Key Points
Skeletal muscle myosin promotes thrombus formation and enhances prothrombin activation by binding factors Xa and Va.
The procoagulant activity of skeletal muscle myosin might contribute to the hypercoagulability in plasmas of acute trauma patients.
Abstract
To test the hypothesis that skeletal muscle myosins can directly influence blood coagulation and thrombosis, ex vivo studies of the effects of myosin on thrombogenesis in fresh human blood were conducted. Addition of myosin to blood augmented the thrombotic responses of human blood flowing over collagen-coated surfaces (300 s−1 shear rate). Perfusion of human blood over myosin-coated surfaces also caused fibrin and platelet deposition, evidencing myosin’s thrombogenicity. Myosin markedly enhanced thrombin generation in both platelet-rich plasma and platelet-poor plasma, indicating that myosin promoted thrombin generation in plasma primarily independent of platelets. In purified reaction mixtures composed only of factor Xa, factor Va, prothrombin, and calcium ions, myosin greatly enhanced prothrombinase activity. The Gla domain of factor Xa was not required for myosin’s prothrombinase enhancement. When binding of purified clotting factors to immobilized myosin was monitored using biolayer interferometry, factors Xa and Va each showed favorable binding interactions. Factor Va reduced by 100-fold the apparent Kd of myosin for factor Xa (Kd ∼0.48 nM), primarily by reducing koff, indicating formation of a stable ternary complex of myosin:Xa:Va. In studies to assess possible clinical relevance for this discovery, we found that antimyosin antibodies inhibited thrombin generation in acute trauma patient plasmas more than in control plasmas (P = .0004), implying myosin might contribute to acute trauma coagulopathy. We posit that myosin enhancement of thrombin generation could contribute either to promote hemostasis or to augment thrombosis risk with consequent implications for myosin’s possible contributions to pathophysiology in the setting of acute injuries.
Introduction
Thrombin is generated in blood by proteolytic activation of prothrombin by factor Xa, factor Va, and Ca++ assembled on a suitable surface.1,2 Insufficient or excessive thrombin generation is associated with bleeding or thrombosis, respectively. Knowledge of the molecular surfaces that regulate thrombin generation is essential. Recent studies emphasize that thrombin generation primarily occurs at the site of damaged endothelium and exposed subendothelium, not primarily on the membranes of activated platelets.3,4 Thus, damaged cells, subendothelial basement membranes, and damaged tissues may provide procoagulant surfaces that contribute to thrombin generation. A variety of molecular and structural elements distinct from phosphatidylserine or phospholipid membranes are procoagulant, including collagens that are prothrombotic via both platelet activation5-7 and contact activation mechanisms.8,9 Murine and primate in vivo thrombosis model studies10,11 are consistent with prothrombotic mechanism for collagens. Collagens bind factor IX,12 and a mouse model with a variant of factor IX defective in collagen binding manifests impaired hemostasis.3 Laminins are procoagulant via both platelet activation and contact activation.5,7-14 Polyphosphates and polynucleotides support contact activation and other procoagulant reactions,2,15 but none of these enhance prothrombinase activity.
In a pilot exomics rare-variant genotyping study targeting low-frequency exomic variants for their potential association with venous thrombosis,16 we linked a cluster of skeletal muscle myosin heavy chain gene rare missense variants to venous thrombosis. However, myosin is not a member of any conventional pathway affecting thrombosis or hemostasis. Thus, here we report our testing of the hypothesis that myosin can directly influence blood coagulation and thrombosis. We report the remarkable direct effects of skeletal muscle myosin on thrombin generation and fibrin formation in ex vivo studies of flowing human blood and in vitro in thrombin generation assays using both plasma assays and purified prothrombinase assays due to myosin’s ability to bind factors Xa and Va. Moreover, myosin is here implicated as potentially contributing to the thrombin generation potential in plasma from severely injured patients presenting with acute traumatic coagulopathy.
Materials and methods
Additional information for some methods is provided in supplemental Materials and Methods (available on the Blood Web site).
Materials
See supplemental Materials and Methods for more information.
Acute trauma plasmas
Citrated plasma from acute trauma patients (N = 26) and healthy controls (N = 10) were obtained from a single level 1 trauma center.17 Blood samples were collected from severely injured acute trauma patients immediately upon arrival at a level 1 trauma center at the emergency department (San Francisco General Hospital). After a waiver of consent was applied for initial blood draws, informed consent was obtained from all patients, as approved by the University of California Committee on Human Research. Healthy control citrated control plasmas came from 20 healthy consenting adults (10 male, 10 female). Ten controls were recruited through the Scripps blood donation program and 10 controls through the University of California, San Francisco level 1 trauma center. Blood plasmas were stored at −70°C. All study subjects provided informed consent according to each institution’s approved protocols.
Ex vivo whole-blood perfusion experiments
The prothrombotic effects of myosin using fresh recalcified whole human blood under flow conditions were tested as described previously for studies presented in Figure 1 18 and supplemental Figure 1.19 The ex vivo whole-blood perfusion studies used in Figure 1 followed procedures described previously.18 For collagen-coated surfaces, 1.0 mg/mL acid insoluble collagen type I was coated on glass slides for 1 hour, washed with 0.02 M HEPES, 0.135 M NaCl, pH 7.4 (HBS), and incubated for 1 hour with 1 mg/mL bovine serum albumin (BSA)/HBS. Alternatively, glass slides were coated with 20 μg/mL (36 nM) freshly prepared rabbit skeletal muscle myosin in 0.6 M NaCl, 50 mM Tris-HCl buffer (pH 7.4) for 1 hour, followed by 1 wash with HBS and 1-hour incubation with 1 mg/mL BSA dissolved in HBS. The coating with BSA blocks activation of the coagulation contact phase by exposure to glass. Thus, as a negative control, glass slides were coated with BSA/HBS only. Mepacrine HCl at 40 μg/mL was added to blood to render platelets fluorescent. Fibrin was visualized by adding to blood anti–human fibrin β-chain monoclonal immunoglobulin G (IgG) (HB-8545) labeled with Alexa Fluor 546 (Molecular Probes, Eugene, OR). Blood was collected from an antecubital vein into one-tenth final volume of citrate-phosphate-dextrose (CPD; 12.88 mM citrate final concentration) and recalcified to 1.15 mM free Ca2+ immediately before perfusion over the coated glass slides assembled at the bottom of a rectangular flow chamber with a 125 μm height maintained by a silicon gasket. Blood was aspirated through the chamber with a syringe pump (Harvard Apparatus Inc., Holliston, MA) at the wall shear rate of 300 s−1 and was followed by Dulbecco’s modified Eagle’s medium to remove red cells and improve platelet and fibrin visualization by confocal z-sectioning with a Zeiss Axiovert 135M/LSM 410 microscope (Carl Zeiss, Jena, Germany). Image processing and analysis was performed with ImageJ64 (downloaded at http://rsbweb.nih.gov/ij/).
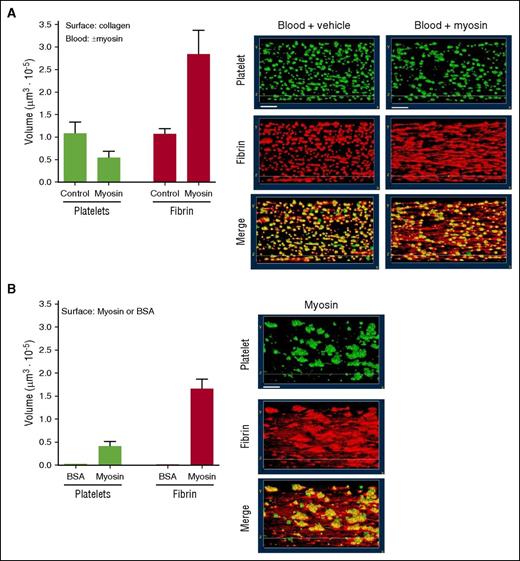
Myosin is thrombogenic in ex vivo studies of fresh human blood under flow. The thrombogenicity of myosin was studied either (A) when myosin (or control vehicle) was added to whole blood that then flowed over collagen-coated surfaces or (B) when whole blood without myosin addition flowed over myosin-coated surfaces. Data represent observations at 4 minutes after flow initiation at 300 s−1 wall shear rate. (A) Either skeletal muscle myosin (50 µg/ml) or control buffer vehicle was added to recalcified blood before its perfusion over a collagen-coated surface. Three-dimensional reconstruction of platelet aggregates and fibrin on a collagen-coated coverslip was generated from confocal z-sections serially collected after blood perfusion. Recalcified flowing human blood contained mepacrine (green) to visualize platelets and Alexa Fluor 546–labeled anti–fibrin antibody (red) to visualize fibrin. Yellow represents superposition of corresponding images at 4 minutes. White bars shown in the top panels indicate 20 μm. The amounts of fibrin and platelet deposition are shown in the bar graphs (average values from 2 experiments). (B) Recalcified human blood was perfused over immobilized myosin at 300 s−1 shear rate. Three-dimensional reconstruction of platelet aggregates and fibrin on myosin or BSA coated coverslip was generated as described for panel A. Images of deposited platelets and fibrin formation after maintaining blood perfusion for 4 minutes are shown. The volumes of fibrin and platelet deposition are shown in the bar graphs (average values from 3 experiments). Human blood perfused over immobilized BSA at 300 s−1 shear rate for 4 minutes did not form detectable platelet aggregates or fibrin (data not shown).
Myosin is thrombogenic in ex vivo studies of fresh human blood under flow. The thrombogenicity of myosin was studied either (A) when myosin (or control vehicle) was added to whole blood that then flowed over collagen-coated surfaces or (B) when whole blood without myosin addition flowed over myosin-coated surfaces. Data represent observations at 4 minutes after flow initiation at 300 s−1 wall shear rate. (A) Either skeletal muscle myosin (50 µg/ml) or control buffer vehicle was added to recalcified blood before its perfusion over a collagen-coated surface. Three-dimensional reconstruction of platelet aggregates and fibrin on a collagen-coated coverslip was generated from confocal z-sections serially collected after blood perfusion. Recalcified flowing human blood contained mepacrine (green) to visualize platelets and Alexa Fluor 546–labeled anti–fibrin antibody (red) to visualize fibrin. Yellow represents superposition of corresponding images at 4 minutes. White bars shown in the top panels indicate 20 μm. The amounts of fibrin and platelet deposition are shown in the bar graphs (average values from 2 experiments). (B) Recalcified human blood was perfused over immobilized myosin at 300 s−1 shear rate. Three-dimensional reconstruction of platelet aggregates and fibrin on myosin or BSA coated coverslip was generated as described for panel A. Images of deposited platelets and fibrin formation after maintaining blood perfusion for 4 minutes are shown. The volumes of fibrin and platelet deposition are shown in the bar graphs (average values from 3 experiments). Human blood perfused over immobilized BSA at 300 s−1 shear rate for 4 minutes did not form detectable platelet aggregates or fibrin (data not shown).
Thrombin generation assay in PRP and PPP
Thrombin generation assays using freshly prepared platelet-rich plasma (PRP) were performed as described20 with some minor modifications. Platelet-poor plasma (PPP) from the same individual donor who provided PRP or pooled normal human PPP were also tested for the plasma thrombin generation assay in the presence or absence of 4 µM phosphatidylcholine-phosphatidylserine (PCPS; 80%:20%) vesicles. Plasmas from acute trauma patients and controls were assayed using tissue factor induced-thrombin generation assays, as described above, in the presence of either polyclonal antiserum against myosin heavy and light chains or nonimmune control antiserum.
Activation of prothrombin by prothrombinase complex
Myosin or PCPS vesicles were incubated with factor Va (5 nM, final) and factor Xa (0.2 nM, final) with 5 mM CaCl2. Thrombin generation was initiated by the addition of prothrombin (0.75 µM, final, unless noted otherwise). The reaction was quenched by 10 mM EDTA, and the rate of thrombin formation was quantified by measuring thrombin concentration as the rate of substrate (Pefa TH) hydrolysis. In separate experiments, des-Gla-domain (DG)-factor Xa was used in place of factor Xa following the same protocols.
Immunoabsorption of myosin using anti–myosin monoclonal antibody–coated beads
Anti–myosin monoclonal antibody (MF-20) or mouse non–immune IgG were immobilized on magnetic beads using Dynabeads Antibody Coupling Kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instruction. The rabbit skeletal muscle myosin in TBSA (50 mM Tris-HCl, 0.15 M NaCl, pH 7.4 containing bovine serum albumin) was incubated at room temperature with either MF-20–coated beads or non–immune mouse IgG–coated beads. Then the beads were separated by DynaMag-Spin, and the supernatants were tested for the prothrombinase enhancement to determine the residual procoagulant activity.
Biolayer interferometry binding studies
The binding kinetics for coagulation proteins (eg, factor Xai, DG-factor Xai, factor Va) binding to immobilized myosin was determined by biolayer interferometry (BLI) using the Octet Red system (Forte Bio, Octet RED96 System, Pall Corporation, see www.fortebio.com/octet-RED96.html). There was negligible nonspecific binding of inactivated factor Xai or DG-factor Xai to anti–myosin antibody–coated sensors in controls in the absence of myosin. Octet Red analysis software (ForteBio) was used to analyze the sensorgram data.
Statistical analysis
Mann-Whitney test and Michaelis constant calculations were performed using Prism 4.03 software (Graph Pad Software Inc., San Diego, CA).
Results
Skeletal muscle myosin promotes thrombus formation in flowing blood
To assay the prothrombotic effects of myosin, recalcified fresh whole human blood was perfused over collagen-coated, BSA-coated, or myosin-coated chambers at the initial wall shear rate of 300 s−1.18 Typical patterns of platelet deposition and fibrin deposition on the collagen-coated surface were seen in the absence of added myosin after 4-minute perfusion as shown by images and by quantification (Figure 1A). The prothrombotic activity of myosin was assessed by adding it to blood before perfusion over a collagen-coated surface; remarkably, addition of myosin to blood resulted in more extensive fibrin strand formation on the collagen surface, but not greater platelet deposition (Figure 1A), indicating myosin under these conditions was procoagulant. To assess if myosin can support platelet deposition and fibrin formation directly, recalcified fresh whole human blood was perfused over a myosin-coated surface. The results show that immobilized myosin supported platelet deposition on which fibrin deposition was enriched in flow-oriented strands (Figure 1B). In controls, recalcified flowing fresh human blood failed to form fibrin strands or to deposit platelets on BSA-coated surfaces (data not shown).
A different experimental design that employed recalcified whole human blood flowing through myosin-coated capillaries and that analyzed thrombus formation after fixation19 was also used to evaluate the myosin’s prothrombotic effects. Recalcified fresh human blood was perfused over the myosin-coated surface under shear at 300 s−1 for 10, 20, and 30 minutes. When thrombus formation was subsequently visually recorded using differential interference contrast microscopy, myosin supported time-dependent increases in platelet adhesion and aggregation and fibrin formation over 10 to 30 minutes (supplemental Figure 1, top). Fibrin strands were visible throughout the myosin-coated capillary at 20 to 30 minutes (supplemental Figure 1). Similarly, fluorescent light microscopy detected fibrin deposition (see blue stain in supplemental Figure 1, bottom) and platelet aggregation (see green for P-selectin deposition and red for integrin αIIb deposition in supplemental Figure 1, bottom) following the perfusion of whole blood over a myosin-coated surface, agreeing with the observed platelet and fibrin deposition detected by differential interference contrast microscopy. In controls, fibrin deposition and platelet aggregation were not visibly detected on BSA-coated surfaces (supplemental Figure 1). Thus, skeletal muscle myosin’s prothrombotic properties were shown using two different methods for visualization of thrombi in two independent ex vivo studies using fresh human blood (Figure 1; supplemental Figure 1).
Effect of rabbit skeletal muscle myosin on thrombin generation in plasma
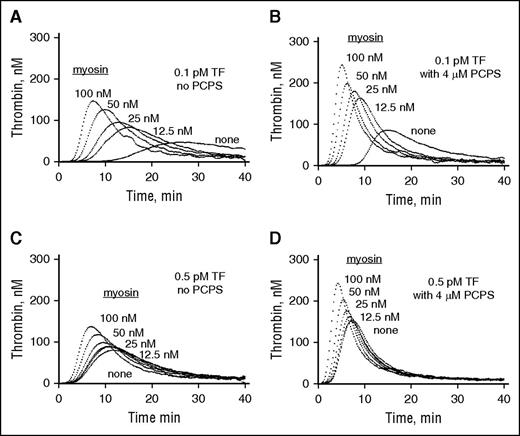
Because there were no previous reports linking myosin to clotting reactions, we studied the effects of skeletal muscle myosin on thrombin generation in plasma. In a concentration-dependent manner, purified rabbit myosin enhanced thrombin generation in PRP when either 0.5 pM tissue factor (TF)/Ca++ ions (Figure 2A) or simple recalcification was used to induce thrombin generation (Figure 2B). Similarly, thrombin generation in PPP was stimulated by myosin when the plasma was freshly prepared from the same source blood used for making PRP and subjected to TF/Ca++ or Ca++ alone (Figure 2C-D). Thus, the ability of myosin to enhance thrombin generation in plasma was primarily independent of platelets. Furthermore, when 0.1 pM or 0.5 pM TF was added to commercially available pooled plasma, myosin concentration-dependently enhanced thrombin generation (Figure 3A,C). Moreover, even when 4 µM phospholipid (PCPS) was present, myosin dose-dependently stimulated thrombin generation in the presence of 0.1 and 0.5 pM TF (Figure 3B,D). Thus, the ability of myosin to enhance TF-initiated thrombin generation in plasma was apparent in the presence as well as the absence of procoagulant phospholipids.
Skeletal muscle myosin promotes thrombin generation in PRP and PPP. Freshly prepared PRP and PPP (30 μL) from the same donor was incubated with various indicated concentrations of myosin for 10 min at 37°C. Then, fluorogenic thrombin substrate solution (I-1140) either with TF (Innovin; final, 0.5 pM) and CaCl2 (final, 11 mM) or with CaCl2 alone (final, 11 mM) was added to the plasma/myosin mixture (total final volume, 110 μL) to initiate thrombin generation at 37°C. For TF-induced thrombin generation assays using PRP and PPP, corn trypsin inhibitor (CTI; final, 50 µg/mL) was added to freshly prepared PRP and PPP immediately after blood was processed to obtain PRP and PPP. Thrombin generation was followed continuously using a SPECTRAmax GEMINI XS fluorometer (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths set at 360 nm and 460 nm, respectively. The first derivative of fluorescence versus time was used to produce thrombin generation curves with the correction for substrate consumption and inner filter effect.20 Thrombin generation is shown for PRP plus 0.5 pM TF-Ca++ and CTI (A) or for only Ca++ addition (C), or for PPP plus 0.5 pM TF-Ca++ and CTI (B) or for only Ca++ addition (D). The same sets of experiments were done for 4 different adult healthy blood donors, and data for 1 donor’s PRP and PPP are shown.
Skeletal muscle myosin promotes thrombin generation in PRP and PPP. Freshly prepared PRP and PPP (30 μL) from the same donor was incubated with various indicated concentrations of myosin for 10 min at 37°C. Then, fluorogenic thrombin substrate solution (I-1140) either with TF (Innovin; final, 0.5 pM) and CaCl2 (final, 11 mM) or with CaCl2 alone (final, 11 mM) was added to the plasma/myosin mixture (total final volume, 110 μL) to initiate thrombin generation at 37°C. For TF-induced thrombin generation assays using PRP and PPP, corn trypsin inhibitor (CTI; final, 50 µg/mL) was added to freshly prepared PRP and PPP immediately after blood was processed to obtain PRP and PPP. Thrombin generation was followed continuously using a SPECTRAmax GEMINI XS fluorometer (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths set at 360 nm and 460 nm, respectively. The first derivative of fluorescence versus time was used to produce thrombin generation curves with the correction for substrate consumption and inner filter effect.20 Thrombin generation is shown for PRP plus 0.5 pM TF-Ca++ and CTI (A) or for only Ca++ addition (C), or for PPP plus 0.5 pM TF-Ca++ and CTI (B) or for only Ca++ addition (D). The same sets of experiments were done for 4 different adult healthy blood donors, and data for 1 donor’s PRP and PPP are shown.
Skeletal muscle myosin promotes thrombin generation when 0.1 pM or 0.5 pM TF is added to pooled normal plasma both in the absence and the presence of 4 µM phospholipid vesicles. CTI (final, 50 µg/mL) was added to freshly thawed pooled normal human plasma (George King Bio Medical Inc., Overland Park, KS). Once-thawed pooled plasma (30 μL) was incubated with various indicated concentrations of myosin for 10 minutes at 37°C. Then, to initiate thrombin generation in the absence (A,C) or presence (B,D) of phospholipid vesicles (4 µM final) (PCPS, 80%/20% wt/wt), fluorogenic thrombin substrate solution (I-1140) with TF at either 0.1 pM (A,B) or 0.5 pM (C,D) (final concentration, as indicated) and CaCl2 (11 mM final) was added.
Skeletal muscle myosin promotes thrombin generation when 0.1 pM or 0.5 pM TF is added to pooled normal plasma both in the absence and the presence of 4 µM phospholipid vesicles. CTI (final, 50 µg/mL) was added to freshly thawed pooled normal human plasma (George King Bio Medical Inc., Overland Park, KS). Once-thawed pooled plasma (30 μL) was incubated with various indicated concentrations of myosin for 10 minutes at 37°C. Then, to initiate thrombin generation in the absence (A,C) or presence (B,D) of phospholipid vesicles (4 µM final) (PCPS, 80%/20% wt/wt), fluorogenic thrombin substrate solution (I-1140) with TF at either 0.1 pM (A,B) or 0.5 pM (C,D) (final concentration, as indicated) and CaCl2 (11 mM final) was added.
Skeletal muscle myosin effects on purified prothrombin activation by purified factors Xa and Va
The final common and most critical step for the extrinsic and intrinsic coagulation pathways involves prothrombin activation to generate thrombin. The potential for myosin to enhance activation of purified prothrombin by purified factor Xa, factor Va, and Ca++ ions (termed the prothrombinase complex) was discovered to be highly noteworthy and led us to perform detailed kinetic studies of prothrombin activation at varying concentrations of myosin and of prothrombin (Figure 4A-B). Similar kinetic studies were performed in parallel on the ability of PCPS phospholipid vesicles containing 20% phosphatidylserine to enhance prothrombinase activity (Figure 4B). Myosin variation, like phospholipid variation, enabled good fits for kinetic parameters for prothrombin activation by the enzymatic complex comprising factors Xa:Va:Ca++. Analysis of the kinetic data yielded the Km of 0.5 μM prothrombin for myosin-enhanced prothrombin activation (supplemental Table 1). Based on curve fitting of data in Figure 4B, for myosin-enhanced prothrombinase, the maximum reaction velocity (Vm) was 836 ± 71/min moles of thrombin per mole of factor Xa. For PCPS-enhanced prothrombinase (Figure 4B), Vm was 429 ± 11/min moles of thrombin per mole of factor Xa.
Skeletal muscle myosin promotes thrombin generation in purified prothrombinase reaction mixtures. (A) The effect of varying concentrations of myosin or prothrombin on the initial rate of prothrombin activation by factor Xa/factor Va. (B) The effects of myosin (●) or PCPS vesicles (Ο) on the initial rate of prothrombin activation by factor Xa/factor Va. (C) The effects on myosin’s enhancement of purified prothrombinase activity for 2 allosteric inhibitors of myosin (8 μM MyoVin-1 and 100 μM trifluoperazine) and for anti–myosin antibodies (abs) (0.9 mg/mL total protein), and the effect of pull-down of myosin using anti–myosin monoclonal antibody beads. (D) The effects of myosin on the initial rate of prothrombin activation by factor Xa/factor Va (●) and by DG-factor Xa/factor Va (Ο). Varying concentrations of myosin or phospholipid vesicles (PCPS, 80%/20% wt/wt) were incubated with factor Va (5 nM, final) and factor Xa (0.2 nM final) in TBS containing 0.5% BSA (TBSA) plus 5 mM CaCl2 at room temperature. Thrombin generation was initiated by the addition of prothrombin (concentration as indicated for panel A, 1.5 μM final for panel B, and 0.75 μM final for panels C and D) in TBSA containing 5 mM CaCl2. The reaction was quenched by adding EDTA (10 mM final) at 5 min (A) or 10 min (C,D). The reactions shown in panel B were quenched by EDTA (10 mM final) at 20, 40, and 60 seconds. Thrombin formation was quantified by the rate of substrate (Pefa TH) hydrolysis. For panel D, DG-factor Xa was used in place of factor Xa following the same protocol.
Skeletal muscle myosin promotes thrombin generation in purified prothrombinase reaction mixtures. (A) The effect of varying concentrations of myosin or prothrombin on the initial rate of prothrombin activation by factor Xa/factor Va. (B) The effects of myosin (●) or PCPS vesicles (Ο) on the initial rate of prothrombin activation by factor Xa/factor Va. (C) The effects on myosin’s enhancement of purified prothrombinase activity for 2 allosteric inhibitors of myosin (8 μM MyoVin-1 and 100 μM trifluoperazine) and for anti–myosin antibodies (abs) (0.9 mg/mL total protein), and the effect of pull-down of myosin using anti–myosin monoclonal antibody beads. (D) The effects of myosin on the initial rate of prothrombin activation by factor Xa/factor Va (●) and by DG-factor Xa/factor Va (Ο). Varying concentrations of myosin or phospholipid vesicles (PCPS, 80%/20% wt/wt) were incubated with factor Va (5 nM, final) and factor Xa (0.2 nM final) in TBS containing 0.5% BSA (TBSA) plus 5 mM CaCl2 at room temperature. Thrombin generation was initiated by the addition of prothrombin (concentration as indicated for panel A, 1.5 μM final for panel B, and 0.75 μM final for panels C and D) in TBSA containing 5 mM CaCl2. The reaction was quenched by adding EDTA (10 mM final) at 5 min (A) or 10 min (C,D). The reactions shown in panel B were quenched by EDTA (10 mM final) at 20, 40, and 60 seconds. Thrombin formation was quantified by the rate of substrate (Pefa TH) hydrolysis. For panel D, DG-factor Xa was used in place of factor Xa following the same protocol.
When Gla domainless (DG)-factor Xa was assayed, myosin retained >30% prothrombinase activity (Figure 4D) whereas, as expected,21-23 DG-factor Xa lost >98% activity in assays using procoagulant phospholipids (supplemental Figure 2). Thus, the Gla domain, which is absolutely essential for phospholipid-enhanced procoagulant activity, was not required for myosin’s enhancement of prothrombinase activity. When prothrombinase assays were done with factor Xa, prothrombin, Ca++ ions, and myosin (2–40 nM) but without addition of factor Va, no significant activation of prothrombin was observed (±15%) (data not shown). Thus, factor Va was required for myosin’s potent enhancement of prothrombinase activity.
To prove that the myosin molecule itself in the skeletal muscle myosin preparation is procoagulant, different myosin-targeting reagents were employed. We used two allosteric inhibitors of myosin, MyoVin-1 and trifluoperazine,24 which alter myosin’s conformation and functional activity, and anti–myosin polyclonal antibodies to study their ability to inhibit myosin’s enhancement of prothrombinase activity. Indeed, each one of the myosin allosteric inhibitors and the anti–myosin antibodies blocked by >80% myosin’s prothrombinase-enhancing action (Figure 4C). In controls, these anti–myosin antibodies did not inhibit prothrombin activation by factor Xa/factor Va in the absence of myosin, nor did they inhibit factor X activation by tissue factor/factor Vlla (supplemental Figure 3). In other studies, pull-down of myosin from myosin solutions using monoclonal anti–myosin heavy chain antibody beads, but not control nonimmune monoclonal antibody beads, removed >85% of myosin’s procoagulant activity (Figure 4C). These combined data indicate that the myosin molecule itself in the myosin preparations is required for the observed enhancement of prothrombin activation by factors Xa and Va in the purified prothrombinase assay mixtures, leading to the hypothesis that myosin binds factors Xa and Va.
BLI binding studies
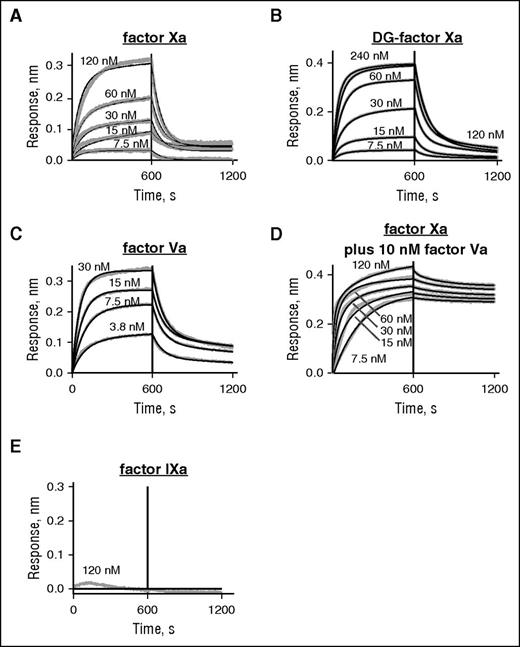
To define direct interactions between myosin and factor Xa or factor Va, we used BLI with the Octet Red system to record the kinetics for binding of inhibited (i) factor Xai and of factor Va to myosin that was immobilized on the Octet Red probe, which carried a monoclonal anti–myosin antibody. Factor Xa was irreversibly inhibited by DEGR-chloromethyl ketone to prevent proteolysis during the study. Factor Xai bound to myosin with a Kd of 51 nM (kon = 3.7 × 105 M−1s−1 and koff = 0.019 s−1) (Figure 5A). Surface plasmon resonance studies for binding of factor Xa to phospholipid vesicles gave similar values.23 In the absence of Ca++, no binding to myosin was observed for factor Xai at up to 120 nM (data not shown). When binding of DG-factor Xai to myosin was analyzed using BLI, the absence of the Gla domain did not ablate binding of the enzyme to immobilized myosin, although the affinity was reduced by one-third (Kd = 175 nM) with moderate differences in rate constants (0.58 × 105 M−1s−1, and 0.010 s−1, respectively) (Figure 5B). This indicates that the primary interaction sites on factor Xa for binding to myosin do not include the Gla domain. Thus, in sharp contrast to procoagulant PS/PC vesicles, myosin’s procoagulant activity and myosin’s ability to bind factor Xa did not require the Gla domain. The specificity for factor Xai was shown by the fact that factor IXai was not detectably bound to immobilized myosin when tested at 120 nM (Figure 5E).
The binding of inactivated factor Xa (Xai) and factor Va to immobilized myosin were determined using BLI and the Octet Red system. Binding studies using BLI were conducted with factor Xai, DG-factor Xai, factor Va, and factor Xai in the presence of factor Va and factor IXai. Proteins were in buffer containing 50 mM Tris (pH 7.4), 0.6 mM MgCl2, 5 mM CaCl2, 0.005% Tween 20, and 0.1% PEG, 300 mM NaCl at room temperature. Skeletal muscle myosin was captured onto the Octet Red probe surface by monoclonal anti–myosin heavy chain (MF20) antibodies, and then after washing, the various proteins were loaded. The protein association and dissociation time courses were then monitored by Octet Red for 30 minutes at room temperature. There was negligible nonspecific binding of inactivated factor Xai or DG-factor Xai or factor Va to anti–myosin antibody coated sensors in the absence of myosin. Octet RED analysis software (ForteBio) was used to analyze the data from sensorgram data. The y-axis indicates the change in optical interference which is a result of the binding response. (A) Sensorgrams depicting the binding of factor Xai to immobilized myosin (ligand concentrations from top to bottom: 120, 60, 30, 15, and 7.5 nM). (B) Sensorgrams depicting the binding of DG-factor Xai to immobilized myosin (ligand concentrations from top to bottom: 240, 120, 60, 30, 15, and 7.5 nM). (C) Sensorgrams depicting the binding of factor Va to immobilized myosin (ligand concentrations from top to bottom: 30, 15, 7.5, and 3.8 nM). (D) Sensorgrams depicting the binding of factor Xai to myosin (ligand concentrations from top to bottom: 120, 60, 30, 15, and 7.5 nM) in the presence of 10 nM factor Va. (E) Sensorgrams depicting the absence of binding of 120 nM factor IXai to immobilized myosin. The solid black lines indicate best fits of the experimental data for association and dissociation reactions and were used to obtain values for kon and koff and calculation of Kd. In the presence of 5 mM EDTA and absence of Ca++ and Mg++ ions, neither factor Xai nor DG-factor Xai detectably bound to myosin (data not shown).
The binding of inactivated factor Xa (Xai) and factor Va to immobilized myosin were determined using BLI and the Octet Red system. Binding studies using BLI were conducted with factor Xai, DG-factor Xai, factor Va, and factor Xai in the presence of factor Va and factor IXai. Proteins were in buffer containing 50 mM Tris (pH 7.4), 0.6 mM MgCl2, 5 mM CaCl2, 0.005% Tween 20, and 0.1% PEG, 300 mM NaCl at room temperature. Skeletal muscle myosin was captured onto the Octet Red probe surface by monoclonal anti–myosin heavy chain (MF20) antibodies, and then after washing, the various proteins were loaded. The protein association and dissociation time courses were then monitored by Octet Red for 30 minutes at room temperature. There was negligible nonspecific binding of inactivated factor Xai or DG-factor Xai or factor Va to anti–myosin antibody coated sensors in the absence of myosin. Octet RED analysis software (ForteBio) was used to analyze the data from sensorgram data. The y-axis indicates the change in optical interference which is a result of the binding response. (A) Sensorgrams depicting the binding of factor Xai to immobilized myosin (ligand concentrations from top to bottom: 120, 60, 30, 15, and 7.5 nM). (B) Sensorgrams depicting the binding of DG-factor Xai to immobilized myosin (ligand concentrations from top to bottom: 240, 120, 60, 30, 15, and 7.5 nM). (C) Sensorgrams depicting the binding of factor Va to immobilized myosin (ligand concentrations from top to bottom: 30, 15, 7.5, and 3.8 nM). (D) Sensorgrams depicting the binding of factor Xai to myosin (ligand concentrations from top to bottom: 120, 60, 30, 15, and 7.5 nM) in the presence of 10 nM factor Va. (E) Sensorgrams depicting the absence of binding of 120 nM factor IXai to immobilized myosin. The solid black lines indicate best fits of the experimental data for association and dissociation reactions and were used to obtain values for kon and koff and calculation of Kd. In the presence of 5 mM EDTA and absence of Ca++ and Mg++ ions, neither factor Xai nor DG-factor Xai detectably bound to myosin (data not shown).
When binding of factor Va alone to immobilized myosin was tested, factor Va bound to myosin with a Kd of 93 nM (kon = 2.5 × 105 M−1s−1 and koff = 0.015 s−1) (Figure 5C). When binding of factor Xai to immobilized myosin in the presence of 10 nM factor Va was analyzed (Figure 5D), data yielded a Kd of 0.48 nM (kon = 7.72 × 105 M−1s−1 and koff = 0.00037s−1), which was due to a 50-fold reduction in koff, indicating that factor Va stabilized the factor Xa:myosin complex, presumably due to ternary complexation.
Myosin-dependent contributions to thrombin generation in acute trauma and normal plasmas
When added to plasma before initiating tissue factor–induced thrombin generation, polyclonal anti–myosin antibodies modestly but significantly reduced thrombin generation in plasma, whereas control polyclonal antibodies did not (supplemental Figure 3). When the effects of anti–myosin antibodies on thrombin generation in plasmas from acute trauma subjects17 were compared with results for healthy control subjects, the reduction of thrombin peak median value by anti–myosin antibodies was 28% for acute traumatic coagulopathy patients, which was significantly greater than the 17% value for healthy control subjects (P = .0004, Mann-Whitney test) (Figure 6A). When the thrombin generation data of this study were quantified in terms of absolute molar amounts of thrombin generation, the reduction of thrombin peak concentration by anti–myosin antibodies was 22 nM, which was almost twofold greater than that for control plasmas (13 nM) (P = .0026, Mann-Whitney test) (Figure 6B), implying that myosin-related thrombin generation potential was almost twice as much in acute trauma plasmas than in healthy subject plasma.
Anti–myosin antibody–induced reduction of thrombin peak values observed for acute trauma coagulopathy plasma and normal control plasma following tissue factor/Ca++ ion addition. The effects of anti–myosin antibodies on the 0.1-pM TF-induced peak of thrombin generation in plasmas from acute traumatic coagulopathy patients (N = 26) and healthy controls (N = 20) were tested using either polyclonal antiserum against myosin heavy and light chains or nonimmune control antiserum, respectively (2 mg/mL protein). The graphs show the reduction of thrombin peak values by anti-myosin antibodies either as percent of control peak (A) or in absolute concentration of the thrombin peak (B). Bars indicate median values.
Anti–myosin antibody–induced reduction of thrombin peak values observed for acute trauma coagulopathy plasma and normal control plasma following tissue factor/Ca++ ion addition. The effects of anti–myosin antibodies on the 0.1-pM TF-induced peak of thrombin generation in plasmas from acute traumatic coagulopathy patients (N = 26) and healthy controls (N = 20) were tested using either polyclonal antiserum against myosin heavy and light chains or nonimmune control antiserum, respectively (2 mg/mL protein). The graphs show the reduction of thrombin peak values by anti-myosin antibodies either as percent of control peak (A) or in absolute concentration of the thrombin peak (B). Bars indicate median values.
Discussion
Studies that were conducted to test the hypothesis that muscle myosin can contribute functional activity to prothrombotic mechanisms16 established the remarkable prothrombotic activities of myosin. Ex vivo experiments using flowing fresh human blood showed that surfaces coated with rabbit skeletal muscle myosin, which is ∼95% homologous to human skeletal muscle myosin, substantially promotes both fibrin deposition and platelet deposition. When myosin is added to flowing blood that is exposed to collagen-coated surfaces, fibrin deposition was greatly increased compared with controls, indicating that myosin particularly promotes fibrin strand formation in flowing blood. The impression that myosin particularly promotes fibrin formation was strengthened by results from in vitro studies showing that skeletal myosin enhances thrombin generation in either PRP or PPP. This finding showed that myosin strongly exerts procoagulant activity, even in plasma without platelets.
To identify potential mechanisms for myosin’s enhancement of thrombin generation, we focused on the most critical step that is common to the extrinsic and intrinsic coagulation pathways, namely prothrombin activation. In prothrombinase assays comprising only myosin and clotting factors, myosin strongly enhances prothrombin activation. This was true whether or not substantial levels of procoagulant phospholipid vesicles (eg, 4 µM PCPS) were added to the reaction mixtures. Moreover, myosin supports the activation of prothrombin by Gla-domainless-factor Xa and factor Va, whereas phospholipid vesicles fail to do so, thereby defining a unique attribute of myosin’s ability to promote thrombin generation.
Consistent with the hypothesis that myosin assembles the prothrombinase factor Xa:factor Va complex on its surface, myosin is capable of binding both factor Xa and factor Va individually, and the latter factor greatly enhances the binding of the former factor such that the dissociation constant of factor Xa for myosin is <1 nM in the presence of factor Va. Thus, the functional assays and biophysical binding studies provide a clear mechanism for the ability of myosin to enhance thrombin generation, namely that myosin assembles a stable prothrombinase complex.
Another remarkable finding in this report is the fact that analysis of the enzyme kinetics for myosin’s enhancement of prothrombinase activity (Figure 4B) yielded a very high value of 836 min−1 for the catalytic rate constant, ie, for the catalytic efficiency of prothrombin activation by the factor Xa:Va:Ca++ complex associated with myosin. This catalytic rate constant value for myosin was somewhat greater than the 429 min−1 value determined in parallel for PCPS (80:20) vesicles using the same reagents and protocol. At 2 μM myosin (Figure 4B), the Vm was 490 moles of thrombin per mole of prothrombinase per minute. Comparison of this observed value to other Vm values measured for phospholipid enhancement of prothrombinase24-27 (see supplemental Table 2) indicates that myosin exerts a procoagulant potency within the generally reported range for PCPS vesicles.
Our findings provoke a potential paradigm shift for how surface-bound prothrombinase activities might contribute to hemostasis or thrombosis when blood encounters damaged tissues that expose procoagulant myosins. Tissue damage exposes cytoskeletal and extracellular matrix structural proteins and intracellular proteins, including laminins, collagens, tissue factor, and polyphosphates that trigger platelet activation and/or that can stimulate activation of intrinsic pathway and extrinsic pathway.1-12 These reactions that are promoted by exposed structural proteins initiate activation of the intrinsic pathway and the extrinsic pathway to generate factor Xa. In turn, the structural protein, myosin, can bind factor Xa with factor Va to form a stable, potent prothrombinase complex (see Figure 7). This new scheme for a novel prothrombinase complex and thrombin generation must be integrated with currently appreciated clotting schemes1-12 and platelet-dependent reactions5-7 to conceptualize the eventual physiologic or pathologic outcome following different types of damage to tissues. The outcome is ultimately determined by the overall integration in time and space of reactions involving cellular and fluid phase components of blood that interact with damaged tissue and with each other under varying flow conditions.
Thrombin generation can be driven by prothrombin activation on the surface of myosin, which binds factors Xa and Va. The intrinsic and extrinsic coagulation pathways converge at the generation of factor Xa, which is the key enzyme that activates prothrombin. As depicted here, both factor Xa and its cofactor, factor Va, bind to myosin, which potently promotes prothrombin activation to generate thrombin. Extensive data in this report lead to this novel scheme for prothrombin activation, which may occur on the surface of myosin independent of any particular cell surface.
Thrombin generation can be driven by prothrombin activation on the surface of myosin, which binds factors Xa and Va. The intrinsic and extrinsic coagulation pathways converge at the generation of factor Xa, which is the key enzyme that activates prothrombin. As depicted here, both factor Xa and its cofactor, factor Va, bind to myosin, which potently promotes prothrombin activation to generate thrombin. Extensive data in this report lead to this novel scheme for prothrombin activation, which may occur on the surface of myosin independent of any particular cell surface.
Myosins are a large family of motor proteins sharing common features of ATP hydrolysis, actin binding, and potential for kinetic energy transduction.28 The conventional myosins consist of a dimer of heterotrimers,28 and they are very abundant and constitute primary contractile elements in the sarcomeres of skeletal, cardiac, and smooth muscles. Skeletal muscle myosin was originally isolated from muscle cells but is broadly found in the body, even in plasma (eg, at 20 nM for conventional skeletal muscle myosin heavy chain and at 50 nM for light chain) (see Multi-Omics Profiling Expression Database [MOPED] at https://www.proteinspire.org/MOPED/). Plasma myosin heavy- or light-chain levels were reported to be relatively elevated by sevenfold to 10-fold above baseline in patients with muscle damage (ie, rhabdomyolysis)29-31 (see also MOPED). Plasma levels of β-type myosin heavy chains are sometimes elevated by 10-fold or more in polymyositis or dermatomyositis patients,32 and polymyositis or dermatomyositis are associated with venous thrombosis risk.33 Acute trauma is a well-recognized risk factor for venous thromboembolism and is associated with derangements of procoagulant and anticoagulant systems, although mechanism(s) for injury-provoked venous thromboembolism remain somewhat unclear due to the complexities of acute trauma.34-37 Much work is needed to define structure–activity relationship for myosin’s procoagulant activities, as nothing is currently known about myosin’s effects on either hemostasis or thrombosis.
One implication of the hypothesis that myosin is procoagulant (Figure 7) is that acute trauma with extensive tissue damage might lead to myosin-induced thrombin generation where factor Xa is generated, potentially with either beneficial or pathologic consequences. Studies of TF-induced thrombin generation in plasma showed that anti–myosin antibodies reduce thrombin generation significantly more in plasma from patients with acute traumatic coagulopathy than in control plasma (ie, by 28% vs 17%; P < .0004). This indicates that myosin, which circulates in human plasma, may contribute significantly more to the procoagulant potential of acute trauma patient plasma than to that of healthy control plasma. Further detailed studies of the potential contributions of myosins to postinjury dyshomeostasis as a result of massive tissue injuries are warranted.
In summary, we discovered that skeletal muscle myosins exert prothrombotic activity in flowing whole blood and procoagulant activity in plasma. Studies using purified clotting factors show that myosin enhances thrombin generation due to binding of factors Xa and Va. Myosin in the plasma of acute trauma patients significantly increases the potential for thrombin generation compared with controls. These findings define a mechanism for myosin’s procoagulant activity and imply that myosin might significantly and physiologically contribute to achieve hemostasis and/or promote thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported, in part, by National Institutes of Health, National Heart, Lung, and Blood Institute grants RO1 HL021544, RO1 HL052246, and PO1 HL031950 (J.H.G.); National Center for Advancing Translational Sciences grant 8UL1 TR000109 (Clinical and Translational Science Award, principal investigator: Eric Topol); National Heart, Lung, and Blood Institute grant UO1 HL077863 and Department of Defense grant W81XWH-10-1-0509 (M.J.C.); National Heart, Lung, and Blood Institute grants PO1 HL031950 and RO1 HL117722 (Z.M.R.); and National Heart, Lung, and Blood Institute grant R01HL101972 and National Institute of General Medical Sciences grant R01GM116184 (O.J.T.M); and by a grant from the Stein Endowment Fund (courtesy of J. W. Kelly, Scripps Research Institute).
Authorship
Contribution: J.H.G. and H.D. participated in the conception of the study and the overall supervision of integrating all studies; J.Z.-R., O.J.T.M., P.M., and Z.M.R. performed ex vivo whole-blood experiments under flow conditions; H.D. and R.K.S performed experiments using purified proteins and plasmas; M.J.C. organized the acute trauma patient plasma study, obtained consent from trauma patients and control subjects, and collected blood specimens; and H.D. and J.H.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John H. Griffin, Department of Molecular and Experimental Medicine, The Scripps Research Institute, MEM180, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: jgriffin@scripps.edu.