Key Points
The frequency of PTS, PE, and DVT recurrence was higher in children with Non-LR DVT than in children with LR DVT.
Thrombus resolution, DVT triggering event, and sex were predictors of LE PTS in children.
Abstract
Pediatric lower extremity deep vein thrombosis (LE-DVT) can lead to postthrombotic syndrome (PTS) and other adverse events. We investigated the outcomes of LE-DVT in children. Three groups were compared: non–line-related (Non-LR) DVT, LR DVT in neonates (LRneonates), and LR DVT in non-neonates (LRnon-neonates). A total of 339 children were included (Non-LR, n = 56; LRneonates, n = 95; and LRnon-neonates, n = 188). We found a statistically significant difference in the frequency of PTS (P = .04; 62.5%, 40.0%, and 46.3% in Non-LR, LRneonates, and LRnon-neonates, respectively), of recurrent LE-DVT (P = .001; 10.7% and 2.0% in Non-LR and LRnon-neonates, respectively), and pulmonary embolism (PE) (P < .001; 19.6% and 3.2% in Non-LR and LRnon-neonates, respectively) among groups. There was no difference in DVT resolution (P = .41). Multivariable analysis showed that DVT resolution, triggering event, and sex predicted Modified Villalta Scale (MVS; for pediatric PTS) scores >1; there was an interaction between DVT triggering event and sex. The time to reach an MVS >1 was significantly different when comparing groups (log-rank test, P < .001). Moreover, we found a significant difference in baseline MVS scores among groups, but the difference did not appear to change over time. In conclusion, LR LE-DVT had more benign outcomes than Non-LR DVT. Sex, DVT triggering event, and DVT resolution predicted LE-PTS in our cohort.
Background
Venous thrombotic events (VTE) are increasingly being diagnosed in the pediatric population. A study reported an escalation from 34 to 58 cases per 10 000 hospital admissions between 2001 and 2007 across tertiary care hospitals in the United States.1 The rising frequency of VTE can be explained by the availability of better diagnostic imaging, increased awareness about thrombotic conditions in children, and the improved survival of otherwise fatal conditions, as a result of which children are exposed to the complications of more aggressive treatments. As a consequence of the increased number of VTE cases, the number of VTE-related complications is also expected to rise and become a relevant healthcare problem in pediatric care.
Potential complications of VTE located in the deep venous system include recurrent thrombosis, pulmonary embolism (PE), postthrombotic syndrome (PTS), and death.2 PTS is defined as “chronic venous symptoms and/or signs secondary to deep vein thrombosis (DVT) and its sequelae.”3,4 This potentially disabling complication has been reported to affect ∼1 in 2 to 5 adult patients following lower extremity (LE) DVT,5 and ∼1 in 4 to 6 children who sustained thrombotic events in either the upper or lower venous territory.6
Given its relatively higher frequency, a large body of research in the adult population has focused on LE-DVT and ensuing PTS. In contrast, little is known about LE-DVT and LE-PTS in children. Whereas during the past decades a great research effort has been devoted to the investigation of risk factors and prevention strategies for LE-PTS affecting adult patients, only recently has the pediatric literature evolved from researching VTE in general to studying of the natural history of VTE at specific sites, such as the upper extremity (UE)7 or LE.8
The present study aimed to describe the outcomes of LE-DVT in children. The main outcome of the study was LE-PTS. We sought to describe its frequency, predictors, and change over time. Secondary objectives included the investigation of DVT resolution, recurrence, PE, and death associated with LE-DVT.
Methods
This retrospective study included children with diagnosis of a first episode of LE-DVT who were followed up at the Thrombosis Clinic at The Hospital for Sick Children between January 2000 and December 2014. The Ethics Review Board approved the study; informed consent was waived.
The health records of clinic patients who had LE-DVT confirmed by Doppler ultrasound (DUS) and/or venography and at least 1 PTS clinical assessment ≥6 months after diagnosis of LE-DVT were reviewed. According to the institutional protocol, patients were assessed for PTS every 18 months from end of DVT therapy until transferred to adult care.7
The following data were extracted from the patients’ health records:
Descriptive variables and predictors
Patient demographics included sex, age at diagnosis of LE-DVT, underlying condition at the time of diagnosis, use of oral contraceptives, and thrombophilia testing results. Thrombophilia testing followed local laboratory protocols (see supplemental Materials, available on the Blood Web site). Results were classified as no, minor, or major thrombophilia. Minor thrombophilia was defined as the documentation of elevated factor VIII (FVIII) on at least 2 occasions, at least 12 weeks apart, elevated plasma lipoprotein(a) on at least 2 occasions and at least 12 weeks apart, and heterozygous factor V Leiden (FVL), or prothrombin gene (PTG) mutation. Major thrombophilia was defined as the presence of protein C (PC), protein S (PS), or antithrombin (AT) deficiency, positive lupus anticoagulant (LAC), or anticardiolipin antibodies (ACLAs) on at least 2 occasions, FVL or PTG homozygous mutation, or double-heterozygous FVL/PTG mutation.
The presence of thrombosis and positive LAC and/or ACLAs at least 12 weeks apart were considered indicative of antiphospholipid antibody syndrome (APS).9
Based on the triggering event of the index thrombosis, patients were classified in 3 groups. The first group included patients with DVT not triggered by a central catheter (Non-LR group). The remaining patients sustained a thrombotic event triggered by the presence of a central catheter, including central venous lines (CVLs), and peripherally inserted central catheters (PICCs). Patients with LR LE-DVT were divided into 2 groups according to age at the time of sustaining LE-DVT: neonates (0-28 days of age, LRneonates group) and non-neonates (>28 days of age, LRnon-neonates group).
Management of index LE-DVT included treatment of the thrombotic event, delay to start therapy, dose (full anticoagulation vs prophylaxis12 ), and length of treatment.
Outcomes
Main outcome: The main outcome of the study was PTS, as measured by the Modified Villalta Scale (MVS) (supplemental Table 1)6,13 in agreement with the recommendations of the International Society of Thrombosis and Hemostasis (ISTH) Pediatric/Neonatal Thrombosis and Haemostasis Subcommittee.13,14 MVS scores were calculated from signs and symptoms recorded at each clinical assessment; PTS severity was classified as mild, moderate, or severe according to the MVS score. To ensure accuracy in outcome assessment, 2 data collectors estimated MVS scores. Disagreements were resolved by a third person.
Secondary outcomes: LE-DVT resolution, recurrent LE-DVT, PE, and LE-DVT–related death were the secondary outcomes of the study. LE-DVT resolution referred to the radiologic resolution documented at the end of therapy, and was classified as complete, partial (wall thickening or worse),15 no resolution, or extension.16 For patients undergoing thrombolysis, “end of therapy” referred to the time immediately after this procedure (ie, end of thrombolysis). For patients receiving anticoagulants but not thrombolysis, “end of therapy” referred to the time when anticoagulants were discontinued (usually 3 to 6 months after DVT diagnosis). For patients who did not receive antithrombotic drugs, resolution was measured at 3 months after diagnosis of LE-DVT. Recurrent LE-DVT was defined according to current ISTH recommendations.16
Statistical analysis
Categorical variables were summarized using percentages and ratios; continuous variables were summarized using appropriate measures of central tendency and dispersion, according to data distribution.
Three main groups, according to the DVT triggering event, were compared throughout the study: Non-LR LE-DVT (Non-LR), LR LE-DVT in neonates (LRneonates), and LR LE-DVT in non-neonates (LRnon-neonates). Patient demographics, characteristics of the index LE-DVT, and management were compared across groups using the χ2 test, Fisher’s exact test, and Kruskal-Wallis test, as appropriate.
Main outcome.
The frequency of PTS at the time of the last clinic follow-up was compared among groups using the Fisher’s exact test.
The association between PTS and the following predictors was investigated using univariable logistic regression: triggering event (Non-LR, LRneonates, LRnon-neonates), sex, thrombophilia (minor, major, none), DVT level (ilio-femoreal, femoro-popliteal), degree of occlusion (at least one occlusive segment at DVT diagnosis: yes, no), treatment modality (anticoagulant, thrombolysis, no treatment), resolution (complete, any other), and recurrence (yes, no); continuous predictors included age at the time of LE-DVT, number of affected venous segments, length of therapy, and delay to start therapy. PTS was categorized as present (MVS score, >1) or absent (MVS score, 0-1). An MVS score >1 was chosen to avoid overcalling PTS, given the retrospective nature of the data.
A multivariable logistic regression model was built following the technique described by Hosmer, Lemeshow, and Sturdivant.17 Multicollinearity among potential predictors and influential outliers were investigated. Model fit was assessed with the Hosmer-Lemeshow test and c-statistics. Different regression models were compared using the Akaike Information Criterion (AIC). The final model was selected according to the lowest AIC and the clinical significance of its predictors.
In addition, taking advantage of the longitudinal nature of the data, the time from DVT to MVS score >1 was analyzed using the Kaplan-Meier method. Survival curves for the 3 main groups were compared using the log-rank test.
Finally, differences in MVS scores at baseline and changes over time, as a function of the 3 main groups, were investigated in all patients who had at least two PTS assessments. To this end, a multilevel model was fit. A random intercept, random slope model was selected for parameter estimation, based on AIC. The autoregressive linear correlation structure on the residuals was chosen, based on AIC and model parsimony. The changes over time in limb circumference difference were also explored.
Secondary outcomes.
The incidence of LE-DVT resolution, LE-DVT recurrence, PE, and LE-DVT–related death were compared across the main groups. Predictors of LE-DVT resolution (triggering event, age at the time of LE-DVT, sex, thrombophilia, number of affected venous segments, degree of occlusion, treatment modality, and DVT level) were investigated using univariable and multivariable logistic regression, as described above.
Significance level was set at 0.05.
Data analysis was generated using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 354 children were diagnosed and followed up at the thrombosis clinic during the study period. Fifteen patients were excluded due to lack of PTS follow-up data (n = 6), cancer-related leg amputation (n = 2), or tumor-thrombus/mass compression (n = 7). Hence, 339 children were included in the present study.
There were 157 additional cases of LE-DVT diagnosed during the study period; 46 of these patients died without ever being seen in clinic (9%), and 111 (22%) were not followed up at our institution.
Patient characteristics and management of the index LE-DVT at diagnosis are shown in Table 1.
Characteristics of the cohort and index thrombotic event per group
| Variable . | Non-LR (N = 56) . | LRneonates (N = 95) . | LRnon-neonates (N = 188) . | P . |
|---|---|---|---|---|
| Age at DVT diagnosis, median (25th, 75th percentile) | 14.5 y (12.2-16.4) | 8 d (4-16) | 198 d (67-931) | <.001*† |
| Follow-up time, median (25th, 75th percentile), y | 2.0 (1.1-4.5) | 4.3 (2.5-9.6) | 3.5 (2.1-9.3) | <.001* |
| Sex ratio (male/female) | 0.7 | 1.4 | 1.4 | .05‡ |
| Underlying conditions at the time of DVT diagnosis, n (%) | ||||
| None | 34 (61%) | 0 (0%) | 0 (0%) | — |
| CHD | 0 (0%) | 60 (63%) | 68 (36%) | |
| Cancer | 2 (3%) | 1 (1%) | 6 (3%) | |
| Autoimmune and inflammatory diseases | 8 (14%) | 1 (1%) | 3 (2%) | |
| Infectious disease | 7 (13%) | 2 (2%) | 29 (15.5%) | |
| Prematurity | 0 (0%) | 8 (8%) | 20 (11%) | |
| Other | 5 (9%) | 9 (10%) | 55 (29%) | |
| CDH | 0 (0%) | 14 (15%) | 1 (0.5%) | |
| HUS | 0 (0%) | 0 (0%) | 6 (3%) | |
| Thrombophilia,§n (%) | ||||
| Major | 18 (34%) | 3 (4%) | 9 (7%) | .001|| |
| Minor | 17 (32%) | 10 (15%) | 9 (7%) | |
| None | 18 (34%) | 55 (81%) | 113 (86%) | |
| Symptoms of acute DVT, n (%) | 51 (91%) | 79 (83%) | 149 (79%) | .11|| |
| DVT location, n (%) | ||||
| Ilio-femoral | 42 (75%) | 95 (100%) | 184 (98%) | <.001|| |
| Femoro-popliteal | 14 (25%) | 0 (0%) | 4 (2%) | |
| Numbers of segments affected, median (25th, 75th percentile) | 3.5 (2-5) | 2 (2-3) | 2 (1-3) | <.001* |
| Occlusive DVT (at least 1 segment), n (%)¶ | 48 (87%) | 80 (88%) | 134 (74%) | .01|| |
| Laterality, n (%) | ||||
| Right | 28 (50%) | 60 (63%) | 107 (57%) | .61‡ |
| Left | 24 (43%) | 29 (31%) | 68 (36%) | |
| Bilateral | 4 (7%) | 6 (6%) | 13 (7%) | |
| Therapeutic modality for DVT, n (%) | ||||
| Anticoagulant treatment | 36 (64%) | 92 (97%) | 172 (91.5%) | <.001|| |
| Thrombolysis | 18 (32%) | 0 (0%) | 1 (0.5%) | |
| No treatment or prophylaxis only | 2 (4%) | 3 (3%) | 15 (8%) | |
| Delay to full dose therapy, median (25th, 75th percentile), d | 0 (0-2) | 0 (0-1) | 0 (0-1) | .11* |
| Length of treatment, median (25th, 75th percentile), d | 365 (181-381) | 91 (83-128) | 92 (87-109) | <.001* |
| Variable . | Non-LR (N = 56) . | LRneonates (N = 95) . | LRnon-neonates (N = 188) . | P . |
|---|---|---|---|---|
| Age at DVT diagnosis, median (25th, 75th percentile) | 14.5 y (12.2-16.4) | 8 d (4-16) | 198 d (67-931) | <.001*† |
| Follow-up time, median (25th, 75th percentile), y | 2.0 (1.1-4.5) | 4.3 (2.5-9.6) | 3.5 (2.1-9.3) | <.001* |
| Sex ratio (male/female) | 0.7 | 1.4 | 1.4 | .05‡ |
| Underlying conditions at the time of DVT diagnosis, n (%) | ||||
| None | 34 (61%) | 0 (0%) | 0 (0%) | — |
| CHD | 0 (0%) | 60 (63%) | 68 (36%) | |
| Cancer | 2 (3%) | 1 (1%) | 6 (3%) | |
| Autoimmune and inflammatory diseases | 8 (14%) | 1 (1%) | 3 (2%) | |
| Infectious disease | 7 (13%) | 2 (2%) | 29 (15.5%) | |
| Prematurity | 0 (0%) | 8 (8%) | 20 (11%) | |
| Other | 5 (9%) | 9 (10%) | 55 (29%) | |
| CDH | 0 (0%) | 14 (15%) | 1 (0.5%) | |
| HUS | 0 (0%) | 0 (0%) | 6 (3%) | |
| Thrombophilia,§n (%) | ||||
| Major | 18 (34%) | 3 (4%) | 9 (7%) | .001|| |
| Minor | 17 (32%) | 10 (15%) | 9 (7%) | |
| None | 18 (34%) | 55 (81%) | 113 (86%) | |
| Symptoms of acute DVT, n (%) | 51 (91%) | 79 (83%) | 149 (79%) | .11|| |
| DVT location, n (%) | ||||
| Ilio-femoral | 42 (75%) | 95 (100%) | 184 (98%) | <.001|| |
| Femoro-popliteal | 14 (25%) | 0 (0%) | 4 (2%) | |
| Numbers of segments affected, median (25th, 75th percentile) | 3.5 (2-5) | 2 (2-3) | 2 (1-3) | <.001* |
| Occlusive DVT (at least 1 segment), n (%)¶ | 48 (87%) | 80 (88%) | 134 (74%) | .01|| |
| Laterality, n (%) | ||||
| Right | 28 (50%) | 60 (63%) | 107 (57%) | .61‡ |
| Left | 24 (43%) | 29 (31%) | 68 (36%) | |
| Bilateral | 4 (7%) | 6 (6%) | 13 (7%) | |
| Therapeutic modality for DVT, n (%) | ||||
| Anticoagulant treatment | 36 (64%) | 92 (97%) | 172 (91.5%) | <.001|| |
| Thrombolysis | 18 (32%) | 0 (0%) | 1 (0.5%) | |
| No treatment or prophylaxis only | 2 (4%) | 3 (3%) | 15 (8%) | |
| Delay to full dose therapy, median (25th, 75th percentile), d | 0 (0-2) | 0 (0-1) | 0 (0-1) | .11* |
| Length of treatment, median (25th, 75th percentile), d | 365 (181-381) | 91 (83-128) | 92 (87-109) | <.001* |
CDH, congenital diaphragmatic hernia; CHD, congenital heart disease; HUS, hemolytic uremic syndrome; IBD, inflammatory bowel disease.
Kruskal-Wallis test.
Same P value for comparison between LR vs Non-LR groups.
χ2 test.
Minor thrombophilia: increased FVIII or plasma lipoprotein(a), heterozygous FVL, or PTG mutation. Major thrombophilia: PC, PS, or AT deficiency, positive LAC or ACLAs, FVL or PTG homozygous mutation, or double-heterozygous FVL/PTG mutation.
Fisher’s exact test.
Information available for Non-LR (n = 55), LRneonates (n = 91), and LRnon-neonates (n = 181).
Patients in the Non-LR group were older than patients in the LR groups, and were followed up for a shorter period of time. There was a predominance of males in the LR groups.
Almost two-thirds of the patients in the Non-LR group (34/56, 61%) had no overt underlying condition at the time of LE-DVT diagnosis. Within this subset of patients, 53% (18/34) were subsequently found to have venous anatomic variants of the LE venous system, 18% (6/34) were later diagnosed with a rheumatologic disease, and 9% (3/34) developed inflammatory bowel disease. Considering all the patients with Non-LR thrombosis (ie, those who had and who did not have an overt underlying condition at the time of LE-DVT diagnosis), 23 were diagnosed with anatomic variants. Thirteen patients had May-Thurner syndrome and 10 patients had anomalies of the inferior vena cava. Of the 33 females in the Non-LR group, 13 (39%) had been receiving oral contraceptives at the time of DVT diagnosis for a median of 4.5 months (range, 1-36 months).
Almost 3/4 of the cohort was tested for thrombophilia (Non-LR, n = 53/56 [95%], LRneonates, n = 68/95 [72%], and LRnon-neonates, n = 131/188 [70%]). In the Non-LR group, minor thrombophilia findings included elevated FVIII (13/53, 25%), and PTG/FVL heterozygosity (4/53, 7.5%); major thrombophilia consisted of LAC and/or ACLAs (9/53, 17%), and PS, PC, and/or AT deficiency (9/53, 17%). Nine of these patients (9/53, 17%) had more than one trait. Ten of 68 tested LRneonates had minor thrombophilia (15%; FVL, n = 8 and elevated FVIII, n = 2), and 3 had major thrombophilia (4%; ACLA, n = 2 and PS deficiency, n = 1). Lastly, among tested LRnon-neonates, minor thrombophilia comprised elevated FVIII (5/131, 4%) and PTG/FVL heterozygous mutations or elevated lipoprotein(a) (4/131, 3%), whereas major thrombophilia included PS, PC, and/or AT deficiency (5/131, 4%) and ACLA/LAC (4/131, 3%).
Anticoagulant treatment was the most common therapeutic modality. Thrombolysis in the Non-LR group was performed using the AngioJet rheolytic thrombectomy with tissue plasminogen activator in 10 patients (56% of the 18 cases submitted to this therapy), the Trellis peripheral infusion system and tissue plasminogen activator in 5 patients (28%), and both devices in 2 patients (11%). Intrathrombus catheter-directed thrombolysis was performed in the remaining case.
Outcomes
PTS.
PTS at last clinic follow up was more frequent in the Non-LR group (Table 2), as compared with patients in the LR groups. Table 3 shows the distribution of clinical findings across groups.
Frequency of primary and secondary outcomes per group
| Outcome . | Non-LR (N = 56) . | LRneonates (N = 95) . | LRnon-neonates (N = 188) . | P . |
|---|---|---|---|---|
| PTS, % (n) | ||||
| No | 37.5% (21) | 60.0% (57) | 53.7% (101) | .04* |
| Mild | 57.1% (32) | 39.0% (37) | 44.7% (84) | |
| Moderate | 5.4% (3) | 1.0% (1) | 1.6% (3) | |
| Resolution,‡% (n) | ||||
| Complete resolution | 19.6% (11) | 30.9% (29) | 30.0% (56) | .41† |
| Partial resolution | 55.4% (31) | 40.4% (38) | 43.8% (82) | |
| No resolution or extension | 25.0% (14) | 28.7% (27) | 26.2% (49) | |
| Recurrence, % (n) | 10.7% (6) | 0 | 2.1% (4) | .001* |
| PE, % (n) | 19.6% (11) | 0 | 3.2% (6) | <.001* |
| Outcome . | Non-LR (N = 56) . | LRneonates (N = 95) . | LRnon-neonates (N = 188) . | P . |
|---|---|---|---|---|
| PTS, % (n) | ||||
| No | 37.5% (21) | 60.0% (57) | 53.7% (101) | .04* |
| Mild | 57.1% (32) | 39.0% (37) | 44.7% (84) | |
| Moderate | 5.4% (3) | 1.0% (1) | 1.6% (3) | |
| Resolution,‡% (n) | ||||
| Complete resolution | 19.6% (11) | 30.9% (29) | 30.0% (56) | .41† |
| Partial resolution | 55.4% (31) | 40.4% (38) | 43.8% (82) | |
| No resolution or extension | 25.0% (14) | 28.7% (27) | 26.2% (49) | |
| Recurrence, % (n) | 10.7% (6) | 0 | 2.1% (4) | .001* |
| PE, % (n) | 19.6% (11) | 0 | 3.2% (6) | <.001* |
Fisher’s exact test.
χ2 test.
One patient in the LRneonate and 1 in the LRnon-neonate group did not have follow-up imaging (n = 94 and n = 187, respectively).
Frequency of clinical findings at last follow-up per group
| . | Non-LR % (n) . | LRneonates % (n) . | LRnon-neonates % (n) . |
|---|---|---|---|
| Increase in limb circumference | 42.8% (24) | 25.3% (24) | 35.1% (66) |
| Collateral vessels | 25.0% (14) | 21.1% (20) | 25.5% (48) |
| Pain or abnormal use | 16.1% (9) | 6.3% (6) | 9.6% (18) |
| Swelling (as a symptom) | 17.9% (10) | 2.1% (2) | 10.6% (20) |
| Varicosities | 5.4% (3) | 2.1% (2) | 1.6% (3) |
| Change in skin color | 1.8% (1) | 0 | 2.1% (4) |
| . | Non-LR % (n) . | LRneonates % (n) . | LRnon-neonates % (n) . |
|---|---|---|---|
| Increase in limb circumference | 42.8% (24) | 25.3% (24) | 35.1% (66) |
| Collateral vessels | 25.0% (14) | 21.1% (20) | 25.5% (48) |
| Pain or abnormal use | 16.1% (9) | 6.3% (6) | 9.6% (18) |
| Swelling (as a symptom) | 17.9% (10) | 2.1% (2) | 10.6% (20) |
| Varicosities | 5.4% (3) | 2.1% (2) | 1.6% (3) |
| Change in skin color | 1.8% (1) | 0 | 2.1% (4) |
The results of univariable logistic regression for potential predictors of PTS are shown in Table 4. The final model (multivariable logistic regression) included 3 predictors: the degree of resolution at the end of treatment, sex, and group. An interaction between the latter 2 predictors was found (Table 4).
ORs for the outcome PTS
| Predictor . | Univariable analysis OR (95% CI) . | P . |
|---|---|---|
| Group | ||
| Non-LR vs LRnon-neonates | 2.17 (1.12-4.20) | .01 |
| LRneonates vs LRnon-neonates | 0.67 (0.34-1.33) | |
| Treatment | ||
| None/prophylaxis vs thrombolysis | 0.20 (0.04-0.90) | .02 |
| Anticoagulants vs thrombolysis | 0.26 (0.10-0.66) | |
| Delay to start therapy, d | 1.03 (0.99-1.06) | .16 |
| Length of therapy, mo | 1.02 (0.98-1.05) | .37 |
| Age at the time of LE-DVT, y | 1.08 (1.03-1.12) | <.001 |
| Sex (male vs female) | 1.38 (0.80-2.38) | .24 |
| Number of affected venous segments | 1.36 (1.12-1.66) | .002 |
| DVT level (femoro-popliteal vs ilio-femoral) | 0.48 (0.11-2.15) | .34 |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 2.20 (0.95-5.09) | .07 |
| Recurrence (yes vs no) | 0.99 (0.21-4.78) | .98 |
| Thrombophilia | ||
| None vs major | 0.71 (0.29-1.72) | .78 |
| Minor vs major | 0.79 (0.25-2.43) | |
| DVT resolution (none/partial vs complete) | 3.13 (1.49-6.67) | .003 |
| Predictor (final model) | Multivariable analysis OR (95% CI) | P |
| Omnibus Likelihood Ratio | <.001 | |
| DVT resolution (none/partial vs complete) | 3.15 (1.47-6.72) | .003 |
| Sex and group interaction | ||
| Male vs female in Non-LR | 0.59 (0.18-1.94) | .04 |
| Male vs female in LR | 2.50 (1.25-5.00) | |
| Non-LR vs LR among males | 1.17 (0.42-3.24) | |
| Non-LR vs LR among females | 4.92 (1.97-12.30) | |
| Predictor . | Univariable analysis OR (95% CI) . | P . |
|---|---|---|
| Group | ||
| Non-LR vs LRnon-neonates | 2.17 (1.12-4.20) | .01 |
| LRneonates vs LRnon-neonates | 0.67 (0.34-1.33) | |
| Treatment | ||
| None/prophylaxis vs thrombolysis | 0.20 (0.04-0.90) | .02 |
| Anticoagulants vs thrombolysis | 0.26 (0.10-0.66) | |
| Delay to start therapy, d | 1.03 (0.99-1.06) | .16 |
| Length of therapy, mo | 1.02 (0.98-1.05) | .37 |
| Age at the time of LE-DVT, y | 1.08 (1.03-1.12) | <.001 |
| Sex (male vs female) | 1.38 (0.80-2.38) | .24 |
| Number of affected venous segments | 1.36 (1.12-1.66) | .002 |
| DVT level (femoro-popliteal vs ilio-femoral) | 0.48 (0.11-2.15) | .34 |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 2.20 (0.95-5.09) | .07 |
| Recurrence (yes vs no) | 0.99 (0.21-4.78) | .98 |
| Thrombophilia | ||
| None vs major | 0.71 (0.29-1.72) | .78 |
| Minor vs major | 0.79 (0.25-2.43) | |
| DVT resolution (none/partial vs complete) | 3.13 (1.49-6.67) | .003 |
| Predictor (final model) | Multivariable analysis OR (95% CI) | P |
| Omnibus Likelihood Ratio | <.001 | |
| DVT resolution (none/partial vs complete) | 3.15 (1.47-6.72) | .003 |
| Sex and group interaction | ||
| Male vs female in Non-LR | 0.59 (0.18-1.94) | .04 |
| Male vs female in LR | 2.50 (1.25-5.00) | |
| Non-LR vs LR among males | 1.17 (0.42-3.24) | |
| Non-LR vs LR among females | 4.92 (1.97-12.30) | |
CI, confidence interval; OR, odds ratio.
Survival analysis showed that the median time to reach an MVS score >1 was 2.0 years (95% CI, 1.1-3.2) for patients in the Non-LR group, 5.1 years (95% CI, 4.1-6.5) for patients in the LRnon-neonates group, and 10.5 years (95% CI, 7.0 to not estimable) for children in the LRneonates group. PTS-free survival was significantly different across groups (log-rank test, P < .001). Kaplan-Meier curves are shown in Figure 1.
Kaplan-Meier curves showing time to PTS according to group. The number of patients at risk according to group is shown at the bottom of the graphic, on the x-axis.
Kaplan-Meier curves showing time to PTS according to group. The number of patients at risk according to group is shown at the bottom of the graphic, on the x-axis.
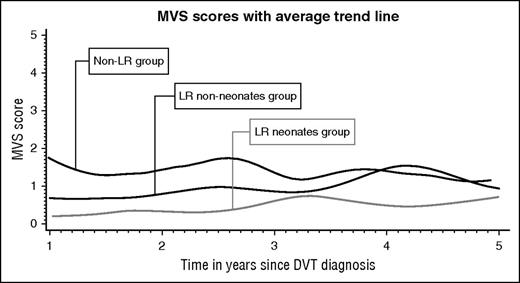
Longitudinal data analysis included 962 PTS assessments corresponding to 247 patients who had at least 2 assessments. The average trajectories per group are plotted in Figure 2. The model (supplemental Table 2) showed that at the time of the first PTS assessment, children in the Non-LR group had an MVS score of 1.33. In comparison, children in both LR groups had a significantly lower MVS score at baseline (1.01 lower, P < .001 for LRneonates and 0.65 lower, P < .001 for LRnon-neonates). Over time, the MVS score across groups increased by 0.05 per year. There was no difference on PTS score variation across groups over time (the time-group interaction was removed from the model).
Longitudinal analysis of the changes in limb circumference difference showed that at the time of the first PTS assessment, children with Non-LR DVT had a 1.63 cm circumference difference, whereas children in the LR groups had a significantly smaller difference (1.04 cm smaller, P < .001 for LRneonates and 0.56 cm smaller, P = .004 for LRnon-neonates). Over time, the circumference difference increased by 0.05 cm per year. There was no difference in change over time among groups.
LE-DVT resolution.
There was no significant difference in DVT resolution among groups (Table 2).
The results of univariable logistic regression analysis for potential predictors of LE-DVT resolution at the end of treatment are shown in Table 5. The only predictor of lack of DVT resolution in multivariable logistic regression was the presence of at least one occlusive segment at DVT diagnosis (Table 5).
ORs for the outcome lack of DVT resolution
| Predictor . | Univariable analysis OR (95% CI) . | P . |
|---|---|---|
| Group | ||
| Non-LR vs LRnon-neonates | 1.75 (0.84-3.63) | .28 |
| LRneonates vs LRnon-neonates | 0.96 (0.56-1.64) | |
| Treatment | ||
| None/prophylaxis vs thrombolysis | 0.22 (0.04-1.23) | .21 |
| Anticoagulants vs thrombolysis | 0.29 (0.07-1.26) | |
| Age at the time of LE-DVT, y | 1.01 (0.97-1.06) | .54 |
| Sex (male vs female) | 0.68 (0.41-1.07) | .09 |
| Number of affected venous segments | 1.57 (1.25-1.99) | <.001 |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 9.25 (5.04-16.98) | <.001 |
| Thrombophilia | ||
| None vs major | 0.96 (0.39-2.39) | .70 |
| Minor vs major | 0.69 (0.23-2.09) | |
| DVT level (femoro-popliteal vs ilio-femoral) | 1.65 (0.62-4.38) | .32 |
| Predictor | Multivariable analysis OR (95% CI) | P |
| Omnibus likelihood ratio | <.001 | |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 9.25 (5.04-16.98) | <.001 |
| Predictor . | Univariable analysis OR (95% CI) . | P . |
|---|---|---|
| Group | ||
| Non-LR vs LRnon-neonates | 1.75 (0.84-3.63) | .28 |
| LRneonates vs LRnon-neonates | 0.96 (0.56-1.64) | |
| Treatment | ||
| None/prophylaxis vs thrombolysis | 0.22 (0.04-1.23) | .21 |
| Anticoagulants vs thrombolysis | 0.29 (0.07-1.26) | |
| Age at the time of LE-DVT, y | 1.01 (0.97-1.06) | .54 |
| Sex (male vs female) | 0.68 (0.41-1.07) | .09 |
| Number of affected venous segments | 1.57 (1.25-1.99) | <.001 |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 9.25 (5.04-16.98) | <.001 |
| Thrombophilia | ||
| None vs major | 0.96 (0.39-2.39) | .70 |
| Minor vs major | 0.69 (0.23-2.09) | |
| DVT level (femoro-popliteal vs ilio-femoral) | 1.65 (0.62-4.38) | .32 |
| Predictor | Multivariable analysis OR (95% CI) | P |
| Omnibus likelihood ratio | <.001 | |
| At least 1 occlusive segment at DVT diagnosis (yes vs no) | 9.25 (5.04-16.98) | <.001 |
Recurrent LE-DVT.
There was a statistically significant difference in the frequency of recurrent LE-DVT across groups (Table 2). All recurrences in the Non-LR group were symptomatic, 2 cases were accompanied by concomitant PE, only 1 patient had a recurrent event in the contralateral limb, and only 1 patient had a recurrence while on anticoagulant treatment. In contrast, all but one of the recurrences in the LRnon-neonate group were DUS-detected asymptomatic events; two occurred while on anticoagulation.
The median time to the diagnosis of recurrent thrombosis among those observed to recur was 20.2 months (range, 3.7-144.0 months) in the Non-LR group and 12.1 months (range, 1.8-96.1 months) in the LRnon-neonate group.
Four of the 6 patients (67%) in the Non-LR group who sustained recurrent events had underlying anatomic variants in the venous system (4/23 patients with anatomic variants, 17%; 2 patients had May-Thurner syndrome and 2 had anomalies of the inferior vena cava). One of the 6 patients had APS, and the remaining patient did not have an identified risk factor.
PE.
A statistically significant difference in the frequency of PE was found among groups (Table 2). Eight of the 11 children (73%) who sustained PE in the Non-LR group had autoimmune or inflammatory conditions associated with the index LE-DVT. The median time to PE diagnosis, among those with PE, was 0 days in Non-LR patients (range, −15 to 3869 days), and 0 days in LRnon-neonate patients (range, −2 to 11 days). Fourteen of the 17 (82%) PE cases were diagnosed within 2 weeks (before or after) the LE-DVT event. Two of the 3 cases that occurred after 2 weeks had anatomic malformations (1 case of May-Thurner syndrome and 1 case of inferior vena cava anomaly); the remaining patient had APS with sustained positive ACLA and LAC, and sustained a PE 2 months after stopping anticoagulation, at the request of the family.
Deaths.
Six patients died on follow up; however, none of these deaths were related to thrombotic events.
Discussion
The present study reports the outcomes of 339 children with LE-DVT who were followed up at a tertiary care institution.
The overall frequency of PTS in the present cohort was similar to that found in a previous study investigating the outcomes of children with UE-DVT7 (47% vs 49%, respectively). Due to the difference in hydrostatic pressure in the venous systems of the UE and LE, PTS was expected to be more frequent in the LE. A potential explanation for the similar overall frequency of PTS is that collateral circulation, an item of the MVS associated with obstruction but not necessarily with venous hypertension, was more frequently observed in children with UE-PTS than in children with LE-PTS (81% vs 52%, respectively), thus increasing the frequency of UE-PTS diagnosis. Moreover, the current pediatric instruments do not allow differentiating the severity of most signs/symptoms, and the presence of more severe findings in LE-PTS as compared with UE-PTS cannot be ruled out.
Other pediatric reports describing the occurrence of PTS following LE-DVT reported a frequency ranging from 8.5% to 78%. It must be pointed out that these studies include either patients with VTE affecting different territories (ie, including organs),2,18-20 patients with UE and LE-DVT,13,21 or patients with LE-DVT only.8,22 Besides the difference in the studied populations, these reports also differ in their PTS assessment approach, and usually combine LR and Non-LR events. Although the overall frequency of PTS found herein (47%) is lower than the 26% weighted mean frequency of PTS reported by Goldenberg et al in their systematic review of pediatric PTS, it is similar to the 41% weighted mean frequency observed in the subset of studies that used a standardized outcome measure.6 Notably, the majority of PTS cases found in the present study were mild in nature in all groups.
In the present cohort, lack of DVT resolution, sex, and DVT triggering event (LR vs Non-LR) were predictors of PTS (MVS score, >1). Lack of DVT resolution was associated with PTS in children with LE-DVT in 1 previously reported pediatric study, and residual thrombosis is a known predictor of LE-PTS in adult patients.15,23-26 In addition, we found an interaction between sex and DVT trigger: (1) male sex was associated with a higher risk of PTS as compared with the female sex in the LR group, but this was not the case in the Non-LR group; and (2) Non-LR DVT were associated with a larger increase in risk of PTS as compared with LR-DVT among females, but this was not the case among males. The research on the association between sex and PTS in adult patients has yielded inconsistent results, and remains unclear. Although in some studies female sex predicted PTS,27,28 others reported a higher risk for male patients24,29 or no association.30,31 Also, although some studies in adult patients27-29 reported that DVT trigger (provoked vs unprovoked) is not a risk factor for PTS, it should be noted that the definition of “provoked” DVT varies between studies, and is not limited to the presence of CVL.
We did not find an association between PTS and ilio-femoral thrombosis, as previously reported in children.8 Ilio-femoral thrombosis, formerly referred to as “proximal DVT,” is also considered a relevant predictor of PTS in adult patients.27-29,32 Nonetheless, an exploratory analysis including only the subset of patients with Non-LR LE-DVT showed that the odds of PTS were higher for patients with ilio-femoral vs femoro-popliteal involvement (OR, 9.8; 95% CI, 1.2-81.5; P = .04), suggesting that older pediatric patients may share some similarities with adults. In addition, femoro-popliteal involvement was extremely infrequent among children with LR LE-DVT, precluding the assessment of the impact of DVT location in these patients.
Similarly, therapeutic modality was not a predictor of PTS in our cohort. Thrombolysis (either systemic or catheter-directed) was associated with a lower frequency of PTS as compared with standard anticoagulation in a selected cohort of 22 children with LE-DVT and positive inflammatory markers.22 In that study, thrombus resolution was also more frequent in patients undergoing thrombolysis, thus suggesting a potential causal sequence of events. In fact, Comerota et al reported an association between residual thrombosis following catheter-directed thrombolysis and PTS severity in adult patients.23 In our cohort, thrombolysis was not associated with complete resolution, which could explain the results; the majority of children undergoing thrombolysis (13/18, 72%) were found to have wall thickening or worse on DUS immediately after treatment.15 Lastly, although DVT recurrence is probably the most important predictor of PTS in adult patients,33 it was not found to be a predictor in our cohort. Because recurrence in adults is thought to be linked to PTS by exerting further damage to venous valves and exacerbating the obstruction of venous outflow, we can hypothesize that the extent of the damage and the degree of recovery after recurrent events are likely to be different in children.
In terms of the longitudinal assessment of PTS, an interesting finding from the survival analysis is that the median time to diagnosis of PTS across groups ranged between 2.0 and 10.5 years, suggesting that longer follow-up after a thrombotic event is warranted in children. The analysis of MVS trajectories showed a statistically significant difference among groups at baseline assessment, and a small increment in MVS scores over time. However, we found no difference in the increment of scores over time among groups; this is consistent with the Villalta score pattern described by Kahn et al in adult patients, in whom no predictor-time interaction was identified.27 The relatively stable mean trajectory found in the study despite the fact that patients were diagnosed with PTS later in their follow up, could be explained by the intermittency of some clinical features (eg, pain and swelling), which would result in patients having lower scorings in subsequent visits. Another contributing factor could be the shorter follow up of some patients, particularly teenagers.
Some comments can be made regarding the secondary outcomes. The overall frequency of DVT resolution and PE in this cohort was only slightly different than those found in children with UE-DVT7 (DVT resolution, 29% vs 36%, P = .13 and PE, 5% vs 1.9%, P = .14 in LE-DVT and UE-DVT, respectively), but DVT recurrence was markedly lower (3% in LE-DVT and 11% in UE-DVT, P = .001). The difference in recurrence between cohorts was seen in non-neonates with LR-DVT, and is likely due to the fact that lines in the UE usually remain in place for a longer period of time. Two of the 4 LE LR-DVT recurrences were associated with the insertion of a new CVL, and 2 were unrelated to CVL.
In comparison with previous pediatric studies reporting on our secondary LE-DVT outcomes, the frequency of complete DVT resolution and recurrence found herein was similar to that reported by Spentzouris et al 8 (29% vs 33% for DVT resolution; 3% vs 7% for recurrence), but lower than the 45% overall resolution22 and 46% nonacute recurrence in patients with LE-DVT21 reported by Goldenberg et al. Both studies by Goldenberg et al included a highly selected cohort of patients, which might have played a role in this discrepancy. Lastly, the frequency of PE observed here in the Non-LR group is close to that reported by Goldenberg et al among 16 children with Non-LR LE-DVT22 (19.6% vs 31.3%); conversely, Spentzouris et al8 found no cases of PE in their cohort of 27 pediatric patients with LE-DVT. Of note, all but 3 cases of PE found in the present study occurred within 2 weeks (before or after) of LE-DVT diagnosis, suggesting that a higher index of suspicion for PE is warranted early in the course of LE-DVT.
There are several knowledge gaps in LE-DVT and pediatric PTS that remain to be solved. For example, the factors that lead to better outcomes in thrombolysis remain to be identified. Also, the scoring systems of pediatric tools need to be improved, to allow monitoring changes in individual clinical features of PTS over time.
The strength of our study is related to the large number of patients that were included and their long scheduled follow up. Nonetheless, its weaknesses must be pointed out. First, its retrospective design requires careful data collection. In order to increase accuracy, outcomes were double-extracted and a third researcher resolved disagreements. Second, almost 10% of patients who sustained LE-DVT during the study period died, and 22% were either transferred out or lost to follow up; we cannot rule-out that disease severity in those patients was different from that of patients included in the study. It is also possible that patients who returned to clinic for follow up had a more severe clinical condition. However, the present cohort reflects clinical practice in many centers. Third, we cannot rule-out confounding by indication when analyzing the effect of therapy on PTS or DVT resolution, particularly in patients with Non-LR DVT. Hence, children with more extensive thrombosis at presentation may have been selected to undergo thrombolytic therapy. To minimize this confounding effect, we also considered the number of affected segments as a marker of thrombotic burden. Fourth, due to the high levels of missing values, we were unable to investigate the role of body mass index as a PTS predictor. Mean body mass index in patients with available data (n = 89) was 16.6 (25th to 75th percentile, 13.1-18.4). Lastly, although there are limitations to the tool used for PTS assessment in this cohort, it remains one of the two tools recommended by the ISTH for evaluation of PTS in pediatric patients.14 Of note, in view of the characteristic of the item scoring of the MVS (supplemental Table 1), we were not able to investigate the progression of clinical features over time, except for the change in limb circumference difference. Different scoring systems should be developed to monitor changes in clinical features over time. Importantly, we did not use the “definitive” classification of PTS suggested by the ISTH, because such a classification could lead to patient selection bias (ie, diagnosis of definitive PTS should be made according to 2 independent assessments at least 3 months apart, and at least 12 months post-event).14
In conclusion, our study showed that LR LE-DVT, particularly among neonates, had a more benign outcome regarding the development of PTS, DVT recurrence, and PE compared with Non-LR LE-DVT. The trigger of the DVT event (LR vs Non-LR), sex, and residual thrombosis were found to be risk factors of PTS development. The time to reach an MVS score >1 was significantly different when comparing LR with Non-LR DVT, and MVS score trajectory was significantly different across groups at baseline, but not over time.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank V. Chan for her input on laboratory testing; H. Said, H. Rotz, and A. Gordanpour for their contribution to data collection; the Comprehensive Research Experience for Medical Students Program/University of Toronto; the Yeo Summer Studentship Funding; the Department of Hematology/University of Toronto; and Sanofi-Aventis for supporting their participation.
Authorship
Contribution: M.L.A. performed outcome assessment, data analyses, and wrote the manuscript; E.P. supervised data analyses/interpretation, and reviewed the manuscript; S.W. reviewed the manuscript; N.Y and P.K. collected data and reviewed the manuscript; and L.R.B. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leonardo R. Brandão, Division of Hematology/Oncology, The Hospital for Sick Children, 555 University Ave, Black Wing, Room 10412, Toronto, ON M5G 1X8, Canada; e-mail: leonardo.brandao@sickkids.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal