Key Points
IL-1 activates signaling and promotes proliferation of primitive CML cells.
IL1RAP antibodies block IL-1–induced effects and mediate cell killing in chronic and blast phase CML in vivo models.
Abstract
Chronic myeloid leukemia (CML) is currently treated with tyrosine kinase inhibitors, but these do not effectively eliminate the CML stem cells. As a consequence, CML stem cells persist and cause relapse in most patients upon drug discontinuation. Furthermore, no effective therapy exists for the advanced stages of the disease. Interleukin-1 receptor accessory protein (IL1RAP; IL1R3) is a coreceptor of interleukin-1 receptor type 1 and has been found upregulated on CML stem cells. Here, we show that primitive (CD34+CD38−) CML cells, in contrast to corresponding normal cells, express a functional interleukin-1 (IL-1) receptor complex and respond with NF-κB activation and marked proliferation in response to IL-1. IL1RAP antibodies that inhibit IL-1 signaling could block these effects. In vivo administration of IL1RAP antibodies in mice transplanted with chronic and blast phase CML cells resulted in therapeutic effects mediated by murine effector cells. These results provide novel insights into the role of IL1RAP in CML and a strong rationale for the development of an IL1RAP antibody therapy to target residual CML stem cells.
Introduction
Chronic myeloid leukemia (CML) is characterized by the BCR/ABL1 fusion gene, resulting from a translocation between chromosomes 9 and 22.1,2 Left untreated, CML progresses from an initial chronic phase (CP) characterized by an exaggerated expansion of mature myeloid cells, into a blast phase (BP) that resembles myeloid or lymphoid acute leukemia with a median survival of only a few months.2 Although most cases of CP CML are successfully managed using tyrosine kinase inhibitors (TKIs), these regimens fail to provide a cure in the majority of patients.3-5 This has been attributed to incomplete eradication of the leukemic stem cells, which are insensitive to TKI treatment.6-8 Thus, even though cessation of TKI therapy has been successfully tried in small cohorts of well-responding patients, the majority still depend on lifelong TKI administration with potential adverse side effects.3-8 In addition, the leukemic stem cells may acquire genetic changes leading to relapse and disease progression,9 and in the advanced BP, TKI treatment does not lead to a sustained remission.10 Hence, there is a need for new therapeutic strategies that more efficiently target the CML stem cells, preferably through a kinase-independent mode of action.
Interleukin-1 receptor accessory protein (IL1RAP; IL1R3) is a coreceptor involved in several signaling pathways including interleukin-1 (IL-1), IL-33, IL-36G, and stem cell factor (SCF).11,12 Signaling by IL-1 requires the formation of a cell surface receptor complex involving IL-1, interleukin-1 receptor type 1 (IL1R1), and IL1RAP. Lack of IL1RAP completely abrogates cellular response to IL-1.13 We have previously shown that IL1RAP is expressed on the cell surface of candidate CML stem cells but not on normal hematopoietic stem cells (HSCs).14 IL1RAP is also upregulated on primitive cells in acute myeloid leukemia and myelodysplastic syndrome.15,16 Moreover, IL1RAP antibodies can induce cell killing of primitive CML and acute myeloid leukemia cells through antibody-dependent cellular cytotoxicity (ADCC).14,15,17 Collectively, these results suggest that targeting IL1RAP is a promising approach to kill leukemic stem cells, but the therapeutic potential of IL1RAP antibodies in CML has so far not been evaluated in vivo.
Herein, we have investigated the effects of IL1RAP antibodies on primitive CML cells and the mechanisms underlying these effects. We report that IL-1-stimulation selectively induces signaling and expansion of primitive CML cells, an effect that could be inhibited by IL1RAP antibodies. In addition, the IL1RAP antibodies displayed in vivo therapeutic efficacy by effector cell–mediated cell killing. These results show that an antibody-based therapy against IL1RAP can be used to efficiently target CML stem cells.
Materials and methods
Generation of IL1RAP antibodies
The monoclonal IL1RAP antibodies mAb81.2 and mAb3F8 were generated by standard hybridoma technique as previously described.15 Fab81.2 was generated by enzymatic cleavage of the Fc-domain of mAb81.2. Polyclonal anti-murine Il1rap antibodies were produced by immunizing rabbits with murine Il1rap and confirmed to bind Il1rap by enzyme-linked immunosorbent assay.
Cell cultures
Healthy volunteers and CML patients donated bone marrow (BM) or peripheral blood (PB) after giving their informed consent. The protocol was in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee. Mononuclear cells (MNCs) were isolated using Lymphoprep (Axis-Shield) and enriched for CD34+ cells by magnetic bead separation (Miltenyi Biotech). Primary cells and BV173 cells (DSMZ) were cultured in medium with or without the addition of cytokines and IL1RAP antibodies as described in the supplemental Methods (available on the Blood Web site). Cells were counted with CountBright beads (Life Technologies). Viable, apoptotic, or dead cells were detected by staining with annexin V and 7-amino-actinomycin D.
Flow cytometry and cell sorting
Fluorescence activated cell sorting (FACS) and flow cytometry was performed on FACS Aria IIu, FACS Canto II, or FACS Fortessa flow cytometers (Becton Dickinson). Geometric mean fluorescence intensity was used to quantify staining intensity. A detailed list of the antibodies used for analysis of human and murine cells is provided in the supplemental Methods.
RNA sequencing and quantitative real-time polymerase chain reaction
For the RNA-sequencing studies and gene set enrichment analysis (GSEA), CD34+CD38− CP CML cells were stimulated with 100 ng/mL IL-1B for 3 or 24 hours before RNA extraction. Sequencing libraries were prepared using Nextera XT (Illumina) and sequenced on a NextSequation 500 (Illumina). Measurement of BCR/ABL1 expression and validation of RNA-sequencing data were performed using custom-ordered probes (Applied Biosystems) with eukaryotic 18S RNA as an endogenous control. Details on the analyses are provided in supplemental Methods.
Antibody treatment studies
Animal experiments were approved by the local animal ethics committee, and mice purchased from Jackson Laboratories. Immunodeficient mice were transplanted with CML cells by tail vein injections. IL1RAP antibodies or isotype control antibodies were administered by intraperitoneal injections biweekly. The mice were euthanized upon signs of serious illness or because of hind limb paralysis in survival studies and at fixed end points for efficacy studies. A detailed description of the antibody treatment studies is provided in the supplemental Methods.
ADCC and ADCP assays
Human natural killer (NK) cells or monocytes were isolated from PB MNCs using magnetic beads. Target cells were stained with PKH26 (Sigma Aldrich) and preincubated with mAb81.2, mAb3F8, or hIgG1 control antibodies. For ADCC analysis, NK cells were used at a 10:1 effector cell/target cell ratio. For antibody-dependent cell phagocytosis (ADCP), monocytes were matured into macrophages using macrophage colony-stimulating factor (Peprotech) and added at a 1:1 effector cell/target cell ratio. The number of dead target cells (ADCC-effect) or PHK26-positive macrophages (ADCP-effect) was determined by flow cytometry following overnight or 90-minute incubation, respectively.
Il1rap-deficient mice and competitive BM transplantations
Il1rap−/− B6;129S1-Il1raptm1Roml/J mice (Jackson Laboratory) were backcrossed into C57 black mice. For competitive BM transplantations, BM cells from Il1rap−/− or Il1rap+/+ littermate control mice (CD45.2) were mixed with equal numbers of B6.SJL (CD45.1) competitor cells and transplanted into lethally irradiated C57xB6.SJL (CD45.1/CD45.2) mice. Primary mice were analyzed 16 weeks, and secondary recipients 10 weeks after transplantation.
Statistical analyses
Prism (GraphPad Software) was used for statistical analyses. Statistical differences were significant if P < .05, and, unless otherwise stated, were tested by 2-tailed Student t test or Mantel Cox log-rank test. All data are presented as mean ± standard error of the mean.
Results
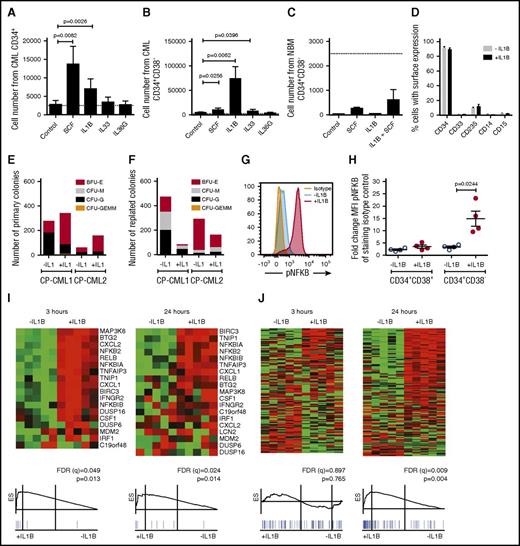
Primitive CP CML cells proliferate and activate NF-κB in response to IL-1B stimulation
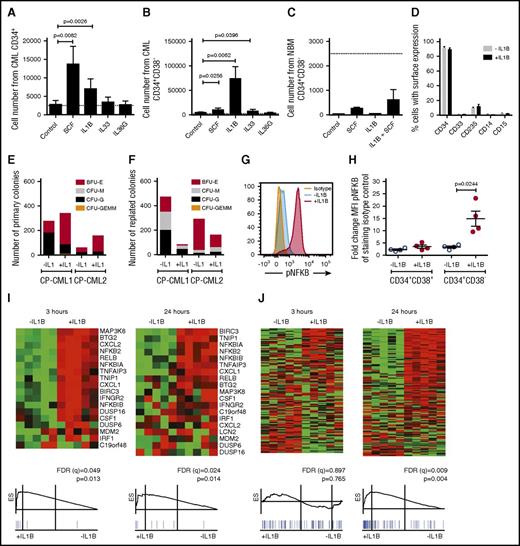
We hypothesized that the upregulation of IL1RAP on primitive (CD34+CD38−) CML cells14 may increase their sensitivity to cytokines. To this end, we stimulated CP CML cells with IL-1B, IL-33, IL-36, or SCF. Both IL-1B and SCF induced a moderate expansion of CD34+ CML progenitor cells (Figure 1A; supplemental Figure 1A). Notably, IL-1B stimulation of CD34+CD38− CML cells resulted in a dramatic 30-fold increase in cell numbers, whereas corresponding normal cells only responded weakly (Figure 1B-C; supplemental Figure 1B-C). The IL-1B-induced expansion of CD34+CD38− CML cells could be suppressed by the addition of IL-1 receptor antagonist (IL1RA; supplemental Figure 1D). IL-1A induced a similar expansion of CML CD34+CD38− cells as IL-1B (supplemental Figure 1E). In spite of the rapid expansion induced by IL-1B, >90% of the seeded CD34+CD38− CML cells retained an immature CD34+ phenotype (Figure 1D). In agreement with previous findings, IL1RAP and IL1R1 were expressed on CD34+CD38− CML cells, whereas corresponding normal cells had low expression of IL1R1 and lacked expression of IL1RAP (supplemental Figure 1F).14,18 IL-1B stimulation of CD34+CD38− CML cells increased the number of primary colonies (Figure 1E). Following replating, IL-1B–stimulated cells generated fewer colonies, but 1 sample displayed a slight increase in myeloid colony numbers (CFU-G and CFU-M; Figure 1F). Normal BM cells generated fewer replated colonies in the presence of IL-1B (supplemental Figure 1G-H).
IL-1B potently stimulates primitive CP CML cells. (A-C) Cells from CML BM (n = 3) or normal BM (n = 3) were sorted based on CD34 and CD38 expression and counted after 7 days culture in serum-free medium supplemented with indicated cytokines. The dotted lines represent seeded cell numbers. Cell numbers from seeded CD34+ CML cells (A), CD34+CD38− CML cells (B), and CD34+CD38− normal BM cells (C). (D) Flow cytometric analysis of cell surface markers following the 7-day culture of CD34+CD38− CML cells in the presence or absence of IL-1B. (E-F) Colony formation of CD34+CD38− CML cells plated in methylcellulose with or without the addition of IL-1B. Primary colony numbers (E) and colony numbers following replating (F). (G-H) CD34+ CML cells were stimulated with IL-1B for 15 minutes and analyzed for phosphorylation of NF-κB within the CD34+CD38+ and CD34+CD38− cell populations by phospho-flow cytometry. The histogram (G) shows the phosphorylation levels in the CD34+CD38− cells from a representative sample, and the diagram (H) summarizes data from 4 patients. (I-J) CD34+CD38− CML cells from 5 patients were incubated in the presence or absence of IL-1B for 3 or 24 hours and analyzed by RNA-sequencing and GSEA. NF-κB target genes (I) and cell cycle–associated genes (J). For complete gene list, see supplemental Tables 1-5. BFU-E, burst forming unit–erythroid; CFU-G, colony forming unit–granulocyte; CFU-GEMM, colony forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte; CFU-M, colony forming unit–macrophage.
IL-1B potently stimulates primitive CP CML cells. (A-C) Cells from CML BM (n = 3) or normal BM (n = 3) were sorted based on CD34 and CD38 expression and counted after 7 days culture in serum-free medium supplemented with indicated cytokines. The dotted lines represent seeded cell numbers. Cell numbers from seeded CD34+ CML cells (A), CD34+CD38− CML cells (B), and CD34+CD38− normal BM cells (C). (D) Flow cytometric analysis of cell surface markers following the 7-day culture of CD34+CD38− CML cells in the presence or absence of IL-1B. (E-F) Colony formation of CD34+CD38− CML cells plated in methylcellulose with or without the addition of IL-1B. Primary colony numbers (E) and colony numbers following replating (F). (G-H) CD34+ CML cells were stimulated with IL-1B for 15 minutes and analyzed for phosphorylation of NF-κB within the CD34+CD38+ and CD34+CD38− cell populations by phospho-flow cytometry. The histogram (G) shows the phosphorylation levels in the CD34+CD38− cells from a representative sample, and the diagram (H) summarizes data from 4 patients. (I-J) CD34+CD38− CML cells from 5 patients were incubated in the presence or absence of IL-1B for 3 or 24 hours and analyzed by RNA-sequencing and GSEA. NF-κB target genes (I) and cell cycle–associated genes (J). For complete gene list, see supplemental Tables 1-5. BFU-E, burst forming unit–erythroid; CFU-G, colony forming unit–granulocyte; CFU-GEMM, colony forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte; CFU-M, colony forming unit–macrophage.
To confirm that IL-1 activated signaling in primary CML cells, we analyzed the phosphorylation of NF-κB and AKT, 2 downstream signaling mediators of IL-1.19,20 Upon IL-1B stimulation, NF-κB and AKT phosphorylation was markedly increased in CD34+CD38− cells but not in CD34+CD38+ CML cells (Figure 1G-H; supplemental Figure 1I-K). RNA-sequencing and GSEA of CD34+CD38− CML cells confirmed transcriptional activation of NF-κB target genes after 3 hours of IL-1B stimulation (Figure 1I). Following 24 hours of stimulation a significant enrichment of cell cycle–associated genes was observed (Figure 1J), consistent with an increased cell proliferation (complete lists of gene sets enriched and the validation of top genes are presented in supplemental Tables 1-5). Collectively, these results show that primitive CP CML cells, but not corresponding normal cells, express a functional IL1R1/IL1RAP receptor complex and are highly responsive to IL-1 signaling.
IL1RAP antibodies, but not imatinib, inhibit IL-1 signaling in primary CP CML cells
To test whether IL1RAP expression and IL-1 signaling are affected by inhibition of the BCR/ABL1 kinase activity, we exposed primary CP CML cells to imatinib. Neither the expression of IL1RAP nor the intracellular response to IL-1 signaling as measured by NF-κB phosphorylation was reduced by imatinib at a dose that inhibited the kinase activity of BCR/ABL1 in the CD34+CD38− cells and in the quiescent CD34+CD38−Ki67− subpopulation of cells (Figure 2A-D; supplemental Figure 2A-G). Also in the CD34+CD38+ cells IL1RAP expression was retained following imatinib exposure (supplemental Figure 2H; supplemental Table 6). To address if IL1RAP antibodies could be used to block the IL-1–induced effects on CML cells, the 2 IL1RAPs targeting monoclonal antibodies mAb3F8 and mAb81.2 were used. In cultures of primary CD34+CD38− CML cells supplemented with IL-1B, mAb3F8, which in contrast to mAb81.2 blocks IL-1 signaling,17 significantly suppressed IL-1B–induced cell proliferation (Figure 2E; supplemental Figure 3A). Consistent with the decreased proliferation, mAb3F8 also caused a significant suppression of IL-1B–induced NF-κB and AKT phosphorylation (Figure 2F-G; supplemental Figure 3B-C). These findings demonstrate that IL1RAP expression and IL-1 signaling are preserved in primitive CML cells during BCR/ABL1 inhibition, and that IL1RAP antibodies can inhibit the IL-1–induced signaling and expansion of these cells.
IL-1 signaling in primary CD34+ CD38− CP CML cells is intact despite BCR/ABL1 inhibition but suppressed by IL1RAP antibodies. (A-D) CD34+CD38− CML cells (n = 2-3 as presented) were incubated in the presence or absence of 5 μM imatinib (IM) for 4 hours. Cell surface expression of IL1RAP (gray, isotype control [no IM]; red, without IM; blue, with IM) (A). NF-κB phosphorylation following 15-minute IL-1B stimulation. The histogram (B) shows the NF-κB phosphorylation in a representative sample, and the diagram (C) summarizes data from 3 patients. Level of tyrosine phosphorylation following 15 minutes of IL-1B stimulation (D). (E) CD34+CD38− CML cells were seeded in serum-free medium supplemented with IL-1B. Total cell numbers were determined following 7 days culture in the presence of the IL1RAP antibodies mAb81.2 and mAb3F8 or an isotype control antibody. Data are presented as fold change in cell numbers relative to conditions without IL-1B (n = 3). (F-G) CD34+CD38− CML BM cells (n = 3) were incubated with the IL-1–blocking mAb3F8 or an isotype control antibody, stimulated with IL-1B, and analyzed for phosphorylation of NF-κB and AKT using phospho-flow. The levels of phosphorylated NF-κB (F) and AKT (G) are presented.
IL-1 signaling in primary CD34+ CD38− CP CML cells is intact despite BCR/ABL1 inhibition but suppressed by IL1RAP antibodies. (A-D) CD34+CD38− CML cells (n = 2-3 as presented) were incubated in the presence or absence of 5 μM imatinib (IM) for 4 hours. Cell surface expression of IL1RAP (gray, isotype control [no IM]; red, without IM; blue, with IM) (A). NF-κB phosphorylation following 15-minute IL-1B stimulation. The histogram (B) shows the NF-κB phosphorylation in a representative sample, and the diagram (C) summarizes data from 3 patients. Level of tyrosine phosphorylation following 15 minutes of IL-1B stimulation (D). (E) CD34+CD38− CML cells were seeded in serum-free medium supplemented with IL-1B. Total cell numbers were determined following 7 days culture in the presence of the IL1RAP antibodies mAb81.2 and mAb3F8 or an isotype control antibody. Data are presented as fold change in cell numbers relative to conditions without IL-1B (n = 3). (F-G) CD34+CD38− CML BM cells (n = 3) were incubated with the IL-1–blocking mAb3F8 or an isotype control antibody, stimulated with IL-1B, and analyzed for phosphorylation of NF-κB and AKT using phospho-flow. The levels of phosphorylated NF-κB (F) and AKT (G) are presented.
IL1RAP antibodies show therapeutic effects in vivo
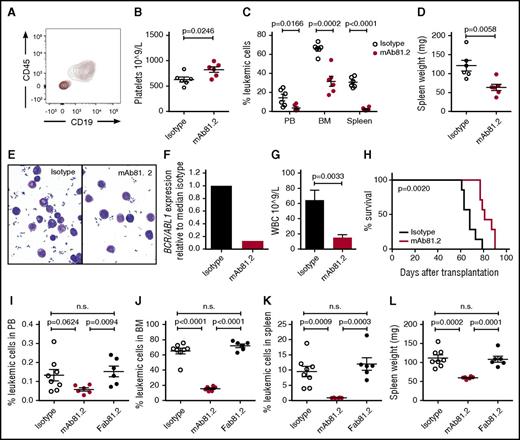
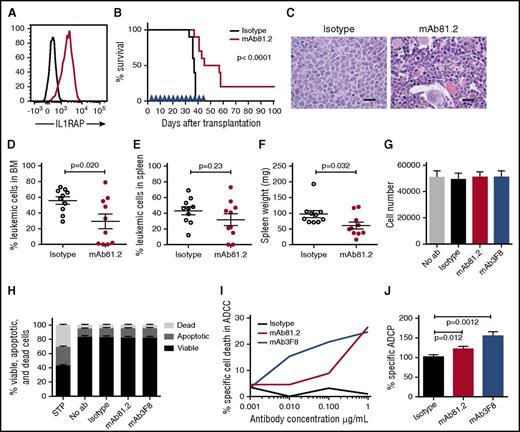
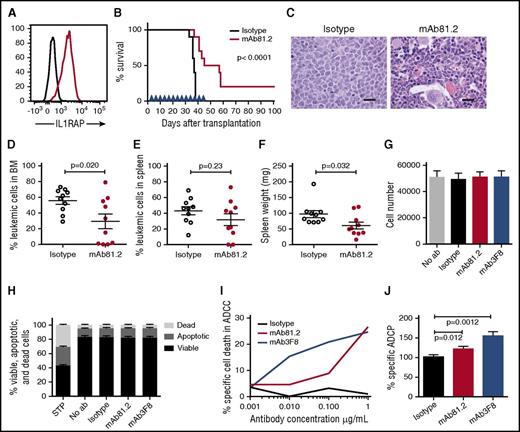
To assess the therapeutic in vivo efficacy of IL1RAP antibodies in CML, we first used IL1RAP-expressing BV173 cells that robustly engraft NOD/SCID mice (Figure 3A; supplemental Figure 4A). Treatment of BV173-transplanted mice with mAb81.2 resulted in a significantly prolonged survival (Figure 3B). Despite treatment discontinuation at day 45, 5 mice treated with mAb81.2 survived for at least 12 additional days, and 2 of these were still healthy at termination day 101. Several of the mAb81.2-treated mice had a normal BM architecture and displayed significantly lower BM leukemic cell levels compared with control mice that showed heavy infiltration of blast cells (Figure 3C-D). The effect was less pronounced in spleen; however, mAb81.2-treated mice displayed significantly lower spleen weights compared with controls (Figure 3E-F). These results, generated in a highly aggressive CML xenograft model, provide the first in vivo proof of concept that IL1RAP antibodies can efficiently target CML cells.
Treatment with IL1RAP antibody shows strong in vivo therapeutic effects in the BV173 xenograft model. (A) Cell surface expression of IL1RAP on BV173 cells (black, isotype control; red, IL1RAP). (B-F) NOD/SCID mice were engrafted with BV173 cells and treated biweekly with 500 μg IL1RAP antibody mAb81.2 or a mIgG2a control antibody (n = 10 per group). Treatment started 3 days after transplantation and was continued for a total of 13 doses. Overall survival with mAb81.2 treatment compared with isotype control (median 51 days vs 37 days); treatment days are indicated with blue arrowheads (B). Hematoxylin and eosin–stained BM sections from representative isotype control and mAb81.2-treated mice (bar represents 200 μm; C). Level of leukemic cells in the BM (D) and spleen (E). Spleen weights (F). (G-H) BV173 cells were cultured in the presence of IL1RAP antibodies or isotype control antibodies and analyzed after 48 hours. Total cell number (n = 3; G) and proportions of viable, apoptotic, and dead cells (n = 4; H). Staurosporine (STP) was used as positive control for inducing apoptosis. (I) BV173 cells were used as target cells in an ADCC assay with IL1RAP antibodies or a hIgG1 control antibody using human NK effector cells. Presented is the percentage of BV173 cells killed by ADCC. One representative experiment out of 3 is displayed. (J) BV173 cells were used as target cells in an ADCP assay with IL1RAP antibodies or a hIgG1 control antibody using human macrophages as effector cells. The ADCP effect is presented as the percentages of macrophages with phagocytosed BV173 cells normalized to the isotype control (n = 4).
Treatment with IL1RAP antibody shows strong in vivo therapeutic effects in the BV173 xenograft model. (A) Cell surface expression of IL1RAP on BV173 cells (black, isotype control; red, IL1RAP). (B-F) NOD/SCID mice were engrafted with BV173 cells and treated biweekly with 500 μg IL1RAP antibody mAb81.2 or a mIgG2a control antibody (n = 10 per group). Treatment started 3 days after transplantation and was continued for a total of 13 doses. Overall survival with mAb81.2 treatment compared with isotype control (median 51 days vs 37 days); treatment days are indicated with blue arrowheads (B). Hematoxylin and eosin–stained BM sections from representative isotype control and mAb81.2-treated mice (bar represents 200 μm; C). Level of leukemic cells in the BM (D) and spleen (E). Spleen weights (F). (G-H) BV173 cells were cultured in the presence of IL1RAP antibodies or isotype control antibodies and analyzed after 48 hours. Total cell number (n = 3; G) and proportions of viable, apoptotic, and dead cells (n = 4; H). Staurosporine (STP) was used as positive control for inducing apoptosis. (I) BV173 cells were used as target cells in an ADCC assay with IL1RAP antibodies or a hIgG1 control antibody using human NK effector cells. Presented is the percentage of BV173 cells killed by ADCC. One representative experiment out of 3 is displayed. (J) BV173 cells were used as target cells in an ADCP assay with IL1RAP antibodies or a hIgG1 control antibody using human macrophages as effector cells. The ADCP effect is presented as the percentages of macrophages with phagocytosed BV173 cells normalized to the isotype control (n = 4).
IL1RAP antibodies mediate effector cell–dependent antileukemic effects
We next explored the possible mechanisms underlying the antileukemic effects of IL1RAP antibodies in vivo. Because mAb81.2 does not block IL-1 signaling and human cells respond poorly to murine IL-1 (supplemental Figure 4B), the IL1RAP antibody mode of action could instead depend on a direct antibody cytotoxic effect or the recruitment of effector cells that would elicit leukemic cell killing. To assess direct cytotoxic effects, we cultured BV173 cells in the presence of IL1RAP antibodies but found no effect on the total cell expansion or apoptosis of the cells (Figure 3G-H). IL-1 stimulation of BV173 cells did not impact the cellular expansion or NF-κB and AKT phosphorylation (supplemental Figure 4C-F). To test if the therapeutic effect in vivo instead was a consequence of interactions between antibodies and effector cells and to explore the mechanisms that would be active in a human setting, we performed ADCC and ADCP assays. Using human NK effector cells, IL1RAP antibodies elicited a dose-dependent ADCC effect on BV173 cells (Figure 3I; supplemental Figure 4G). In addition, IL1RAP antibodies directed macrophages to phagocytosis of the CML cells (Figure 3J). Overall, these results show that the IL1RAP antibodies lack direct toxic effects but mediate ADCC and ADCP, suggesting that the observed in vivo antileukemic effects were effector cell mediated.
IL1RAP antibodies show therapeutic effects in a primary CP CML xenograft model
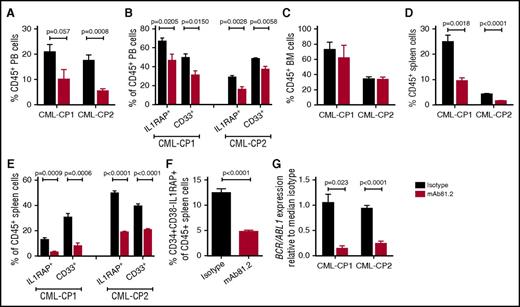
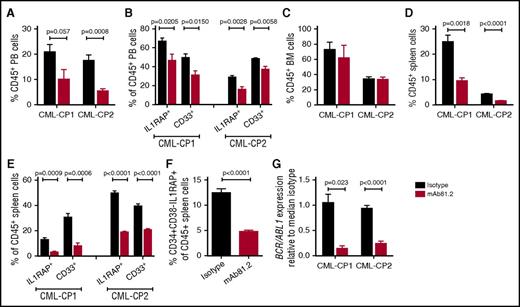
In vivo studies of primary CP CML cells are challenging because of transient engraftment of the leukemic cells and because cotransplanted healthy cells may have a selective growth advantage.21,22 To investigate the effect of IL1RAP antibodies on primary human CML cells in vivo, we transplanted CD34+ CP CML cells into highly immunodeficient NSGS mice that lack functional NK cells, thereby allowing for higher engraftment than less immunodeficient strains but also having a reduced repertoire of effector cells to elicit antibody-mediated killing. In this xenograft model, mAb81.2 treatment resulted in approximately a twofold reduction of human CD45+ cells in PB compared with controls (Figure 4A). Moreover, lower levels of IL1RAP-expressing cells and a reduction of myeloid cells was observed, suggesting that the treatment targeted the leukemic graft (Figure 4B). Although no significant reduction of human cells was seen in BM, likely because of the low effector cell function, mAb81.2 induced a marked antileukemic effect in spleen (Figure 4C-E). Notably, mAb81.2 treatment reduced the levels of candidate CML stem cells (CD34+CD38−IL1RAP+ cells; Figure 4F). BCR/ABL1 transcript levels were ∼10-fold lower in the spleens of mAb81.2-treated mice than in controls (Figure 4G). These findings demonstrate that IL1RAP antibodies show therapeutic efficacy on primary human CP CML cell in vivo, including the candidate CML stem cells.
Primary CP CML cells are killed by IL1RAP antibodies in vivo. NSGS mice were transplanted with CD34+ CP CML cells from 2 patients and treated biweekly with 50 μg IL1RAP antibody mAb81.2 or a mIgG2a isotype control antibody until euthanization 40 days after transplantation (mice given mAb81.2 or control antibody: n = 4 and n = 5 in CP-CML1, n = 5 and n = 5 in CP-CML2, respectively). Treatment was started 1 (CP-CML1) or 3 days (CP-CML2) after transplantation. (A-E) The levels of human (CD45+) cells and proportions of IL1RAP expressing and myeloid (CD33+) cells within the human cell population is presented in PB (A-B), BM (C), and spleen (D-E). (F) Levels of CD34+CD38−IL1RAP+ CML cells within the CD45+ population in the spleen. (G) Relative levels of BCR/ABL1 transcripts in spleens.
Primary CP CML cells are killed by IL1RAP antibodies in vivo. NSGS mice were transplanted with CD34+ CP CML cells from 2 patients and treated biweekly with 50 μg IL1RAP antibody mAb81.2 or a mIgG2a isotype control antibody until euthanization 40 days after transplantation (mice given mAb81.2 or control antibody: n = 4 and n = 5 in CP-CML1, n = 5 and n = 5 in CP-CML2, respectively). Treatment was started 1 (CP-CML1) or 3 days (CP-CML2) after transplantation. (A-E) The levels of human (CD45+) cells and proportions of IL1RAP expressing and myeloid (CD33+) cells within the human cell population is presented in PB (A-B), BM (C), and spleen (D-E). (F) Levels of CD34+CD38−IL1RAP+ CML cells within the CD45+ population in the spleen. (G) Relative levels of BCR/ABL1 transcripts in spleens.
BP CML cells express IL1RAP and can be targeted for ADCC by IL1RAP antibodies
BP CML cells represent a major clinical challenge, particularly in patients with the BCR/ABL1 T315I mutation that renders the CML cells resistant to most TKIs.23 We detected surface expression of IL1RAP on all 5 primary BP CML samples investigated (Figure 5A-B). The expression of IL1RAP was similar in the CD34+CD38− and CD34+CD38+ populations and was unaffected by imatinib treatment (Figure 5B; supplemental Table 6). As BP CML stem cells may reside outside the CD34+CD38− population,24,25 we used MNCs when studying the response to IL-1B, IL-33, IL-36G, and SCF. Of these cytokines, stimulation with IL-1B had the largest effect on cell numbers (Figure 5C). NF-κB activation was detected in ∼10% of the MNCs upon IL-1B stimulation (Figure 5D), suggesting that these IL-1–sensitive cells caused the increase in total cell numbers. Of 2 analyzed BP CML samples, 1 showed imatinib-independent phosphorylation of NF-κB in response to IL-1B, whereas the other displayed a higher baseline level of NF-κB phosphorylation, which was not increased by IL-1B (supplemental Figure 5A). Both samples were sensitive to imatinib (supplemental Figure 5B). IL-1B significantly increased the colony-forming capacity in 1 of 3 tested BP CML samples (supplemental Figure 5C-D). Notably, IL1RAP antibodies specifically mediated ADCC in all 3 analyzed BP CML samples including the one with a T315I mutation (Figure 5E-F). We conclude that the response to IL-1 varies between BP CML samples, but that all samples tested expressed IL1RAP and were sensitive to ADCC-mediated killing using IL1RAP antibodies.
Primary BP CML cells respond to IL-1 and can be killed by ADCC in vitro. (A) IL1RAP cell surface expression on MNCs from 3 BP CML BM samples, 2 of lymphoid and 1 of myeloid phenotype, and including 1 with T315I mutation (black, isotype control; red, IL1RAP). (B) Cell surface expression of IL1RAP on CD34+CD38− and CD34+CD38+ cells from 2 additional BP CML patients after 4 hours incubation with or without 5 μM IM (gray, isotype control [no IM]; red, without IM; blue, with IM). (C) BP CML MNCs were seeded in serum-free medium supplemented with indicated cytokines. The dotted line represents the seeded cell number. Total cell numbers following 7 days culture are presented (n = 4). (D) NF-κB and AKT phosphorylation in BP CML MNCs after 15-minute stimulation with IL-1B (blue, without IL-1B; red, with IL-1B). (E-F) BP CML BM MNCs were analyzed in an ADCC assay using IL1RAP antibodies or a hIgG1 control antibody and with human NK effector cells. Presented is the percentage of target cells killed by ADCC for CML-BP1 and CML-BP2 (E) and CML-BP3 cells (F).
Primary BP CML cells respond to IL-1 and can be killed by ADCC in vitro. (A) IL1RAP cell surface expression on MNCs from 3 BP CML BM samples, 2 of lymphoid and 1 of myeloid phenotype, and including 1 with T315I mutation (black, isotype control; red, IL1RAP). (B) Cell surface expression of IL1RAP on CD34+CD38− and CD34+CD38+ cells from 2 additional BP CML patients after 4 hours incubation with or without 5 μM IM (gray, isotype control [no IM]; red, without IM; blue, with IM). (C) BP CML MNCs were seeded in serum-free medium supplemented with indicated cytokines. The dotted line represents the seeded cell number. Total cell numbers following 7 days culture are presented (n = 4). (D) NF-κB and AKT phosphorylation in BP CML MNCs after 15-minute stimulation with IL-1B (blue, without IL-1B; red, with IL-1B). (E-F) BP CML BM MNCs were analyzed in an ADCC assay using IL1RAP antibodies or a hIgG1 control antibody and with human NK effector cells. Presented is the percentage of target cells killed by ADCC for CML-BP1 and CML-BP2 (E) and CML-BP3 cells (F).
BP CML cells can be killed by IL1RAP antibodies in vivo
Next, we investigated whether IL1RAP antibodies would have therapeutic effects on primary BP CML cells in vivo and if CML stem cells could be targeted. NOD/SCID mice were successfully engrafted with CML cells from a lymphoid BP sample (CML-BP1; Figure 6A). Mice treated with mAb81.2 displayed higher platelet counts indicative of a less disturbed normal hematopoiesis and significantly reduced levels of leukemic cells in the PB, BM, and spleen, accompanied by lower spleen weights (Figure 6B-D). The leukemic cells displayed similar blastlike morphology in both treatment groups; however, the level of BCR/ABL1 transcript in mAb81.2-treated mice was fivefold lower (Figure 6E-F). To assess the therapeutic effects on BP CML stem cells, spleen cells from primary mice treated with mAb81.2 or control antibody were transplanted into secondary NSG recipient mice. Secondary recipients that received cells from mAb81.2-treated mice displayed significantly lower white blood cell counts and prolonged survival compared with control mice (Figure 6G-H). In a separate experiment, mAb3F8 treatment resulted in similar antileukemic effects (supplemental Figure 6A-B). To experimentally investigate if the therapeutic effect of IL1RAP antibodies in mice was effector cell mediated, we used a Fab fragment of mAb81.2, designated Fab81.2. The Fab fragment lacks the FcγR binding domain and is therefore unable to mediate cell death by binding to effector cells. In contrast to mAb81.2, Fab81.2 did not exhibit therapeutic effects in mice engrafted with BP CML cells (Figure 6I-L). Taken together, these data show that IL1RAP antibodies kill BP CML cells in vivo through effector cell–mediated mechanisms, and that the antibodies therapeutically target the leukemic stem cells.
IL1RAP antibodies kill primary BP CML cells in vivo by effector cell–mediated mechanisms. (A-F) NOD/SCID mice engrafted with CML-BP1 cells were treated biweekly with 100 μg IL1RAP antibody mAb81.2 or a mIgG2a control antibody (n = 6 per group). Treatment was initiated 3 days after transplantation, and the mice were euthanized 33 days after transplantation. Contour plot showing a distinct human cell population of CD45+CD19+ cells in both mAb81.2-treated (red) and isotype control–treated (black) mice (A). Platelet counts (B) and levels of leukemic cells in PB, BM, and spleen (C). Spleen weight (D). Morphology of cells from representative mAb81.2-treated and isotype control–treated mice (E). Levels of BCR/ABL1 transcripts in the spleens from mAb81.2 and isotype control–treated mice (F). (G-H) Spleen cells from mAb81.2-treated and mIgG2a control antibody–treated mice were harvested and transplanted into NSG mice (n = 7 per group). White blood cell (WBC) counts in secondary recipients 6 weeks after transplantation (G). Survival of mice transplanted with cells from mAb81.2 or mIgG2a control antibody–treated mice (median 81 days vs 68 days; H). (I-L) NOD/SCID mice engrafted with CML-BP1 cells were treated biweekly with the IL1RAP antibody mAb81.2, the Fab fragment of mAb81.2 (Fab81.2), or a mIgG2a control antibody (n = 6-8 per group) until euthanized 29 days after transplantation. Treatment was initiated 3 days after transplantation. Levels of leukemic cells in PB (I), BM (J), and spleen (K). Spleen weight (L). n.s., not significant.
IL1RAP antibodies kill primary BP CML cells in vivo by effector cell–mediated mechanisms. (A-F) NOD/SCID mice engrafted with CML-BP1 cells were treated biweekly with 100 μg IL1RAP antibody mAb81.2 or a mIgG2a control antibody (n = 6 per group). Treatment was initiated 3 days after transplantation, and the mice were euthanized 33 days after transplantation. Contour plot showing a distinct human cell population of CD45+CD19+ cells in both mAb81.2-treated (red) and isotype control–treated (black) mice (A). Platelet counts (B) and levels of leukemic cells in PB, BM, and spleen (C). Spleen weight (D). Morphology of cells from representative mAb81.2-treated and isotype control–treated mice (E). Levels of BCR/ABL1 transcripts in the spleens from mAb81.2 and isotype control–treated mice (F). (G-H) Spleen cells from mAb81.2-treated and mIgG2a control antibody–treated mice were harvested and transplanted into NSG mice (n = 7 per group). White blood cell (WBC) counts in secondary recipients 6 weeks after transplantation (G). Survival of mice transplanted with cells from mAb81.2 or mIgG2a control antibody–treated mice (median 81 days vs 68 days; H). (I-L) NOD/SCID mice engrafted with CML-BP1 cells were treated biweekly with the IL1RAP antibody mAb81.2, the Fab fragment of mAb81.2 (Fab81.2), or a mIgG2a control antibody (n = 6-8 per group) until euthanized 29 days after transplantation. Treatment was initiated 3 days after transplantation. Levels of leukemic cells in PB (I), BM (J), and spleen (K). Spleen weight (L). n.s., not significant.
Mice deficient for Il1rap display normal HSC function
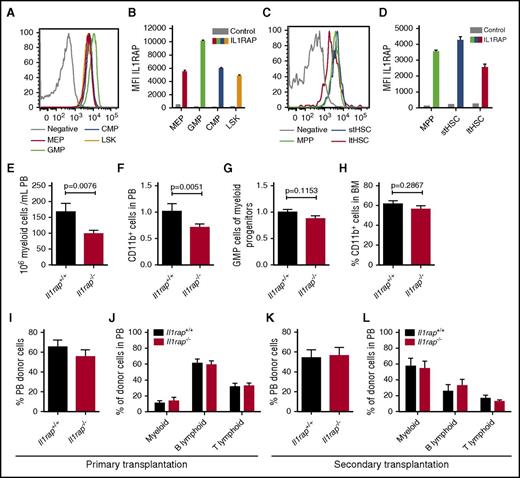
The role of IL1RAP in normal hematopoiesis has not been studied before, and, depending on its function, interfering with this receptor by antibodies could result in impaired hematopoiesis. To investigate the importance of IL1RAP in normal hematopoiesis, we used an Il1rap-deficient (Il1rap−/−) mouse.13 First, we examined the expression of Il1rap on normal murine hematopoietic cells. Similar to human cells,14 Il1rap expression was higher on the granulocyte macrophage progenitor (GMP) population compared with other HSC and progenitor populations (Figure 7A-B). However, in contrast to human hematopoiesis, Il1rap was expressed also on murine HSCs (Figure 7C-D; supplemental Table 6).
Il1rap is dispensable for normal HSC function. (A-D) IL1RAP expression on myeloid progenitor populations (CMP, common myeloid progenitor; LSK, lineage negative, Sca1 positive, c-kit positive cells; MEP, megakaryocyte erythrocyte progenitor) and stem and multipotent progenitor populations (ltHSC, long-term HSC; MPP, multipotent progenitor; stHSC, short-term HSC). Histograms display the stains from 1 representative sample, and diagrams summarize the data (n = 3). Stained BM cells from an Il1rap−/− mouse were used as negative controls. (E) Numbers of PB myeloid cells in Il1rap+/+ and Il1rap−/− mice. (F) Levels of CD11b+ cells in PB, normalized to mean of Il1rap+/+ mice. The P value was calculated by a 1-sided Student t test to confirm the data presented in panel E. (G) Relative size of the GMP population among myeloid progenitors, normalized to the mean of the Il1rap+/+ mice. (H) Proportion of CD11b+ BM cells in Il1rap+/+ and Il1rap−/− mice. (I-J) Donor reconstitution in PB and lineage distribution within the donor cell population 16 weeks after competitive BM transplantations of Il1rap+/+ and Il1rap−/− cells. (K-L) Donor reconstitution and lineage distribution within the donor population 10 weeks after transplantation of BM into secondary recipient mice.
Il1rap is dispensable for normal HSC function. (A-D) IL1RAP expression on myeloid progenitor populations (CMP, common myeloid progenitor; LSK, lineage negative, Sca1 positive, c-kit positive cells; MEP, megakaryocyte erythrocyte progenitor) and stem and multipotent progenitor populations (ltHSC, long-term HSC; MPP, multipotent progenitor; stHSC, short-term HSC). Histograms display the stains from 1 representative sample, and diagrams summarize the data (n = 3). Stained BM cells from an Il1rap−/− mouse were used as negative controls. (E) Numbers of PB myeloid cells in Il1rap+/+ and Il1rap−/− mice. (F) Levels of CD11b+ cells in PB, normalized to mean of Il1rap+/+ mice. The P value was calculated by a 1-sided Student t test to confirm the data presented in panel E. (G) Relative size of the GMP population among myeloid progenitors, normalized to the mean of the Il1rap+/+ mice. (H) Proportion of CD11b+ BM cells in Il1rap+/+ and Il1rap−/− mice. (I-J) Donor reconstitution in PB and lineage distribution within the donor cell population 16 weeks after competitive BM transplantations of Il1rap+/+ and Il1rap−/− cells. (K-L) Donor reconstitution and lineage distribution within the donor population 10 weeks after transplantation of BM into secondary recipient mice.
Because Il1rap was highly expressed on myeloid progenitors, we investigated whether loss of Il1rap would alter the steady-state levels of myeloid cells and found a modest reduction of myeloid cell numbers in PB of Il1rap−/− mice (Figure 7E-F). The BM cellularity and HSC compartment in the Il1rap−/− mice were similar to controls (supplemental Figure 7A-B), and no significant difference in the frequency of GMPs and myeloid CD11b+ cells was observed (Figure 7G-H). Furthermore, Il1rap−/− and control cells displayed similar colony-forming capacity (supplemental Figure 7C).
To address if Il1rap regulates HSC function, we performed competitive BM repopulation assays. Similar PB and BM repopulation of Il1rap−/− and Il1rap+/+ donor cells was observed after 16 weeks, and no difference in hematopoietic lineages was seen (Figure 7I-J; supplemental Figure 7D-F). In addition, secondary transplantations did not reveal differences in repopulation capacity or lineage distribution (Figure 7K-L).
Altogether, our data indicate that Il1rap deficiency results in a modest reduction of myeloid cell numbers in PB, but that Il1rap is dispensable for normal HSC and progenitor cell function, even under conditions of hematopoietic stress.
Discussion
Therapeutic antibodies have emerged as promising therapies in a wide variety of malignancies.26 However, despite accumulating evidence that CML stem cells are insensitive to TKI treatment, no clinical attempts have been made to target these cells using recombinant antibodies. Herein, we provide critical in vivo evidence that IL1RAP is a promising therapeutic target on CML stem cells.
Normal human HSCs do not express IL1RAP, and we hypothesized that the upregulation of IL1RAP on primitive CD34+CD38− CP CML cells compared with corresponding normal cells would increase their sensitivity to cytokines that signal through receptor complexes involving IL1RAP. We found that IL-1 stimulation resulted in a pronounced and selective expansion of primitive CML cells, relative to normal CD34+CD38− BM cells. Thus, the upregulation of IL1RAP on primitive CML cells, resulting in a functional IL1R1/IL1RAP receptor complex, is likely the critical mechanism sensitizing these cells to IL-1-stimulation. The importance of altered cytokine signaling for the regulation of CML stem cell expansion is supported by a study using a BCR/ABL1 transgenic mouse model, in which leukemia-induced abnormalities in cytokine expression, including IL-1, resulted in a selective enhanced growth of CML stem cells.27 Moreover, it was recently reported that secretion of IL-6, a cytokine induced by IL-1/NF-κB signaling, alters normal hematopoiesis to promote CML progression.28 Both CD34+CD38− and CD34+CD38+ CML cells expressed similar levels of IL1R1 and IL1RAP, but primitive CML cells expanded more strongly to IL-1 stimulation. The finding that IL-1 activated NF-κB and AKT selectively within the CD34+CD38− cell population shows that these cells are intrinsically different in their response to IL-1.
Because primitive CP CML cells express IL1RAP and are sensitized for IL-1 stimulation, we evaluated the antileukemic effects of IL-1–blocking IL1RAP antibodies and found that such antibodies can inhibit IL-1 signaling and expansion of primitive CML cells. This suggests that IL1RAP antibodies may provide a new therapeutic strategy against CML stem cells. Previous studies on CML MNCs demonstrate an increased colony-forming capacity in response to IL-1 that could be blocked by IL1RA, soluble IL1R, or IL-1 antibodies.29 We here found an increased colony-forming capacity of primitive CML cells following IL-1 stimulation. Although our results suggest that IL-1 does not increase the numbers of CML cells with replating potential under the in vitro conditions tested, previous studies using quiescent CML stem cells from BCR/ABL1 transgenic mice demonstrate that IL-1 significantly increase the growth of these cells.27 Interestingly, Zhang et al show that the addition of IL-1 blockade to TKI enhances the elimination of CML stem cells in BCR/ABL1 transgenic mice, lending further support to our conclusion that IL-1 blockade may be a viable strategy to target CP CML stem cells.30 As to the importance of IL-1 signaling in BP CML, we found that all samples tested expressed IL1RAP, whereas the response to IL-1 varied between samples. This would suggest that IL-1 signaling blockade also may be beneficial in selected cases of more advanced CML but will require further investigations on larger numbers of samples given the genetic heterogeneity of BP CML.
Apart from blocking signaling, antibodies may also recruit effector cells that elicit tumor cell killing, as shown for several therapeutic antibodies including rituximab and trastuzumab.26 Using xenograft models of human CP and BP CML cells, we here demonstrate antileukemic effects and prolonged survival upon administration of IL1RAP antibodies. Treatment with IL1RAP antibodies did not completely eradicate the leukemic cells in most mice. However, because the immunodeficient mice used have reduced effector cell function, it is likely that the therapeutic effect is underestimated and would be more pronounced in an immunocompetent host. Moreover, we were not able to reliably investigate the effects of IL-1 blockade in vivo because murine IL-1 poorly cross-reacts with human cells (supplemental Figure 4B). That the in vivo therapeutic effects indeed were effector cell mediated was demonstrated by the use of Fab81.2, which lacks the FcγR binding domain and thereby the ability to direct effector cells to target cell killing. In a human context, we show that the IL1RAP antibodies are able to direct human NK cells and macrophages to cell killing by mediating ADCC and ADCP. Importantly, it has been shown that NK cells persist during TKI treatment in CML,31-33 and that the ability of autologous NK cells to kill primary CML cells by ADCC is equivalent to allogeneic NK cells from healthy donors.34 Hence, in addition to the effects of IL-1 signaling blockade, our data suggest that effector cell–mediated killing would be an effective therapeutic mechanism of an IL1RAP antibody–based treatment in CML.
To date, only a few cell surface markers apart from IL1RAP have been shown to be upregulated on primitive CML cells. Additional markers include CD25, CD26, CD117, CD123, and ST2, although CD117, CD123, and ST2 are also expressed on normal HSCs.14,35-38 Of these markers, only CD123 has been evaluated as a therapeutic target in CML models using antibodies, but in vivo administration of CD123 antibodies has not been studied.34
In a clinical setting, we speculate that IL1RAP antibodies would be beneficial following initial treatment with TKIs. If successful, the IL1RAP antibodies would eradicate residual CML stem cells and thereby provide a cure for CML. In contrast to anakinra (Kineret), a modified form of IL1RA used to treat inflammatory disorders,39 IL1RAP antibodies would elicit therapeutic effects both by directing effector cells to killing and by blocking IL-1 signaling. Our previous studies using forced expression of BCR/ABL1 in cord blood cells and TKI treatment of KU812 cells suggested that the IL1RAP expression at least partially is regulated by the BCR/ABL1 kinase activity.14 Here, we found that neither IL1RAP expression nor NF-κB phosphorylation was reduced by imatinib in primary CML cells. Although these results may seem contradictory, they reflect different experimental settings and cell types used. Importantly, the results showing retained IL1RAP expression on primary primitive CML cells in the presence of imatinib indicate that these cells can be targeted by IL1RAP antibodies in patients undergoing TKI treatment. Our finding that Il1rap is dispensable for normal HSCs in mice, and that human HSCs lack IL1RAP expression, suggest that therapeutic IL1RAP antibodies would not cause severe negative effects on normal hematopoiesis. In summary, our results show that IL1RAP is a promising therapeutic target in CML and provide a strong rationale for the clinical development of an antibody-based therapy targeting IL1RAP on CML stem cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Göran Forsberg, Anki Malmborg Hager, Karin von Wachenfeld, and Kjell Sjöström for fruitful discussions; Cantargia AB for contributing the antibodies used in the study; and Junia V. Melo for help with editing the manuscript.
This work was supported by the Swedish Cancer Society, the Swedish Children’s Cancer Foundation, the Medical Faculty of Lund University, the Swedish Research Council, BioCARE, the Finnish Cancer Institute, and a contract research grant from Cantargia AB.
Authorship
Contribution: H.Å., N.H., M.J., and T.F. contributed to designing the study; H.Å., N.H., S.v.P., C.S., K.R., C.K., N.L., M.A., C.H., and M.R. performed the experiments; H.Å., N.H., S.v.P., C.S., K.R., C.K., H.L., C.H., M.J., and T.F. analyzed the data; K.P., H.W., S.M., and J.R. collected patient material and clinical data; and H.Å., N.H., M.J., and T.F. wrote the manuscript, and all other authors contributed with valuable comments.
Conflict-of-interest disclosure: M.J. and T.F. are cofounders and have equity ownership in Cantargia AB (Medicon Village, Lund, Sweden) formed together with Lund University Bioscience AB. J.R. owns shares in Cantargia. The remaining authors declare no competing financial interests.
Correspondence: Thoas Fioretos, Department of Clinical Genetics, Lund University, Klinikgatan 28, SE-22184 Lund, Sweden; e-mail: thoas.fioretos@med.lu.se; and Helena Ågerstam, Department of Clinical Genetics, Lund University, Klinikgatan 28, SE-22184 Lund, Sweden; e-mail: helena.agerstam@med.lu.se.
References
Author notes
H.Å. and N.H. contributed equally to this study.
M.J. and T.F. are joint senior authors.

![Figure 2. IL-1 signaling in primary CD34+ CD38− CP CML cells is intact despite BCR/ABL1 inhibition but suppressed by IL1RAP antibodies. (A-D) CD34+CD38− CML cells (n = 2-3 as presented) were incubated in the presence or absence of 5 μM imatinib (IM) for 4 hours. Cell surface expression of IL1RAP (gray, isotype control [no IM]; red, without IM; blue, with IM) (A). NF-κB phosphorylation following 15-minute IL-1B stimulation. The histogram (B) shows the NF-κB phosphorylation in a representative sample, and the diagram (C) summarizes data from 3 patients. Level of tyrosine phosphorylation following 15 minutes of IL-1B stimulation (D). (E) CD34+CD38− CML cells were seeded in serum-free medium supplemented with IL-1B. Total cell numbers were determined following 7 days culture in the presence of the IL1RAP antibodies mAb81.2 and mAb3F8 or an isotype control antibody. Data are presented as fold change in cell numbers relative to conditions without IL-1B (n = 3). (F-G) CD34+CD38− CML BM cells (n = 3) were incubated with the IL-1–blocking mAb3F8 or an isotype control antibody, stimulated with IL-1B, and analyzed for phosphorylation of NF-κB and AKT using phospho-flow. The levels of phosphorylated NF-κB (F) and AKT (G) are presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2015-11-679985/4/m_blood679985f2.jpeg?Expires=1765288763&Signature=kCW~o141~tVCdnhZJpaN46hfQzvH-aNle3fJrKhR-a8AZvR6zuo-jphAL0hen3qV1XPvRwHcQcIjXlsm-7Tl9xs5-STJX3gmTTgvujzc7dyHL6VLjTC4ATmLynEkVaQngXpAoOnoSnqBztOIldON07Yq7W~uda53gIQdnvi4NRprVR81F-ncAC32ETrCxCs-PHlCsx2gaPIBi67uR8clLIHj7DmuAMUszZ1oSL27eHmN3hil8OI04rLAJyUroIC-ZTmKJx~6EU3iBc0G8ZFV97jwxmOfpB8HuoW7odGy0H6mNA3piHtmbPmGeLjfAQGlut9HYXgFKhb4vU9lSZdfwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Primary BP CML cells respond to IL-1 and can be killed by ADCC in vitro. (A) IL1RAP cell surface expression on MNCs from 3 BP CML BM samples, 2 of lymphoid and 1 of myeloid phenotype, and including 1 with T315I mutation (black, isotype control; red, IL1RAP). (B) Cell surface expression of IL1RAP on CD34+CD38− and CD34+CD38+ cells from 2 additional BP CML patients after 4 hours incubation with or without 5 μM IM (gray, isotype control [no IM]; red, without IM; blue, with IM). (C) BP CML MNCs were seeded in serum-free medium supplemented with indicated cytokines. The dotted line represents the seeded cell number. Total cell numbers following 7 days culture are presented (n = 4). (D) NF-κB and AKT phosphorylation in BP CML MNCs after 15-minute stimulation with IL-1B (blue, without IL-1B; red, with IL-1B). (E-F) BP CML BM MNCs were analyzed in an ADCC assay using IL1RAP antibodies or a hIgG1 control antibody and with human NK effector cells. Presented is the percentage of target cells killed by ADCC for CML-BP1 and CML-BP2 (E) and CML-BP3 cells (F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2015-11-679985/4/m_blood679985f5.jpeg?Expires=1765288763&Signature=CKnyMJYzWD9utgzf-39X2BMQ9RJyQgzBunYCZnA77H1vkgQeA74Vsuvk8dst829YPAxqhulWE7IxRxp2bMz1uH~dF8RDzj~GfMDlhEO00RbQKT5NM-It9FcpKFsYY7STkMH1gGspWPYKMxF57xJy1q0EHQT52vBeSrehUUyt4uxHXoHusMR9cLN01FYQoHG8yV0s1WMTowuHIHnOyqkchuYFa01Fpe31VMcHnESifaug6ORVdN6FJFsPvks1Pl3iHee8m6ZdI1om2VnJbUsrh3aUQafLWokVjs4Sa7l2Fsoxm~ubVLz5BKJKQf3DON6tuXUTBatgBG6iwMHAr6zJfg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. IL-1 signaling in primary CD34+ CD38− CP CML cells is intact despite BCR/ABL1 inhibition but suppressed by IL1RAP antibodies. (A-D) CD34+CD38− CML cells (n = 2-3 as presented) were incubated in the presence or absence of 5 μM imatinib (IM) for 4 hours. Cell surface expression of IL1RAP (gray, isotype control [no IM]; red, without IM; blue, with IM) (A). NF-κB phosphorylation following 15-minute IL-1B stimulation. The histogram (B) shows the NF-κB phosphorylation in a representative sample, and the diagram (C) summarizes data from 3 patients. Level of tyrosine phosphorylation following 15 minutes of IL-1B stimulation (D). (E) CD34+CD38− CML cells were seeded in serum-free medium supplemented with IL-1B. Total cell numbers were determined following 7 days culture in the presence of the IL1RAP antibodies mAb81.2 and mAb3F8 or an isotype control antibody. Data are presented as fold change in cell numbers relative to conditions without IL-1B (n = 3). (F-G) CD34+CD38− CML BM cells (n = 3) were incubated with the IL-1–blocking mAb3F8 or an isotype control antibody, stimulated with IL-1B, and analyzed for phosphorylation of NF-κB and AKT using phospho-flow. The levels of phosphorylated NF-κB (F) and AKT (G) are presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2015-11-679985/4/m_blood679985f2.jpeg?Expires=1765510553&Signature=VB3mvYl9dTN7M6FznaFlSKGRUmEJh-K1FXZ4HBWbpJmpjWYFohYNHgyYVJ7AQvkD85C36c6wniGIcVPxe7uoJi1Hm7DlcQu8MTQM5DU00c-wMcQmDuOnYCMeC0nLkwn9LFmvKkBSfK7Ij6x2HJXcsyzWcEitzZ8vwstQReBtRYDR9-tCHXr~Ky-jRhMFx85w7g6TiJwvLBhj4eUd5KkyLd0~9lYpI-ViycdwnU-ketTS8x5B6heIE7b7DyqG1ZnSi4DIOQOKS~POg2T2pTAoHbrY45yqKeSk5bRjW65nUa~AWrni98vHO9~Qo7ZY0hoORKHYmrmTiPalHeP~5Ue7AA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Primary BP CML cells respond to IL-1 and can be killed by ADCC in vitro. (A) IL1RAP cell surface expression on MNCs from 3 BP CML BM samples, 2 of lymphoid and 1 of myeloid phenotype, and including 1 with T315I mutation (black, isotype control; red, IL1RAP). (B) Cell surface expression of IL1RAP on CD34+CD38− and CD34+CD38+ cells from 2 additional BP CML patients after 4 hours incubation with or without 5 μM IM (gray, isotype control [no IM]; red, without IM; blue, with IM). (C) BP CML MNCs were seeded in serum-free medium supplemented with indicated cytokines. The dotted line represents the seeded cell number. Total cell numbers following 7 days culture are presented (n = 4). (D) NF-κB and AKT phosphorylation in BP CML MNCs after 15-minute stimulation with IL-1B (blue, without IL-1B; red, with IL-1B). (E-F) BP CML BM MNCs were analyzed in an ADCC assay using IL1RAP antibodies or a hIgG1 control antibody and with human NK effector cells. Presented is the percentage of target cells killed by ADCC for CML-BP1 and CML-BP2 (E) and CML-BP3 cells (F).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2015-11-679985/4/m_blood679985f5.jpeg?Expires=1765510553&Signature=KHUlG-WeP0yQYGG3WsQNXGgpob6MNZZGmyGixzz3r57QcmuixnrxzTdH~KHWT7f6JHYZq1hUR4sAzF7Z8TrLqHhZwruFuASalVz4dNjivfoTPgCqpgpefCw97or~3QSRbSa2mZzsyXw7TJMoP2ADv6qfKo2ICMFqSsyLJfbieD6EoktiVankQguP6ZHyeMQqPf~PLyss7YZzVx03Dl8UpEN8jJkDmqARvMLwRc7U0F6FzeXfiPfSK-~Ljn05-dDbCQVhQTBBrhNXuiyGecBYckjqrRjBrA-UvHkJHDz87Agv6IVSINbnqpRLhj8JLy4memdymZCQkPGMnu6y4kVM7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)