Key Points
A high D-dimer level strongly predicts symptomatic venous and arterial thrombosis in newly diagnosed AML.
Thrombosis occurs in up to 10% of patients with newly diagnosed AML.
Abstract
Venous thromboembolism is a common complication in patients with cancer, but only limited data are available in acute myeloid leukemia (AML). In a prospective study in a cohort of 272 adult patients (aged 18-65) and an independent validation cohort of 132 elderly adults (aged >60) with newly diagnosed AML, we assessed markers of disseminated intravascular coagulation (DIC) (fibrinogen, D-dimer, α-2-antiplasmin, antitrombin, prothrombin time, and platelet count) and the DIC score according the International Society of Thrombosis and Haemostasis and their associations with the occurrence of venous and arterial thrombosis during follow-up. The prevalence of thrombosis was 8.7% (4.7% venous, 4.0% arterial) in the younger adults over a median follow-up of 478 days and 10.4% (4.4% venous, 5.9% arterial) in elderly patients. Most thrombotic events (66%) occurred before the start of the second course of chemotherapy. The calculated DIC score significantly predicted venous and arterial thrombosis with a hazard ratio (HR) for a high DIC score (≥5) of 4.79 (1.71-13.45). These results were confirmed in the validation cohort of elderly patients with AML (HR 11.08 [3.23-38.06]). Among all DIC parameters, D-dimer levels are most predictive for thrombosis with an HR of 12.3 (3.39-42.64) in the first cohort and an HR of 7.82 (1.95-31.38) in validation cohort for a D-dimer >4 mg/L vs ≤4 mg/L. It is concluded that venous and arterial thrombosis may develop in ∼10% of AML patients treated with intensive chemotherapy, which to a large extent can be predicted by the presence of DIC at time of AML diagnosis.
Introduction
Venous thromboembolism (VTE) is a common complication in patients with cancer. The incidence of VTE depends on type of malignancy, time since cancer diagnosis, and stage of disease. Lung cancer, gastrointestinal cancer, and hematological malignancies are associated with the highest risk.1,2
Among patients with hematological malignancies, those with acute lymphoid leukemia and multiple myeloma are particularly prone to develop VTE with incidences of 6% during induction chemotherapy and 10% to 28% during induction chemotherapy, respectively.3,4 Regimens containing doxorubicin, dexamethasone, and thalidomide may increase the VTE rate during induction treatment in association with other risk factors. Several recent studies reported that hematological malignancies, including multiple myeloma and Hodgkin lymphoma, are also associated with arterial thrombotic events (ATEs),5,6 especially in the first months after diagnosis.
It has been reported that acute myeloid leukemia (AML) is associated with a slightly increased risk of VTE with an incidence of 1.7-8.9%, but only limited data are available.7-10 The mechanism of the occurrence of thrombosis in hematological disorders is still unresolved. Disseminated intravascular coagulation (DIC) is associated with VTE and bleeding in acute promyelocytic leukemia and acute lymphoblastic leukemia.7-12 Although DIC has also been reported in AML, no data exist on the relationship between DIC and VTE and ATE in AML patients.7,13
We hypothesized that the presence of DIC at diagnosis of AML may contribute to the risk of both venous and arterial thrombosis in AML. Therefore, we studied a large cohort of adult patients with newly diagnosed AML aged <65 years by measuring DIC parameters at diagnosis prior to treatment and assessing the occurrence of both venous and arterial thrombosis during follow-up. The findings of this study were validated in a second large cohort of patients with newly diagnosed elderly AML patients aged >60 years.
Methods
Selection of participants
Study cohort.
A total of 276 consecutive newly diagnosed patients aged 18 to 65 years with AML or high-risk myelodysplasia (MDS) (refractory anemia with excess blasts [RAEB] with International Prognostic Scoring System [IPSS] ≥1.5) admitted to the Department of Hematology of the Erasmus University Medical Center Rotterdam from 2001 until 2010 were analyzed. Patients with acute promyelocytic leukemia were excluded. At the time of inclusion in the study, AML was classified according to the French–American–British (FAB) classification. One hundred fifty-five patients, aged 18 to 60, participated in the Dutch-Belgian Hematology-Oncology Cooperative group (HOVON) 42 trial (Netherlands Trial Register number, NTR230).14 Eighty-four patients participated in the multicenter HOVON 42A15 trial, a prospective, multicenter, randomization for granulocyte colony-stimulating factor priming in patients aged 18 to 60 years with AML or MDS RAEB, RAEB–in transformation (RAEB-t) with an International Prognostic Score Index score ≥1.5. Thirty-seven patients, aged 18 to 65, participated in the multicenter HOVON 92 trial (Netherlands Trial Register number, NTR1446; not yet published), a randomized study to assess the added value of Laromustine in combination with the same control treatment that had been used in the above-mentioned HOVON-42 trial.
External validation cohort.
A second cohort, consisting of 135 consecutive newly diagnosed patients older than 60 years (elderly) with AML or MDS RAEB with IPSS ≥ 1.5 admitted to the Department of Hematology of the Erasmus University Medical Center Rotterdam between 2000 and 2009, was analyzed to validate the results obtained from the first cohort. One hundred three patients participated in the multicenter HOVON 4316 trial, a randomized induction and postinduction therapy phase III study. Thirty-two patients participated in the multicenter HOVON 8117 trial, a phase II multicenter study to assess the tolerability and efficacy of the addition of Bevacizumab to standard induction therapy of AML. These studies were approved by the medical ethics committee of the Erasmus Medical Center and were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants. Details about the HOVON 42, 43, 81, and 92 protocols can be found at http://www.hovon.nl.
Blood collection and laboratory analysis
Blood samples were collected before start of treatment in 410 newly diagnosed AML patients. Venous blood was collected using a vacutainer system in citrate (0.105 M; Becton-Dickinson, Plymouth, UK) and centrifuged at 4°C at 2000 g for 10 minutes. The following DIC parameters were determined: D-dimer (missing values in 4.9% of patients), prothrombin time (PT), and, if not available, the PT was calculated from available international normalized ratio (INR) values in 117 patients (in total 2.2% missing), antithrombin (AT; missing 6.3%), fibrinogen (missing 36.9%), and α-2-antiplasmin (missing 45.6%). The DIC score according to the International Society on Thrombosis and Haemostasis (ISTH) scoring system for DIC was determined as shown in Table 1.18
ISTH-DIC scoring system
| Risk assessment: Does the patient have an underlying disorder known to be associated with overt DIC |
| If yes, proceed; if no, do not use this algorithm |
| Order global coagulation tests (platelet count, PT, fibrinogen, soluble fibrin monomers, or fibrin degradation products) |
| Score global coagulation test results |
| Platelet count (>100 × 109/L, 0; <100, 1; <50, 2) |
| Elevated fibrin-related marker (eg, soluble fibrin monomers/fibrin degradation products) (no increase = 0; moderate increase = 2; strong increase = 3) |
| Prolonged PT (<3 s, 0; >3 s but <6 s, 1, >6 s, 2) |
| Fibrinogen level (>1.0 g/L = 0; <1.0 g/L = 1) |
| Calculate score |
| If ≥5, compatible with overt DIC |
| Risk assessment: Does the patient have an underlying disorder known to be associated with overt DIC |
| If yes, proceed; if no, do not use this algorithm |
| Order global coagulation tests (platelet count, PT, fibrinogen, soluble fibrin monomers, or fibrin degradation products) |
| Score global coagulation test results |
| Platelet count (>100 × 109/L, 0; <100, 1; <50, 2) |
| Elevated fibrin-related marker (eg, soluble fibrin monomers/fibrin degradation products) (no increase = 0; moderate increase = 2; strong increase = 3) |
| Prolonged PT (<3 s, 0; >3 s but <6 s, 1, >6 s, 2) |
| Fibrinogen level (>1.0 g/L = 0; <1.0 g/L = 1) |
| Calculate score |
| If ≥5, compatible with overt DIC |
A fibrinogen level <1.0 g/L is counted as 1 point from a maximum of 8 in the ISTH-DICscore. When fibrinogen levels were missing, they were categorized as 0 points in the ISTH-DIC score (as if fibrinogen >1.0 g/L). Fibrinogen was measured as described by von Clauss. Antithrombin activity levels were determined using a chromogenic substrate. α-2-Antiplasmin activity was determined in a kinetic test (Dade Behring, Marburg, Germany). D-dimer levels were measured using an enzyme-linked immunoassay (Biopool, Bray, Ireland). The lower limit of sensitivity was 0.25 mg/mL. PT was measured on a Sysmex CA-1500 (Dade Thrombin Reagent; Siemens Diagnostics, Leusden, The Netherlands).
Diagnosis of thrombosis or bleeding events
None of the study patients received anticoagulant prophylaxis. In case a patient developed symptoms of venous or arterial thrombosis, imaging studies were performed to confirm the diagnosis of thrombotic events, ie, compression ultrasonography for deep vein thrombosis of the leg or arm and/or computerized tomography for pulmonary embolism. Patients with asymptomatic catheter thrombosis were not classified as VTE. Myocardial infarction or unstable angina pectoris was diagnosed according to clinical, enzymatic, and electrocardiographic criteria. Ischemic stroke was defined as the onset of rapidly developing symptoms and signs of loss of cerebral function that lasted at least 24 hours and had no apparent nonvascular cause. Furthermore, it had to be confirmed by means of computed tomography or magnetic resonance imaging. If a cerebral event resolved completely within 24 hours without signs of cerebral lesions on scanning, it was classified by a neurologist as a transient ischemic attack (TIA). Peripheral arterial disease (PAD) had to be symptomatic and proven by contrast angiography. Major bleeding was defined as a fatal bleeding, symptomatic bleeding in a critical area or organ, bleeding causing a fall in hemoglobin level of 2 g/dL or more, and/or bleeding leading to transfusion of 2 or more units of whole blood or red cells.19
Statistical analysis
Continuous variables were summarized as median values and ranges; categorical data were summarized as frequencies and percentages. Univariate and multivariate Cox regression analysis was used to evaluate the impact of baseline characteristics on (time to) event. All P values are 2-sided, and P values <.05 were considered statistically significant.
Results
Baseline characteristics and thrombosis
The prevalence of thrombosis in the total group of 276 younger AML patients was 8.7%, of which 4.7% were venous thrombosis and 4.0% were arterial thrombosis. Of the AML patients, 2.9% had pulmonary embolism, 1.4% venous thrombosis of the leg, and 0.4% had thrombosis of the upper extremity. With regard to arterial thrombosis, 1.4% had a myocardial infarction/acute coronary event, 1.4% had ischemic stroke, 0.4% had TIA, and 0.7% had another ATE. Two patients (0.7%) developed an ATE shortly after the occurrence of a venous thrombosis (within 8 and 11 days, respectively). No other patient had a recurrent thrombotic event during follow-up.
A total of 272 (98.6%) younger patients with AML with a mean age of 47 years (range, 18-65) were included in the analysis of the association between DIC parameters and thrombosis (see Table 2 for patient characteristics). Four patients were excluded from this analysis because they already presented with VTE at the time of AML diagnosis. During a median follow-up of 478 days (range, 0-109 months), 18 patients (6.6%) developed a symptomatic venous thrombosis (n = 9) or an ATE (n = 9). One patient had a thrombotic event 4 days before start of chemotherapy. Twelve patients (67%) developed thrombosis after the start of the first and before the second chemotherapy course (median 8 days after start of the first chemotherapy course; range, 2-60 days) and 5 patients (28%) developed thrombosis after the start of the second course (median 32 days after start of the second chemotherapy course; range, 6-47 days). Two patients suffered a fatal cerebral vascular accident (0.7%). Incidences of thrombotic events were similar across different study protocols and between control and study treatment groups. The detailed characteristics of all thrombotic events are presented in Table 3.
Characteristics of 272 younger and 132 elderly AML patients
| . | Younger (test cohort) . | Elderly (validation cohort) . | ||||
|---|---|---|---|---|---|---|
| . | VTE . | AT . | No events . | VTE . | AT . | No events . |
| . | n = 9 . | n = 9 . | n = 254 . | n = 3 . | n = 8 . | n = 121 . |
| Male sex, n (%) | 2 (22) | 5 (56) | 135 (53) | 2 (67) | 4 (50) | 63 (52) |
| Mean age at enrollment, y (range) | 46 (24-60) | 48 (30-58) | 47 (18-65) | 67 (63-72) | 69 (61-74) | 68 (61-77) |
| Overall survival, mean in mo (range) | 31 (3-69) | 32 (1-79) | 28 (1-108) | 13 (4-14) | 7 (0-44) | 12 (0-158) |
| FAB | ||||||
| M0, n (%) | 0 | 0 | 13 (5) | 0 | 1(13) | 2 (2) |
| M1, n (%) | 1 (11) | 1 (11) | 39 (15) | 1 (33) | 2 (25) | 20 (17) |
| M2, n (%) | 3 (33) | 2 (22) | 70 (28) | 1 (33) | 1 (33) | 42 (35) |
| M4, n (%) | 1 (11) | 1 (11) | 30 (12) | 1 (33) | 3 (38) | 9 (7) |
| M5, n (%) | 3 (33) | 3 (33) | 40 (16) | 0 | 1 (33) | 11 (9) |
| M6, n (%) | 0 | 0 | 5 (2) | 0 | 0 | 7 (6) |
| RAEB, n (%) | 0 | 0 | 14 (6) | 0 | 0 | 9 (7) |
| RAEB-t, n (%) | 1 (11) | 2 (22) | 33 (13) | 0 | 0 | 20 (17) |
| Unknown, n (%) | 0 | 0 | 10 (4) | 0 | 0 | 1 (1) |
| Karyotype | ||||||
| t(8;21), n (%) | 1 (11) | 1 (11) | 7 (3) | 0 | 0 | 5 (4) |
| inv(16), n (%) | 0 | 0 | 15 (6) | 0 | 1 (13) | 4 (3) |
| CN –X-Y, n (%) | 3 (33) | 6 (67) | 132 (52) | 2 (67) | 3 (38) | 52 (43) |
| CA Rest, n (%) | 2 (22) | 1 (11) | 68 (27) | 1 (33) | 3 (38) | 43 (36) |
| MK, n (%) | 2 (22) | 1 (11) | 31 (12) | 0 | 0 | 14 (12) |
| Unknown, n (%) | 1 (11) | 0 | 1 (0) | 0 | 1 (13) | 3 (2) |
| Leukocytes, mean; range | 47.1 (1.7-175) | 18.5 (1.0-94) | 26.8 (0.5-232) | 45.1 (8.4-89) | 58.9 (1.2-144) | 26.6 (0.7-510) |
| Platelets, mean; range | 82 (29-212) | 107 (17-400) | 84 (6-701) | 74 (14-173) | 68.1 (32-109) | 84 (6-449) |
| Blasts in bone marrow, mean; range | 69 (10-97) | 62 (26-92) | 51 (2-97) | 74.3 (49-95) | 58.6 (29-95) | 44.9 (2-94) |
| DIC parameters* | ||||||
| D-dimer (mg/L), mean; range | 7.6 (0.2-18.5) | 3.3 (0.1-19.2) | 1.2 (0.0-18.5) | 2.2 (0.4-5.6) | 3.2 (0.3-8.7) | 1.2 (0.1-21.8) |
| PT (s), mean; range | 14.7 (11.8-19.8) | 15.0 (12.4-20.5) | 13.9 (10.9-35.3) | 15.3 (14.2-16.3) | 16.6 (11-26.7) | 13.9 (10.5-20.2) |
| Antithrombin (IU/mL), mean; range | 0.87 (0.51-1.23) | 0.89 (0.73-1.07) | 0.97 (0.40-1.41) | 0.8 (0.7-1.0) | 0.9 (06-1.1) | 0.9 (0.4-1.4) |
| Fibrinogen (g/L), mean; range | 3.2 (0.3-4.6) | 3.5 (2.2-4.4) | 4.2 (0.7-8.0) | 3.3 (3.3-3.3) | 3.8 (2.8-4.8) | 4.5 (1.7-9.7) |
| α-2-Antiplasmin (IU/mL), mean; range | 0.75 (0.15-1.11) | 0.99 (0.65-1.19) | 0.98 (0.37-1.52) | 1.0 (1.0-1.0) | 0.9 (0.8-1.1) | 1.0 (0.4-1.3) |
| Site of venous thrombosis | ||||||
| DVT of the leg, n | 2 | 1 | ||||
| Pulmonary embolism, n | 6 | 2 | ||||
| DVT upper extremity, n | 1 | 1 | ||||
| Site of arterial thrombosis | ||||||
| Myocardial infarction/acute coronary event, n | 3 | 3 | ||||
| CVA, n | 3 | 4 | ||||
| TIA, n | 1 | 0 | ||||
| Other, n | 2 | 1† | ||||
| Major bleeding n (%) | 0 (0) | 1 (5) | 21 (8) | 0 | 0 | 18 (15) |
| . | Younger (test cohort) . | Elderly (validation cohort) . | ||||
|---|---|---|---|---|---|---|
| . | VTE . | AT . | No events . | VTE . | AT . | No events . |
| . | n = 9 . | n = 9 . | n = 254 . | n = 3 . | n = 8 . | n = 121 . |
| Male sex, n (%) | 2 (22) | 5 (56) | 135 (53) | 2 (67) | 4 (50) | 63 (52) |
| Mean age at enrollment, y (range) | 46 (24-60) | 48 (30-58) | 47 (18-65) | 67 (63-72) | 69 (61-74) | 68 (61-77) |
| Overall survival, mean in mo (range) | 31 (3-69) | 32 (1-79) | 28 (1-108) | 13 (4-14) | 7 (0-44) | 12 (0-158) |
| FAB | ||||||
| M0, n (%) | 0 | 0 | 13 (5) | 0 | 1(13) | 2 (2) |
| M1, n (%) | 1 (11) | 1 (11) | 39 (15) | 1 (33) | 2 (25) | 20 (17) |
| M2, n (%) | 3 (33) | 2 (22) | 70 (28) | 1 (33) | 1 (33) | 42 (35) |
| M4, n (%) | 1 (11) | 1 (11) | 30 (12) | 1 (33) | 3 (38) | 9 (7) |
| M5, n (%) | 3 (33) | 3 (33) | 40 (16) | 0 | 1 (33) | 11 (9) |
| M6, n (%) | 0 | 0 | 5 (2) | 0 | 0 | 7 (6) |
| RAEB, n (%) | 0 | 0 | 14 (6) | 0 | 0 | 9 (7) |
| RAEB-t, n (%) | 1 (11) | 2 (22) | 33 (13) | 0 | 0 | 20 (17) |
| Unknown, n (%) | 0 | 0 | 10 (4) | 0 | 0 | 1 (1) |
| Karyotype | ||||||
| t(8;21), n (%) | 1 (11) | 1 (11) | 7 (3) | 0 | 0 | 5 (4) |
| inv(16), n (%) | 0 | 0 | 15 (6) | 0 | 1 (13) | 4 (3) |
| CN –X-Y, n (%) | 3 (33) | 6 (67) | 132 (52) | 2 (67) | 3 (38) | 52 (43) |
| CA Rest, n (%) | 2 (22) | 1 (11) | 68 (27) | 1 (33) | 3 (38) | 43 (36) |
| MK, n (%) | 2 (22) | 1 (11) | 31 (12) | 0 | 0 | 14 (12) |
| Unknown, n (%) | 1 (11) | 0 | 1 (0) | 0 | 1 (13) | 3 (2) |
| Leukocytes, mean; range | 47.1 (1.7-175) | 18.5 (1.0-94) | 26.8 (0.5-232) | 45.1 (8.4-89) | 58.9 (1.2-144) | 26.6 (0.7-510) |
| Platelets, mean; range | 82 (29-212) | 107 (17-400) | 84 (6-701) | 74 (14-173) | 68.1 (32-109) | 84 (6-449) |
| Blasts in bone marrow, mean; range | 69 (10-97) | 62 (26-92) | 51 (2-97) | 74.3 (49-95) | 58.6 (29-95) | 44.9 (2-94) |
| DIC parameters* | ||||||
| D-dimer (mg/L), mean; range | 7.6 (0.2-18.5) | 3.3 (0.1-19.2) | 1.2 (0.0-18.5) | 2.2 (0.4-5.6) | 3.2 (0.3-8.7) | 1.2 (0.1-21.8) |
| PT (s), mean; range | 14.7 (11.8-19.8) | 15.0 (12.4-20.5) | 13.9 (10.9-35.3) | 15.3 (14.2-16.3) | 16.6 (11-26.7) | 13.9 (10.5-20.2) |
| Antithrombin (IU/mL), mean; range | 0.87 (0.51-1.23) | 0.89 (0.73-1.07) | 0.97 (0.40-1.41) | 0.8 (0.7-1.0) | 0.9 (06-1.1) | 0.9 (0.4-1.4) |
| Fibrinogen (g/L), mean; range | 3.2 (0.3-4.6) | 3.5 (2.2-4.4) | 4.2 (0.7-8.0) | 3.3 (3.3-3.3) | 3.8 (2.8-4.8) | 4.5 (1.7-9.7) |
| α-2-Antiplasmin (IU/mL), mean; range | 0.75 (0.15-1.11) | 0.99 (0.65-1.19) | 0.98 (0.37-1.52) | 1.0 (1.0-1.0) | 0.9 (0.8-1.1) | 1.0 (0.4-1.3) |
| Site of venous thrombosis | ||||||
| DVT of the leg, n | 2 | 1 | ||||
| Pulmonary embolism, n | 6 | 2 | ||||
| DVT upper extremity, n | 1 | 1 | ||||
| Site of arterial thrombosis | ||||||
| Myocardial infarction/acute coronary event, n | 3 | 3 | ||||
| CVA, n | 3 | 4 | ||||
| TIA, n | 1 | 0 | ||||
| Other, n | 2 | 1† | ||||
| Major bleeding n (%) | 0 (0) | 1 (5) | 21 (8) | 0 | 0 | 18 (15) |
DVT, deep venous thrombosis; LMWH, low-molecular-weight heparin.
DIC parameters in 272 patients.
This patient developed a pulmonary embolism 1 d later; only the first thrombotic event was taken into account.
Characteristics of younger and elderly (E) AML patients with arterial or venous thrombosis
| Patient . | Type . | Age at onset, y . | Sex . | Course . | Onset in days post chemo . | Laboratory in days before onset . | D-dimer . | PT/INR . | Platelets . | Fibrinogen . | DIC score . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PE | 34 | F | 1 | 28 | 30 | 18.5 | 18.6 | 29 | 0.3 | 7 |
| 2 | PE | 39 | F | 2 | 64 | 75 | 2.4 | 12.7 | 32 | 4.3 | 4 |
| 3 | DVT | 40 | F | 1 | 2 | 6 | 13.1 | 16.7 | 44 | 1.6 | 6 |
| 4 | PE | 53 | M | 1 | 31 | 41 | 0.2 | 13.3 | 43 | 4.6 | 2 |
| 5 | PE | 60 | F | 1 | 4 | 7 | 17 | 19.8 | 51 | 2.4 | 6 |
| 6 | DVT | 24 | F | 1 | 3 | 8 | 0.6 | INR 1.1 | 195 | nm | 2 |
| 7 | PE | 51 | M | pre | −4 | 7 | 1.2 | 11.8 | 212 | 3.3 | 2 |
| 8 | Upper-extremity DVT | 57 | F | 1 | 33 | 35 | 2.1 | 13.6 | 44 | 4.5 | 4 |
| 9 | PE | 52 | F | 2 | 84 | 87 | 13.0 | 12.8 | 89 | 4.4 | 4 |
| 10 | CVA | 49 | M | 1 | 10 | 21 | 0.8 | INR 1.2 | 130 | nm | 2 |
| 11 | TIA | 55 | F | 2 | 6 | 44 | 1.1 | 13.6 | 26 | nm | 4 |
| 12 | MI | 57 | M | 1 | 6 | 21 | 0.2 | 14.9 | 43 | 4.4 | 2 |
| 13 | ACS | 49 | M | 1 | 2 | 4 | 19.2 | 17 | 17 | 2.9 | 6 |
| 14 | Splenal infarction | 30 | F | 1 | 4 | 6 | 4.9 | 20.5 | 45 | 2.2 | 7 |
| 15 | MI | 47 | F | 1 | 14 | 16 | 0.6 | 13.4 | 160 | 4.0 | 2 |
| 16 | PAD | 36 | F | 2 | 73 | 79 | 0.2 | INR 1.1 | 115 | nm | 0 |
| 17 | CVA | 53 | M | 2 | 37 | 112 | 0.1 | INR 1.0 | 29 | nm | 2 |
| 18 | CVA | 58 | M | 1 | 60 | 61 | 2.2 | 15.5 | 400 | 4.0 | 2 |
| 19* | DVT + CVA | 47 | F | pre | na | −8 | 8.9 | 16.1 | 36 | 1.2 | na |
| 20* | DVT + MI | 56 | M | pre | na | −1 | 2.8 | 13.7 | 74 | nm | na |
| 21* | PE | 50 | M | pre | na | −2 | 1.1 | VKA | 202 | nm | na |
| 22* | PE | 24 | F | pre | na | 1 | 19.2 | 14.2 | 101 | 1.7 | na |
| 23 E | PE | 72 | M | 1 | 10 | 21 | 0.4 | 16.3 | 14 | 3.3 | 3 |
| 24 E | Upper extremity DVT | 63 | F | 2 | 145 | 154 | 0.5 | 14.2 | 173 | nm | na |
| 25 E | PAD + PE | 67 | M | 1 | 1 | 3 | 7.9 | 14.8 | 44 | 3.3 | 5 |
| 26 E | DVT | 65 | M | 2 | 71 | 71 | 5.6 | INR 1.3 | 35 | nm | na |
| 27 E | CVA | 66 | F | 1 | 3 | 5 | 8.8 | 15.3 | 99 | 3.8 | 4 |
| 28 E | CVA | 71 | F | 1 | 1 | 10 | >4 | 14.8 | 65 | 4.8 | 4 |
| 29 E | CVA | 74 | F | 2 | 102 | 125 | .9 | 17 | 32 | 2.8 | 5 |
| 30 E | CVA | 74 | M | 1 | 36 | 52 | .8 | 26.7 | 42 | 3.4 | 6 |
| 31 E | MI | 67 | F | 2 | 61 | 65 | 2.8 | 11 | 109 | 4.4 | 2 |
| 32 E | MI | 70 | M | 2 | 60 | 70 | .35 | INR 1.1 | 89 | na | na |
| 33 E | MI | 61 | M | 1 | 3 | 11 | .34 | INR 0.9 | 65 | na | na |
| 34*E | DVT | 72 | pre | na | 0 | 0.8 | 14.2 | 82 | 6.3 | na | |
| 35*E | DVT | 67 | pre | na | 0 | 1.1 | 13.0 | 101 | 3.8 | na |
| Patient . | Type . | Age at onset, y . | Sex . | Course . | Onset in days post chemo . | Laboratory in days before onset . | D-dimer . | PT/INR . | Platelets . | Fibrinogen . | DIC score . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PE | 34 | F | 1 | 28 | 30 | 18.5 | 18.6 | 29 | 0.3 | 7 |
| 2 | PE | 39 | F | 2 | 64 | 75 | 2.4 | 12.7 | 32 | 4.3 | 4 |
| 3 | DVT | 40 | F | 1 | 2 | 6 | 13.1 | 16.7 | 44 | 1.6 | 6 |
| 4 | PE | 53 | M | 1 | 31 | 41 | 0.2 | 13.3 | 43 | 4.6 | 2 |
| 5 | PE | 60 | F | 1 | 4 | 7 | 17 | 19.8 | 51 | 2.4 | 6 |
| 6 | DVT | 24 | F | 1 | 3 | 8 | 0.6 | INR 1.1 | 195 | nm | 2 |
| 7 | PE | 51 | M | pre | −4 | 7 | 1.2 | 11.8 | 212 | 3.3 | 2 |
| 8 | Upper-extremity DVT | 57 | F | 1 | 33 | 35 | 2.1 | 13.6 | 44 | 4.5 | 4 |
| 9 | PE | 52 | F | 2 | 84 | 87 | 13.0 | 12.8 | 89 | 4.4 | 4 |
| 10 | CVA | 49 | M | 1 | 10 | 21 | 0.8 | INR 1.2 | 130 | nm | 2 |
| 11 | TIA | 55 | F | 2 | 6 | 44 | 1.1 | 13.6 | 26 | nm | 4 |
| 12 | MI | 57 | M | 1 | 6 | 21 | 0.2 | 14.9 | 43 | 4.4 | 2 |
| 13 | ACS | 49 | M | 1 | 2 | 4 | 19.2 | 17 | 17 | 2.9 | 6 |
| 14 | Splenal infarction | 30 | F | 1 | 4 | 6 | 4.9 | 20.5 | 45 | 2.2 | 7 |
| 15 | MI | 47 | F | 1 | 14 | 16 | 0.6 | 13.4 | 160 | 4.0 | 2 |
| 16 | PAD | 36 | F | 2 | 73 | 79 | 0.2 | INR 1.1 | 115 | nm | 0 |
| 17 | CVA | 53 | M | 2 | 37 | 112 | 0.1 | INR 1.0 | 29 | nm | 2 |
| 18 | CVA | 58 | M | 1 | 60 | 61 | 2.2 | 15.5 | 400 | 4.0 | 2 |
| 19* | DVT + CVA | 47 | F | pre | na | −8 | 8.9 | 16.1 | 36 | 1.2 | na |
| 20* | DVT + MI | 56 | M | pre | na | −1 | 2.8 | 13.7 | 74 | nm | na |
| 21* | PE | 50 | M | pre | na | −2 | 1.1 | VKA | 202 | nm | na |
| 22* | PE | 24 | F | pre | na | 1 | 19.2 | 14.2 | 101 | 1.7 | na |
| 23 E | PE | 72 | M | 1 | 10 | 21 | 0.4 | 16.3 | 14 | 3.3 | 3 |
| 24 E | Upper extremity DVT | 63 | F | 2 | 145 | 154 | 0.5 | 14.2 | 173 | nm | na |
| 25 E | PAD + PE | 67 | M | 1 | 1 | 3 | 7.9 | 14.8 | 44 | 3.3 | 5 |
| 26 E | DVT | 65 | M | 2 | 71 | 71 | 5.6 | INR 1.3 | 35 | nm | na |
| 27 E | CVA | 66 | F | 1 | 3 | 5 | 8.8 | 15.3 | 99 | 3.8 | 4 |
| 28 E | CVA | 71 | F | 1 | 1 | 10 | >4 | 14.8 | 65 | 4.8 | 4 |
| 29 E | CVA | 74 | F | 2 | 102 | 125 | .9 | 17 | 32 | 2.8 | 5 |
| 30 E | CVA | 74 | M | 1 | 36 | 52 | .8 | 26.7 | 42 | 3.4 | 6 |
| 31 E | MI | 67 | F | 2 | 61 | 65 | 2.8 | 11 | 109 | 4.4 | 2 |
| 32 E | MI | 70 | M | 2 | 60 | 70 | .35 | INR 1.1 | 89 | na | na |
| 33 E | MI | 61 | M | 1 | 3 | 11 | .34 | INR 0.9 | 65 | na | na |
| 34*E | DVT | 72 | pre | na | 0 | 0.8 | 14.2 | 82 | 6.3 | na | |
| 35*E | DVT | 67 | pre | na | 0 | 1.1 | 13.0 | 101 | 3.8 | na |
ACS, acute coronary syndrome; CVA, cerebrovascular accident; F, female; M, male; MI, myocardial infarction; na, not applicable; nm, not measured; PE, pulmonary embolism; pre, before start of chemotherapy.
Excluded from analysis.
Independent validation cohort of elderly patients with AML
The validation cohort consisted of 135 newly diagnosed elderly AML patients. The prevalence of thrombosis in this group was 10.4% (4.4% venous and 5.9% arterial). Of the AML patients, 1.5% had pulmonary embolism, 2.2% had venous thrombosis of the leg, and 0.7% had thrombosis of the upper extremity. With regard to arterial thrombosis, 2.2% had a myocardial infarction, 2.9% had ischemic stroke, and 0.7% had PAD. One patient with an arterial event was diagnosed with a venous thrombosis 1 day later. No other patient had a recurrent thrombotic event during follow-up (Table 3). A total of 132 elderly patients with AML with a mean age of 68 years (range, 61-77) were included in the analysis on the association between DIC parameters and thrombosis. Three patients were excluded from this analysis because they had thrombosis at the time of AML diagnosis (n = 2) or because the blood sample was taken after the start of chemotherapy (n = 1). The detailed characteristics of all thrombotic events are presented in Table 3.
DIC in AML patients
Markers of DIC (D-dimer, PT, fibrinogen, α-2-antiplasmin, AT, and platelet count) were assessed before start of chemotherapy (median 6 days before start chemotherapy; range, 0-37 days). The ISTH-DIC score was available in 270 (99%) patients of the young cohort (Table 4). Twenty-three (8.5%) patients had an overt DIC (defined as DIC score ≥5) at presentation as shown in Table 5. There was no apparent difference regarding AML cytogenetic features among patients with and without DIC (P = .25). The AML-FAB types correlated with DIC risk (P = .003); in particular, DIC was more frequent in the case of AML of FAB type M5. Twelve of 46 patients with an AML M5 classification (26%) presented with DIC compared with 11 of 224 patients with other FAB types (4.9%). Furthermore, mean white blood cell and blast count in bone marrow were significantly higher in patients with DIC (P = .005 and P < .001, respectively).
Characteristics of 270 younger and 126 elderly AML patients with and without DIC at diagnosis
| . | YOUNGER (test cohort) . | . | ELDERLY (validation cohort) . | . | ||
|---|---|---|---|---|---|---|
| . | DIC n = 23 . | No DIC n = 247 . | P value . | DIC n = 8 . | No DIC n = 118 . | P value . |
| Male sex, n (%) | 11 (48) | 129 (52) | .828 | 5 (63) | 58 (49) | .717 |
| Mean age at enrollment, y (range) | 45 (20-61) | 47 (18-65) | 69 (65-74) | 67 (61-77) | ||
| FAB | .003 | .069 | ||||
| M0, n (%) | 2 (9) | 11 (4) | 1 (13) | 2 (2) | ||
| M1, n (%) | 3 (13) | 38 (15) | 3 (38) | 20 (17) | ||
| M2, n (%) | 3 (13) | 72 (29) | 0 | 43 (36) | ||
| M4, n (%) | 2 (9) | 30 (12) | 2 (25) | 11 (9) | ||
| M5, n (%) | 12 (52) | 34 (14) | 1 (13) | 10 (8) | ||
| M6, n (%) | 0 | 5 (2) | 0 | 5 (4) | ||
| RAEB, n (%) | 0 | 13 (5) | 0 | 8 (7) | ||
| RAEB-t, n (%) | 0 | 35 (14) | 1 (13) | 18 (15) | ||
| Unknown, n (%) | 1 (4) | 9 (4) | 0 | 1 (1) | ||
| Karyotype | .245 | .32 | ||||
| t (8;21), n (%) | 1 (4) | 8 (3) | 0 | 5 (4) | ||
| inv (16), n (%) | 2 (9) | 13 (5) | 1 (13) | 4 (3) | ||
| CN –X-Y, n (%) | 8 (35) | 133 (54) | 5 (63) | 50 (42) | ||
| CA Rest, n (%) | 7 (30) | 62 (25) | 1 (13) | 44 (37) | ||
| MK, n (%) | 5 (22) | 29 (12) | 1 (13) | 12 (10) | ||
| Unknown, n (%) | 0 | 2 (1) | 0 | 3 (3) | ||
| Leukocytes 109/L, mean; range | 64.1 (1.0-215) | 23.9 (0.5-232) | .005 | 65.7 (1.2-144) | 23.6 (0.7-200) | .04 |
| Platelets 109/L, mean; range | 36 (6-69) | 89 (6-701) | .000 | 34.8 (6-59) | 86.6 (9-449) | .025 |
| Blasts in bone marrow, mean; range, % | 74 (5-97) | 50 (2-95) | .000 | 73 (18-95) | 46 (2-95) | .009 |
| Event group, n (%) | .015 | .002 | ||||
| Venous thrombosis | 3 (13) | 6 (2) | 1 (13) | 2 (2) | ||
| Arterial thrombosis | 2 (9) | 7 (3) | 3 (38) | 5 (4) | ||
| Major bleeding, n (%) | 1 (4) | 10 (4) | 1.00 | 0 | 16 (13) | |
| . | YOUNGER (test cohort) . | . | ELDERLY (validation cohort) . | . | ||
|---|---|---|---|---|---|---|
| . | DIC n = 23 . | No DIC n = 247 . | P value . | DIC n = 8 . | No DIC n = 118 . | P value . |
| Male sex, n (%) | 11 (48) | 129 (52) | .828 | 5 (63) | 58 (49) | .717 |
| Mean age at enrollment, y (range) | 45 (20-61) | 47 (18-65) | 69 (65-74) | 67 (61-77) | ||
| FAB | .003 | .069 | ||||
| M0, n (%) | 2 (9) | 11 (4) | 1 (13) | 2 (2) | ||
| M1, n (%) | 3 (13) | 38 (15) | 3 (38) | 20 (17) | ||
| M2, n (%) | 3 (13) | 72 (29) | 0 | 43 (36) | ||
| M4, n (%) | 2 (9) | 30 (12) | 2 (25) | 11 (9) | ||
| M5, n (%) | 12 (52) | 34 (14) | 1 (13) | 10 (8) | ||
| M6, n (%) | 0 | 5 (2) | 0 | 5 (4) | ||
| RAEB, n (%) | 0 | 13 (5) | 0 | 8 (7) | ||
| RAEB-t, n (%) | 0 | 35 (14) | 1 (13) | 18 (15) | ||
| Unknown, n (%) | 1 (4) | 9 (4) | 0 | 1 (1) | ||
| Karyotype | .245 | .32 | ||||
| t (8;21), n (%) | 1 (4) | 8 (3) | 0 | 5 (4) | ||
| inv (16), n (%) | 2 (9) | 13 (5) | 1 (13) | 4 (3) | ||
| CN –X-Y, n (%) | 8 (35) | 133 (54) | 5 (63) | 50 (42) | ||
| CA Rest, n (%) | 7 (30) | 62 (25) | 1 (13) | 44 (37) | ||
| MK, n (%) | 5 (22) | 29 (12) | 1 (13) | 12 (10) | ||
| Unknown, n (%) | 0 | 2 (1) | 0 | 3 (3) | ||
| Leukocytes 109/L, mean; range | 64.1 (1.0-215) | 23.9 (0.5-232) | .005 | 65.7 (1.2-144) | 23.6 (0.7-200) | .04 |
| Platelets 109/L, mean; range | 36 (6-69) | 89 (6-701) | .000 | 34.8 (6-59) | 86.6 (9-449) | .025 |
| Blasts in bone marrow, mean; range, % | 74 (5-97) | 50 (2-95) | .000 | 73 (18-95) | 46 (2-95) | .009 |
| Event group, n (%) | .015 | .002 | ||||
| Venous thrombosis | 3 (13) | 6 (2) | 1 (13) | 2 (2) | ||
| Arterial thrombosis | 2 (9) | 7 (3) | 3 (38) | 5 (4) | ||
| Major bleeding, n (%) | 1 (4) | 10 (4) | 1.00 | 0 | 16 (13) | |
The influence of DIC parameters on the occurrence of a thrombotic event (Cox regression)
| . | Younger (test cohort) . | Elderly (validation cohort) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | VTE . | AT . | No event . | HR (95% CI) . | HR (95% CI) . | ||||
| Risk factor . | . | . | . | VTE . | AT . | Any event . | VTE . | AT . | Any event . |
| Total | 9 | 9 | 252 | ||||||
| PT | P = .076 | P = .21 | P = .020 | P = .092 | P = .39 | P = .092 | |||
| <16.3 s, n | 6 | 7 | 232 | ||||||
| ≤19.3 s, n | 2 | 1 | 17 | 4.38 (0.88-21.69) | 1.88 (0.23-15.26) | 3.03 (0.86-10.64) | 10.31 (1.45-73.40) | 1.58 (0.19-13.13) | 4.39 (1.13-16.98) |
| >19.3 s, n | 1 | 1 | 3 | 12.22 (1.46-102.10) | 11.28 (1.37-92.70) | 11.71 (2.63-52.17) | na | 5.91 (0.71-49.37) | 5.10 (0.62-41.63) |
| D-dimer | P = .002 | P = .24 | P < .001 | P = .013 | P = .046 | P = .01 | |||
| ≤0.5 mg/L, n | 1 | 3 | 134 | ||||||
| 0.5-4.0 mg/L, n | 4 | 4 | 98 | 5.58 (0.62-49.97) | 1.86 (0.42-8.33) | 2.79 (0.84-9.28) | na | 1.41 (0.24-8.47) | 0.73 (0.16-3.24) |
| >4.0 mg/L, n | 4 | 2 | 15 | 32.05 (3.58-286.83) | 5.35 (0.89-32.03) | 12.03 (3.39-42.64) | 7.66 (1.07-54.80) | 9.52 (1.59-57.12) | 7.82 (1.95-31.38) |
| Platelet count 109/L | P = .72 | P = .031 | P = .12 | P = .25 | P = .15 | P = .31 | |||
| <50, n | 5 | 5 | 108 | ||||||
| 51-100, n | 2 | 0 | 81 | 0.54 (0.10-2.78) | 0.00 (0.00-) | 0.27 (0.06-1.23) | na | 6.67 (0.74-59.79) | 3.54 (0.65-19.39) |
| >100, n | 2 | 4 | 63 | 0.66 (0.13-3.38) | 1.32 (0.35-4.92) | 0.99 (0.36-2.71) | 2.66 (0.28-25.61) | 2.92 (0.26-24.30) | 2.22 (0.43-11.46) |
| Fibrinogen score | P = .079 | P = .031 | P = .12 | ||||||
| >1 | 8 | 9 | 250 | ||||||
| <1 | 1 | 0 | 2 | 12.38 (1.54-99.18) | 0.00 (0.00) | 5.79 (0.77-43.56) | na | na | na |
| α-2-Antiplasmin | P = .033 | P = .45 | P = .029 | ||||||
| >0.8 IU/mL, n | 4 | 3 | 128 | ||||||
| ≤0.8 IU/mL, n | 3 | 1 | 15 | 5.99 (1.34-26.76) | 2.59 (0.27-24.91) | 4.52 (1.32-15.44) | na | na | na |
| Antithrombin | P = .13 | P = .093 | P = .025 | P = .92 | P = .46 | P = .7 | |||
| >0.8 IU/mL, n | 6 | 5 | 212 | ||||||
| ≤0.8 IU/mL, n | 3 | 3 | 32 | 3.16 (0.79-12.62) | 3.80 (0.91-15.90) | 3.45 (1.27-9.32) | 1.12 (0.12-10.75) | 0.48 (0.06-3.92) | 0.74 (0.16-3.43) |
| DIC score | P = .025 | P = .16 | P = .009 | P = .077 | P = .0016 | P = .0013 | |||
| <5, n | 6 | 7 | 234 | ||||||
| ≥5, n | 3 | 2 | 18 | 6.21 (1.55-24.85) | 3.57 (0.74-17.19) | 4.79 (1.71-13.45) | 16.99 (1.06-272.58) | 19.15 (3.86-95.11) | 11.08 (3.23-38.06) |
| . | Younger (test cohort) . | Elderly (validation cohort) . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| . | VTE . | AT . | No event . | HR (95% CI) . | HR (95% CI) . | ||||
| Risk factor . | . | . | . | VTE . | AT . | Any event . | VTE . | AT . | Any event . |
| Total | 9 | 9 | 252 | ||||||
| PT | P = .076 | P = .21 | P = .020 | P = .092 | P = .39 | P = .092 | |||
| <16.3 s, n | 6 | 7 | 232 | ||||||
| ≤19.3 s, n | 2 | 1 | 17 | 4.38 (0.88-21.69) | 1.88 (0.23-15.26) | 3.03 (0.86-10.64) | 10.31 (1.45-73.40) | 1.58 (0.19-13.13) | 4.39 (1.13-16.98) |
| >19.3 s, n | 1 | 1 | 3 | 12.22 (1.46-102.10) | 11.28 (1.37-92.70) | 11.71 (2.63-52.17) | na | 5.91 (0.71-49.37) | 5.10 (0.62-41.63) |
| D-dimer | P = .002 | P = .24 | P < .001 | P = .013 | P = .046 | P = .01 | |||
| ≤0.5 mg/L, n | 1 | 3 | 134 | ||||||
| 0.5-4.0 mg/L, n | 4 | 4 | 98 | 5.58 (0.62-49.97) | 1.86 (0.42-8.33) | 2.79 (0.84-9.28) | na | 1.41 (0.24-8.47) | 0.73 (0.16-3.24) |
| >4.0 mg/L, n | 4 | 2 | 15 | 32.05 (3.58-286.83) | 5.35 (0.89-32.03) | 12.03 (3.39-42.64) | 7.66 (1.07-54.80) | 9.52 (1.59-57.12) | 7.82 (1.95-31.38) |
| Platelet count 109/L | P = .72 | P = .031 | P = .12 | P = .25 | P = .15 | P = .31 | |||
| <50, n | 5 | 5 | 108 | ||||||
| 51-100, n | 2 | 0 | 81 | 0.54 (0.10-2.78) | 0.00 (0.00-) | 0.27 (0.06-1.23) | na | 6.67 (0.74-59.79) | 3.54 (0.65-19.39) |
| >100, n | 2 | 4 | 63 | 0.66 (0.13-3.38) | 1.32 (0.35-4.92) | 0.99 (0.36-2.71) | 2.66 (0.28-25.61) | 2.92 (0.26-24.30) | 2.22 (0.43-11.46) |
| Fibrinogen score | P = .079 | P = .031 | P = .12 | ||||||
| >1 | 8 | 9 | 250 | ||||||
| <1 | 1 | 0 | 2 | 12.38 (1.54-99.18) | 0.00 (0.00) | 5.79 (0.77-43.56) | na | na | na |
| α-2-Antiplasmin | P = .033 | P = .45 | P = .029 | ||||||
| >0.8 IU/mL, n | 4 | 3 | 128 | ||||||
| ≤0.8 IU/mL, n | 3 | 1 | 15 | 5.99 (1.34-26.76) | 2.59 (0.27-24.91) | 4.52 (1.32-15.44) | na | na | na |
| Antithrombin | P = .13 | P = .093 | P = .025 | P = .92 | P = .46 | P = .7 | |||
| >0.8 IU/mL, n | 6 | 5 | 212 | ||||||
| ≤0.8 IU/mL, n | 3 | 3 | 32 | 3.16 (0.79-12.62) | 3.80 (0.91-15.90) | 3.45 (1.27-9.32) | 1.12 (0.12-10.75) | 0.48 (0.06-3.92) | 0.74 (0.16-3.43) |
| DIC score | P = .025 | P = .16 | P = .009 | P = .077 | P = .0016 | P = .0013 | |||
| <5, n | 6 | 7 | 234 | ||||||
| ≥5, n | 3 | 2 | 18 | 6.21 (1.55-24.85) | 3.57 (0.74-17.19) | 4.79 (1.71-13.45) | 16.99 (1.06-272.58) | 19.15 (3.86-95.11) | 11.08 (3.23-38.06) |
na, not applicable.
In the validation cohort, the ISTH-DIC score was available of 126 (95%) patients. Eight (6.3%) patients had a DIC score ≥5 at presentation (Table 4). In the latter series of patients, there was no significant association of frequency of DIC with AML FAB subtypes (P = .069). Mean white blood cell and blast count in bone marrow were significantly higher in patients with DIC (P = .04 and P = .009, respectively). Mean D-dimer levels were similar in the younger-age study cohort (1.2 mg/L [0.0-18.5]) and the older-age cohort (1.2 mg/L [0.1-21.8]).
Association between DIC, thrombosis, and bleeding
The individual DIC parameters, PT, D-dimer, AT, and α-2-antiplasmin, were significantly associated with thrombosis risk in the cohort of younger AML patients (Table 5). Hazard ratios (HRs) were 3.03 (95% confidence interval [CI] 0.86-10.64) for PT ≤ 19.3 seconds, 11.71 (2.63-52.17) for a PT > 19.3 (compared with PT < 16.3 seconds), 2.79 (0.84-9.28) for D-dimer between 0.5 and 4.0 mg/L (defined as a moderate increase in the ISTH-DIC scoring system), 12.03 (3.39-42.64) for D-dimer >4.0 mg/L (defined as a strong increase in the ISTH-DIC scoring system) compared with <0.5 mg/L (normal range). The c-statistic value for a D-dimer >4.0 mg/L was 0.72. When we considered D-dimer values as a continuous variable in the analysis, the mean D-dimer levels were significantly higher in symptomatic patients (D-dimer 5.4 mg/L, standard error of the mean, 1.67) than those in asymptomatic patients (D-dimer 1.2 mg/L, standard error of the mean, 0.15; P < .001). HRs for laboratory parameters of DIC that are not part of the ISTH-DIC score were 3.45 (1.27-9.32) for AT plasma level below 0.8 IU/mL (which is the lower limit of normal in our laboratory) vs >0.8 IU/mL and 4.52 (1.32-15.44) for α-2-antiplasmin level below 0.8 IU/mL (lower limit of normal) compared with >0.8 IU/mL. Fibrinogen and platelet counts were not significantly associated with thrombosis. Also, other characteristics of patients, such as age, sex, phenotype of AML (FAB type), karyotype, leukocyte, and platelet counts at diagnosis, and kind of treatment, were not associated with thrombosis.
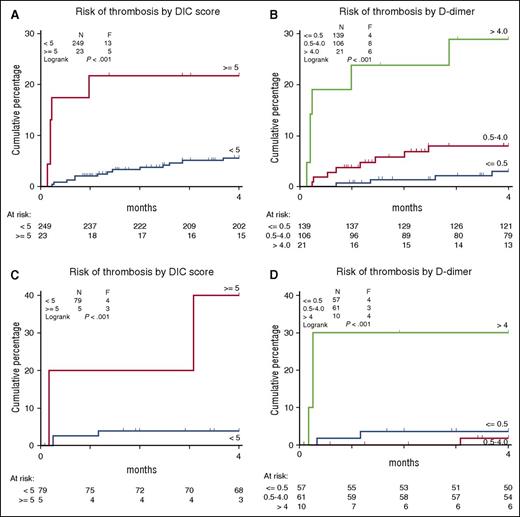
The ISTH scoring system for DIC was a strong predictor for the occurrence of thrombosis with an HR of 4.79 (1.71-13.45) for a DIC score ≥5. The cumulative incidences of a thrombotic event among patients with a DIC score ≥5 vs DIC score <5 and D-dimer <0.5 mg/L, 0.5 to 4.0 mg/L, and >4.0 mg/L are shown in Figure 1. The association between DIC and thrombosis appears even stronger for DIC parameters and thrombotic events within 30 days (HR 18 [CI: 3.1-106.1] for DIC score ≥5). Twenty-two patients (8%) developed major bleeding. Major bleeding did not occur more often in patients with a thrombotic event and was not associated with any of the DIC parameters at diagnosis.
Cumulative incidence of thrombosis in 2 cohorts of AML patients with DIS score ≥5 vs DIC score <5 and D-dimer <0.5, 0.5 to 4.0, and >4.0. (A-B) Data for the test cohort of younger AML patients. (C-D) Data for the validation cohort of elderly AML patients.
Cumulative incidence of thrombosis in 2 cohorts of AML patients with DIS score ≥5 vs DIC score <5 and D-dimer <0.5, 0.5 to 4.0, and >4.0. (A-B) Data for the test cohort of younger AML patients. (C-D) Data for the validation cohort of elderly AML patients.
We also evaluated the relationship between thrombotic risk with DIC parameters in the validation cohort of elderly individuals with AML. Of the individual DIC parameters, only D-dimer was significantly associated with thrombosis in this cohort (Table 4). HRs were 0.73 (0.16-3.24) for D-dimer between 0.5 and 4.0 mg/L (defined as a moderate increase in the ISTH scoring system) and 7.82 (1.95-31.38) for D-dimer >4.0 mg/L (defined as a strong increase in the ISTH scoring system compared with normal D-dimer levels [<0.5 mg/L]).
Also in this cohort, the ISTH scoring system for DIC was a strong predictor for the occurrence of thrombosis with an HR of 11.08 (CI: 3.23-38.06) for DIC score ≥5. The cumulative incidence of a thrombotic event in patients with a DIC score ≥5 vs DIC score <5 and D-dimer <0.5 mg/L, 0.5 to 4.0 mg/L, and >4.0 mg/L is shown in Figure 1. Major bleeding did not occur more often in patients with a thrombotic event and was not associated with any of DIC parameters at diagnosis.
Discussion
The results of the current observational study in a substantial group of adults with newly diagnosed AML who were treated according to a standard protocol of intensive chemotherapy reveal that venous and arterial thrombosis occurs in 8% to 10% of patients during remission induction treatment. The occurrence of thrombosis was significantly higher in patients with laboratory evidence of DIC prior to initiation of treatment. Of all parameters, the D-dimer level is distinctively highly predictive of occurrence of thrombosis.
Although it is well known that acute promyelocytic leukemia is frequently complicated by DIC and thrombotic events, the relation between DIC parameters and thrombosis in AML patients has not been reported before. In clinical practice, their common prevalence may be underestimated. Although DIC in AML has been reported, it is generally thought that VTE in AML is relatively rare.7 De Stefano et al reported a venous thrombosis rate of 3% as a presenting symptom and 1% in the first 6 months from diagnosis in 279 patients diagnosed with AML. They reported arterial thrombosis in only 1% of the individuals. Data on prothrombotic factors were obtained only from symptomatic patients, hampering a case-control comparison.20 Ku et al examined VTE in 5394 patients with AML, including AML M3, using codes from hospital discharge administrative datasets. The 2-year incidence of deep vein thrombosis or pulmonary embolism was estimated at 3.6%, and most events occurred within the first 3 months after AML diagnosis. Reported risk factors for VTE were female sex, older age, and presence of a central venous catheter. Laboratory data were not available in that study.21 Our study is unique in the sense that, in addition to detailed clinical information, pretreatment laboratory coagulation tests were collected. In our study, a comparable incidence of venous thrombosis (4.7% young cohort, 4.4% elderly cohort), but a higher incidence of arterial thrombosis (4.0% young cohort, 5.9% elderly cohort) than reported by De Stefano et al,20 was observed. No other data on arterial thrombosis and AML were available.
Only a few studies have reported on the occurrence of DIC in other types of AML than acute promyelocytic leukemia with an incidence of 7% to 18% depending on the definition of DIC.10,22-24 In previously published reports, the following risk factors for developing DIC were identified: high levels of C-reactive protein, high leukocyte counts, negative expressions of CD-13 and HLA-DR, and cytogenetics with a normal karyotype or 11q23 abnormality.22 None of these previous studies reported on thrombosis or bleeding complications in relation to DIC. We found a comparable DIC incidence (8.5% younger cohort and 6.3% elderly cohort) in our series of patients. We also noted that higher leukocyte counts were strongly associated with DIC in both younger and elderly AML patients. AML M5 morphology phenotype is strongly associated with DIC only in the younger cohort, as has previously been reported.25,26 The DIC parameter that was most strongly associated with the development of thrombosis was D-dimer. Associations between D-dimer levels and future thrombotic events have been reported in the general population.27,28 In patients with cancer, Ay et al showed that elevated levels of D-dimer and prothrombin fragment 1+2 were independently associated with an increased risk for occurrence of VTE.29 More recently, Pabinger et al reviewed the use of biomarkers in prediction of VTE in cancer patients. They also observed that D-dimer is associated with VTE in various types of cancer, including hematological malignancies; however, AML patients were not included.30 Thus, specific information regarding the clinical syndrome of both VTE and ATE in patients with AML who are frequently severely thrombocytopenic at diagnosis and/or during remission induction therapy was lacking.
So far, no standardized tests exist for the determination of DIC in patients with acute leukemia. Two of 4 parameters of the most commonly used ISTH-DIC score seem of little value in AML patients. First, low platelet counts are very common in acute leukemia and are not due to coagulation activation. Also, fibrinogen levels are not very informative, because as documented in our study, a strongly reduced fibrinogen level <1 g/L occurs very rarely in AML. Previously, the study that prospectively validated the DIC score showed that the sensitivity and specificity of the DIC score hardly change when fibrinogen levels were excluded.31
We found that a strongly increased D-dimer level (>4.0 mg/L) is the best predictor for a thrombotic event in our study population. Ten of 29 patients (34%) with a thrombotic event had a d-dimer >4.0 mg/L. Also, α-2-antiplasmin and AT levels appear as valuable parameters of DIC in AML and seem to be better markers than fibrinogen.
A unique feature of our study is that we measured DIC parameters in a well-defined cohort of consecutive AML patients at diagnosis before the start of chemotherapy and before occurrence of thrombosis that allowed us to identify patients at high risk of a symptomatic thrombotic event. In addition, we were able to validate our results in an external control group of elderly AML patients.
A limitation of our study is that we did not determine other acquired and inherited thrombophilic factors, such as Factor V Leiden mutation or the prothrombin gene variant that might have contributed to the thrombotic risk.32,33 A possible bias is the use of anticoagulant therapy in 22 participants (8%) during follow-up in the test cohort. Twenty-one of these had anticoagulant therapy for other reasons than previous thrombosis, such as atrial fibrillation. In addition, one patient used aspirin before the start of the study. Our results however remain significant after exclusion of these patients (HR 5.6 [95% CI; 1.8-17.8] for DIC score ≥5). Another limitation is that fibrinogen levels were not available in 36.9% of the patients. In the case of missing fibrinogen levels, we scored 0 in the calculation of the DIC score assuming a fibrinogen level of >1.0 g/ L. The use of score 0 if fibrinogen was not measured does not seem unreasonable because only 3 of the 260 fibrinogen measurements (1.1%) were <1 g/L, and 2 of these patients had an overt DIC even without accounting for fibrinogen value. When patients with missing fibrinogen levels were excluded from analysis, the ISTH scoring system for DIC remained a strong predictor for the occurrence of thrombosis with an HR of 5.9 (95% CI: 1.87-18.60) for DIC score ≥5 (P = .002) in the cohort of young AML patients and 10.74 (95% CI 3.14-62.87) in the cohort of elderly AML patients.
Our study clearly revealed that we were able to identify AML patients at high risk of thrombosis by measuring DIC parameters at diagnosis of AML. These results were confirmed in a second large cohort of elderly AML patients. It seems therefore of potential clinical interest to perform prospective studies to evaluate the potential role of prophylactic anticoagulant therapy in patients at high risk of thrombosis,34 especially during the first month after the start of treatment. A recent survey among North American clinicians involved in leukemia care reported the use of pharmacologic thrombosis prophylaxis in about half of the acute leukemia patients; however, most of them stopped in the case of a platelet count <50 000/µL.35 Obviously, the potential benefit of prophylactic anticoagulation must be carefully balanced against the possible enhanced risk of bleeding due to thrombocytopenia. The cause of DIC and the contribution of other DIC parameters, such as α-2-antiplasmin and AT, to the diagnosis of DIC in relation to thrombotic complications may also merit further study.
In conclusion, both venous and arterial thromboses are frequently occurring complications in AML especially during induction chemotherapeutic treatment. A high D-dimer level as a parameter of DIC strongly predicts the occurrence of symptomatic arterial and venous thrombosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J.L. and C.P.W.K. collected and analyzed data and wrote the paper; F.W.G.L. conceived and designed the study and wrote the paper; B.L., M.J.K., and P.S. advised on study design and revised the paper; Y.v.N. performed statistical analysis and revised the paper; and M.P.M.d.M. contributed to laboratory assessment and interpretation of DIC parameters and revised the paper.
Conflict-of-interest disclosure: P.S. is a consultant for Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Frank W. G. Leebeek, Department of Hematology (Rm L-435), Erasmus University Medical Center, PO Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal