 |
| Ln(albumin, g/dL) | 0.14 | 0.044-0.42 | < .001 |
| No. of prior treatments | 1.12 | 1.02-1.23 | .017 |
| Fludarabine refractory vs others | 1.9 | 1.3-2.8 | < .001 |
| PFS (with cytogenetic analysis, n = 177) | |||
| Serum creatinine, mg/L | 2.0 | 1.2-3.2 | .0074 |
| Ln(platelets, ×109/L) | 0.66 | 0.49-0.88 | .0043 |
| Abnormality of chromosome 17 | 4.6 | 2.5-8.2 | < .001 |
| Complex karyotype, not chromosome 17* | 2.6 | 1.5-4.4 | < .001 |
| 11q deletion | 3.0 | 1.6-5.7 | < .001 |
| No. of prior treatments | 1.12 | 1.009-1.25 | .033 |
| Fludarabine refractory vs others | 2.3 | 1.5-3.5 | < .001 |
| OS (with cytogenetic analysis, n = 182) | |||
| Age, y | 1.03 | 1.005-1.05 | .019 |
| Serum creatinine, mg/L | 2.3 | 1.4-3.8 | .0020 |
| Ln(platelets, ×109/L) | 0.59 | 0.44-0.80 | < .001 |
| Abnormality of chromosome 17 | 5.2 | 2.8-9.6 | < .001 |
| Complex karyotype, not chromosome 17* | 1.9 | 1.1-3.2 | .015 |
| Prior treatments (> 3 vs 3 or less) | 1.7 | 1.008-2.6 | .047 |
| Fludarabine refractory vs others | 1.8 | 1.16-2.7 | .0082 |
| Pretreatment characteristic . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| PFS (n = 278) | |||
| Serum creatinine, mg/L | 1.5 | 1.1-2.0 | .0034 |
| Ln(platelets, ×109/L) | 0.62 | 0.49-0.80 | < .001 |
| Ln(LDH, IU/L) | 1.7 | 1.2-2.5 | .0040 |
| Ln(albumin, g/dL) | 0.14 | 0.044-0.42 | < .001 |
| No. of prior treatments | 1.12 | 1.02-1.23 | .017 |
| Fludarabine refractory vs others | 1.9 | 1.3-2.8 | < .001 |
| PFS (with cytogenetic analysis, n = 177) | |||
| Serum creatinine, mg/L | 2.0 | 1.2-3.2 | .0074 |
| Ln(platelets, ×109/L) | 0.66 | 0.49-0.88 | .0043 |
| Abnormality of chromosome 17 | 4.6 | 2.5-8.2 | < .001 |
| Complex karyotype, not chromosome 17* | 2.6 | 1.5-4.4 | < .001 |
| 11q deletion | 3.0 | 1.6-5.7 | < .001 |
| No. of prior treatments | 1.12 | 1.009-1.25 | .033 |
| Fludarabine refractory vs others | 2.3 | 1.5-3.5 | < .001 |
| OS (with cytogenetic analysis, n = 182) | |||
| Age, y | 1.03 | 1.005-1.05 | .019 |
| Serum creatinine, mg/L | 2.3 | 1.4-3.8 | .0020 |
| Ln(platelets, ×109/L) | 0.59 | 0.44-0.80 | < .001 |
| Abnormality of chromosome 17 | 5.2 | 2.8-9.6 | < .001 |
| Complex karyotype, not chromosome 17* | 1.9 | 1.1-3.2 | .015 |
| Prior treatments (> 3 vs 3 or less) | 1.7 | 1.008-2.6 | .047 |
| Fludarabine refractory vs others | 1.8 | 1.16-2.7 | .0082 |
CI indicates confidence intervals; Ln, natural logarithm; LDH, lactate dehydrogenase; PFS, progression-free survival; and OS, overall survival.
Complex karyotype excluding abnormalities of chromosome 17.
Time to progression and minimal residual disease
Among 211 patients achieving PR or better, 131 (62%) have progressed with a median TTP of 37 months. Higher number of prior treatments (hazard ratio [HR] 1.4, P < .001), chromosome 17 abnormalities (HR 4.3, P = .017), 11q deletion (HR 2.9, P = .0087) and shorter time from last therapy in months (HR 0.99, P = .040) were independently associated with shorter TTP by multivariate regression analysis. Because IGHV mutation testing was not readily available at the initiation of the study only 72 of 211 responders had IGHV mutation analysis results. In patients who achieved a remission, the presence of unmutated IGHV gene was associated with significantly shorter TTP compared with mutated IGHV gene (28 vs 84 months, n = 51 vs n = 21, P = .0044). We did not incorporate IGHV in a multivariate analysis of factors associated with shorter TTP because of the high number of missing values.
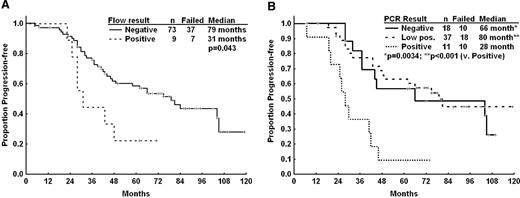
The association of MRD status by flow or PCR was correlated with clinical outcomes for patients achieving CR but not patients achieving PR or nPR. Of 86 patients who achieved CR, 82 had available flow cytometry MRD results and 66 patients had PCR MRD data. Patients in CR who were flow MRD-negative experienced longer remission duration compared with flow MRD–positive patients (Figure 3A). By IGHV PCR, patients who were negative or low positive also had significantly longer TTP than patients with positive PCR MRD results (Figure 3B). However, there was no significant difference in OS for patients achieving PCR or flow MRD–negative status (data not shown). There was no difference in TTP or OS according to flow or PCR MRD status in patients whose best responses were PR or nPR.
Time to progression by MRD. (A) Time-to-progression by flow cytometry (Flow) minimal residual disease (MRD) status for patients achieving a complete response (n = 82 evaluable). (B) Time-to-progression by polymerase chain reaction (PCR) MRD status for patients achieving a complete response (n = 66 evaluable).
Time to progression by MRD. (A) Time-to-progression by flow cytometry (Flow) minimal residual disease (MRD) status for patients achieving a complete response (n = 82 evaluable). (B) Time-to-progression by polymerase chain reaction (PCR) MRD status for patients achieving a complete response (n = 66 evaluable).
Overall survival
Among 284 patients, 192 (68%) have died with a median follow-up time for all patients of 43 months (range, 0-122 months). The estimated median survival time for all patients was 46.7 months (95% CI, 41.2-53.4 months; Figure 1) and 100 months for patients who achieved CR or nPR (Figure 2B). Pretreatment characteristics associated with longer survival by univariable analyses are shown in supplemental Table 5. The presence of unfavorable chromosome abnormalities was associated with shorter OS including patients with chromosome 17 abnormalities who had a median OS of 10 months (Figure 2D). We constructed 2 multivariate models for OS, excluding or including cytogenetic analysis (n = 278 or n = 182, respectively). The pretreatment patient characteristics independently associated with shorter OS in the former model were older age, higher serum creatinine, higher LDH, lower platelet count, lower serum albumin, and refractoriness to fludarabine (data not shown). Including results of cytogenetic analysis in the multivariate model; older age, presence of chromosome 17 or complex cytogenetic abnormalities, lower platelet count, higher serum creatinine, refractoriness to fludarabine, and more than 3 prior treatments were independently associated with shorter survival (Table 4). Causes of mortality are detailed in supplemental Table 6. Infection and progressive CLL were the most common causes of mortality.
Number of courses of therapy to achieve a response
Of 224 patients who received at least 3 courses of FCR, 211 had an evaluable response assessment after 3 courses, including 29 CR (14%), 39 nPR (18%), 112 PR (53%), and 31 patients with no objective response. There were 142 patients with response evaluation both after 3 courses and 5 or more courses of therapy; 66 (46%) patients improved their response including 28 from PR to CR, 19 PR to nPR, 19 nPR to CR and 3 achieving a PR. Because of the small patient numbers confirmed to be in MRD-negative CR (n = 31) after 3 courses, our study was not powered to detect differences in remission duration or survival between patients who received 6 courses of FCR and those patients who received less.
Toxicity
One hundred twenty patients (42%) completed all 6 courses of FCR, 103 (36%) received full dose; 169 (60%) received more than 3 courses. At least 1 dose reduction was required for 42 patients. Patients older than 70 years were significantly less likely to complete 3 or more courses of therapy compared with younger patients, with 5 of 40 patients (13%) over the age of 70 years completing 6 courses and 11 (28%) receiving more than 3 courses of FCR (Figure 4). The most common reasons for early treatment cessation were myelosuppression, infection, and lack of response (supplemental Table 7). In a multivariate analysis, patient pretreatment characteristics independently associated with lower likelihood of completing all 6 courses included having received more than 3 prior chemotherapy regimens and age older than 70 years. Patients who had received up to 3 prior regimens were also less likely to complete 6 courses of therapy if they had Rai stage IV disease before FCR; however, Rai stage was not an independent factor in patients who had received more than 3 prior therapies.
Percentage of patients completing courses of FCR. Patients have been divided into subgroups of age 70 years or younger and age over 70 years. Significant differences in proportion of patients per age group completing corresponding courses of therapy denoted by asterisks (*P < .05, **P < .001).
Percentage of patients completing courses of FCR. Patients have been divided into subgroups of age 70 years or younger and age over 70 years. Significant differences in proportion of patients per age group completing corresponding courses of therapy denoted by asterisks (*P < .05, **P < .001).
The most common toxicity was hematologic, with grade 3 and 4 neutropenia complicating 22% and 34% of treatment courses, respectively. The rate of grade 3 or 4 neutropenia remained consistent across courses. Grade 3 and 4 thrombocytopenia was associated with 12.5% and 7.0% of treatment courses, respectively. By CTC version 2.0 criteria, grade 2 anemia was associated with 11.2% of treatment courses; grade 3 or 4 anemia was rare (0.46%). Using CTC version 2.0 criteria, anemia is calculated as a proportional decrease from baseline and therefore may underestimate proportion of anemia with hemoglobin less than 8 g/dL. Using CTC version 3.0 grading criteria for anemia, grade 3 and 4 anemia was noted in 7.1% and 4.7% of treatment courses, respectively (supplemental Table 8). There were 18 (6%) patients who developed clinically significant autoimmune hemolytic anemia that required treatment during or after therapy including 3 patients who had evidence of active hemolysis before therapy.
Serious infections were defined as grade 3 or 4 infections by CTC version 2.0 grading criteria. The most common serious infections were sepsis and pneumonia, associated with 1.0% and 3.3% of courses, respectively. Forty-six patients (16%) experienced 1 or more episode of pneumonia or sepsis associated with treatment. Pretreatment variables associated with serious infection by univariable analysis were serum albumin (HR 0.02, P < .001) or absolute neutrophil count (HR 0.87, P = .027). Low albumin was the only variable independently associated with an increased risk of infection. Minor infections and fever of unknown origin occurred with 8.5% and 10.3% of treatment courses. Herpes simplex virus and herpes zoster reactivation were associated with 0.8% and 0.7% of courses, respectively. Elderly patients were more likely to discontinue therapy before 6 courses following an infectious event; 24% of patients 70 years or older discontinued therapy following an infection compared with 9% of younger patients (supplemental Table 9).
Twelve patients (4.2%) developed Richter transformation after FCR with a median time from initiation of treatment to transformation of 17 months (2-81 months). Nine of these 12 patients have died with an estimated median OS of 40 months from FCR (9-84 months). Nine patients (3%) developed secondary MDS or acute myeloid leukemia (n = 1) with a median of 20 months from starting FCR. Eight of these 9 patients died with a median OS time of 23 months from FCR therapy.
Historic comparison with combined fludarabine and cyclophosphamide
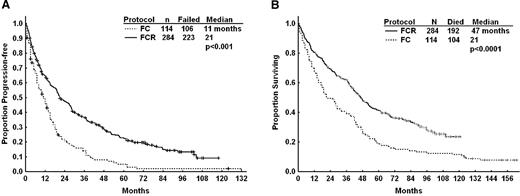
We compared the clinical responses, PFS (Figure 5A), and OS (Figure 5B) of patients in this study with a historical cohort of 114 relapsed patients who received FC as salvage therapy. Pretreatment characteristics with known prognostic significance were not significantly different between the FCR versus FC patients including median age, platelet count, LDH, and creatinine, or proportion with advanced clinical stage, ≥ 4 prior treatment regimens (data not shown); however, there was a trend toward a higher proportion of fludarabine-refractory patients in the FC versus FCR cohort (29% vs 19%, P = .059). The ORR was similar for patients treated with FCR versus FC (75% vs.68%, P = .13) but FCR achieved a higher quality of response (CR or nPR, 46% vs 23%, P < .001). Patients receiving FCR also experienced longer PFS (21 months vs 11 months, P < .001; Figure 5A) and overall survival (47 months vs 21 months, P < .001; Figure 5B) compared with FC. Although FCR was associated with a significant improvement in PFS in patients not refractory to fludarabine (28 months vs 13 months, P < .0001), there was no significant improvement in median PFS in patients who were refractory to fludarabine (8 months vs 4 months, P = .23). Patients who had received up to 3 prior treatments experienced improved median PFS and OS compared with FC (PFS: 28 vs 12 months, P < .001; OS: 52 vs 28 months, P < .001) whereas patients who had more than 3 prior treatments did not benefit significantly in either PFS (P = .99) or OS (P = .24). After adjusting for pretreatment characteristics by multivariate regression analysis, FCR remained one of the strongest predictors of PFS (HR 0.48, P < .001) with presence of chromosome 17 abnormality or complex karyotype (HR 2.24, P < .001), β-2-microglobulin (HR 1.6, P < .021), hemoglobin (HR 0.89, P < .0083), number of prior treatments (HR 1.10, P = .045), and fludarabine refractoriness (HR 1.7, P = .0034) as significant covariates.
Progression-free and overall survival for patients receiving fludarabine and cyclophosphamide (FC), and FCR as salvage therapy at MD Anderson Cancer Center. (A) Progression-free survival (PFS) for relapsed/refractory patients treated with FCR versus a historic group treated with fludarabine and cyclophosphamide (FC). (B) Overall survival (OS) for relapsed/refractory patients treated with FCR versus a historic group treated with FC.
Progression-free and overall survival for patients receiving fludarabine and cyclophosphamide (FC), and FCR as salvage therapy at MD Anderson Cancer Center. (A) Progression-free survival (PFS) for relapsed/refractory patients treated with FCR versus a historic group treated with fludarabine and cyclophosphamide (FC). (B) Overall survival (OS) for relapsed/refractory patients treated with FCR versus a historic group treated with FC.
Discussion
The FCR combination is an effective regimen for treatment of relapsed patients with CLL. The estimated median PFS of 21 months compares favorably with published data of therapies for relapsed patients including purine analog combinations.23-25 Historical comparison of patients with relapsed or refractory CLL at MDACC, demonstrated improvement in quality of responses, duration of response and OS with FCR therapy, compared with FC9 ; this is confirmed in this updated analysis. These results are supported by the phase 3 randomized REACH study demonstrating superior PFS for patients in first relapse treated with FCR versus FC.
We identified subgroups of patients with favorable outcomes following treatment with FCR. These analyses aimed to identify relapsed patients for whom FCR was beneficial. This is particularly important because the REACH trial excluded patients who had received more than 1 prior treatment and those previously treated with either FC or rituximab. Patients in our study who had received 3 or fewer prior therapies, not resistant to fludarabine or patients without high-risk cytogenetic abnormalities (presence of chromosome 17 or complex cytogenetic abnormalities) demonstrated better response duration and survival after therapy.
To explore the effect of prior therapy on response, we developed a hierarchical model exploring the likelihood of response according to the intensity of prior treatment. Patients who were previously exposed to antibody therapy, including rituximab, or purine analogues but not alkylating agents had the highest response rates and survival. Therefore, these patients are ideal candidates for salvage therapy with FCR. In our hierarchy, patients previously exposed to fludarabine and alkylating agents, either sequentially or concurrently, demonstrated acceptable response rates as long as they were not fludarabine-refractory. In these patients, FCR would still be considered a reasonable treatment option. Patients refractory to fludarabine or those who had received more than 3 prior therapies experienced short PFS. Such patients are likely to demonstrate poor responses with currently available chemotherapy regimens and should be considered for investigational therapies or stem cell transplantation when available.13,25
Patients with 11q deletion demonstrated shorter PFS and OS compared with low-risk cytogenetic groups; however, in the REACH trial, patients with 11q deletion by FISH experienced significantly longer PFS after FCR compared with FC.13 The benefit of adding rituximab to FC in patients with 11q deletion was also noted in the GCLLSG frontline CLL8 trial.15 In our study, 11q deletion was defined by metaphase karyotype whereas in the 2 randomized trials 11q deletion was identified by fluorescence in situ hybridization (FISH) analysis. In addition, patients with 11q deletion in our study were a highly pretreated group of patients who had received a median of 3 prior treatments. This small subgroup of patients is therefore not comparable with the patients in the REACH trial. Based on the results of the REACH study patients with 11q deletion should be considered for salvage therapy with FCR.
The lack of data on newer prognostic markers is a limitation of this study. Unmutated IGHV gene status was independently associated with shorter PFS and TTP in a multivariable analysis; however, we did not incorporate this into our multivariate model because of the high number of missing values. There were an insufficient number of patients with FISH or ZAP70 results for meaningful multivariable statistical analyses.
Because of the age of this study, few patients in our study had received prior FCR; however, we demonstrate in this study that FCR is an effective therapy after FC if patients were not refractory to this therapy. In a recent analysis of patients who relapsed after the frontline FCR study by Keating et al, patients who were retreated with FCR as first salvage experienced durable responses if they experienced a response duration of at least 3 years after the initial regimen.26 Retreatment with FCR should be considered cautiously in elderly patients with CLL because of the potential for prolonged myelotoxicity. From this data, younger patients with an initial response for at least 3 years after frontline FCR may be successfully re-treated with salvage FCR.
This study was not designed to assess the optimal number of courses of FCR for patients with relapsed CLL. All patients were expected to receive 6 courses unless they had toxicity or lack of response, therefore comparison of patients who completed therapy before 6 courses to those who completed planned therapy is confounded by selection of patients with toxic events or inadequate responses causing early cessation of treatment. A controlled clinical trial should be conducted to explore the optimal number of courses of FCR in patients who achieve a flow cytometry–negative CR after 3 courses. This is of particular relevance in the relapsed setting as cumulative myelotoxicity following chemotherapy may have a relatively greater impact on survival than in frontline therapy. A significant number of patients who achieved a PR after 3 courses of therapy did improve their response to nPR or CR after 5 or 6 courses of therapy. In the absence of further evidence, it is reasonable to continue therapy for patients who tolerate therapy and achieve a PR after 3 courses.
MRD measurement has become an increasingly important tool in determining response to chemotherapy or chemoimmunotherapy in CLL. MRD negativity using high-sensitivity flow cytometry following therapy has been associated with longer time to progression and survival after therapy.27-30 Although we used flow cytometry to further assess patients with complete remissions; our 2-color flow cytometry assay is limited by low sensitivity in identifying residual CLL cells compared with newer protocols which are able to detect around 1 in 10 000 residual CLL cells. This lack of sensitivity and in particular the low numbers of flow-positive CR limited our ability to detect significant differences in survival outcomes between MRD-positive and -negative patients. MRD assessment was also performed using a PCR assay of higher sensitivity than 2-color flow cytometry. The results of this assay were also limited by the lower number of patients with MRD-positive CR. In addition, computer-assisted tomography scans were not performed to confirm responses which may have led to patients assessed clinically as CR as having residual CLL in abdominal or other lymph node areas. Future studies assessing outcomes in MRD patients should use newer more sensitive and standardized approaches for MRD measurement as described by the European Research Initiative on CLL (ERIC).31
Patients over 70 years were underrepresented in this study. Elderly patients were less likely to complete more than 3 courses of FCR, had a lower CR rate, and experienced more infectious complications. Fludarabine-based combinations are associated with high incidence of myelosuppression and toxicity in elderly patients.32 Patients in this study did not routinely receive growth factor support and this may have played a role in the ability of this group of patients to tolerate therapy. Although patients were required to have good performance status, assessment of comorbidities was not required for protocol entry and may also impact the ability of elderly patients to tolerate FCR. Further research is needed to determine the role of dose-reduced FCR,33 pentostatin, cyclophosphamide, and rituximab24 or bendamustine and rituximab34 in elderly patients with relapsed CLL. We recommend this regimen be used with caution in patients older than the age of 70, with assessment of comorbidity status, dose adjustments, and consideration of growth factor support.35-37
FCR is an effective and safe therapy in patients without high-risk features (refractory to therapy or chromosome 17 abnormalities). Whereas the REACH trial confirmed the benefit gained by the addition of rituximab in CLL patients in first salvage after single-agent (F or C) therapy,13 we demonstrate its effectiveness in patients with up to 3 prior treatments and patients who have received prior FC combination therapy. Further research is required to determine optimal salvage strategies for high-risk and elderly patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the contribution of physicians within The University of Texas M. D. Anderson Cancer Center and in the community who assisted with the clinical care of patients on this trial, and Dr T. Kipps and the CLL Research Consortium (CRC), University of California–San Diego (La Jolla, CA) for provision of data on ZAP70 and IGHV mutation status.
W.G.W. is a Leukemia & Lymphoma Society Clinical Scholar.
Authorship
Contribution: X.C.B. analyzed results and wrote the paper; M.J.K. and W.G.W. designed, performed, and analyzed the trial and coauthored the paper; X.W. analyzed data and coauthored paper; S.M.O., A.F., S.F., J.B., C.K., and H.K. provided clinical care to patients, assisted in the analysis of data and development of critical themes, and coauthored the paper; and S.L. collected and verified patient information, analyzed data, and coauthored the paper.
Conflict-of-interest disclosure: M.J.K. has served as consultant and received honoraria from Hoffmann-La Roche, Genzyme, and Genentech. S.M.O. has received consultancy honoraria and research funding from Biogen-Idec and Genentech. A.F. has received speaker's honoraria and research funding from Genentech. W.G.W. is the principal investigator and has received speaker's honoraria from Genentech, Genzyme, and Hoffmann-La Roche, and served as consultant on scientific advisory boards for Genentech and Genzyme, and the data safety monitoring board for Genentech. The remaining authors declare no competing financial interests.
Correspondence: William G. Wierda, Leukemia Department, Unit 428, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: wwierda@mdanderson.org.