In this issue of Blood, Deguchi et al have established a mechanism by which skeletal muscle promotes thrombin generation through binding of factor Xa and factor Va to myosin.1
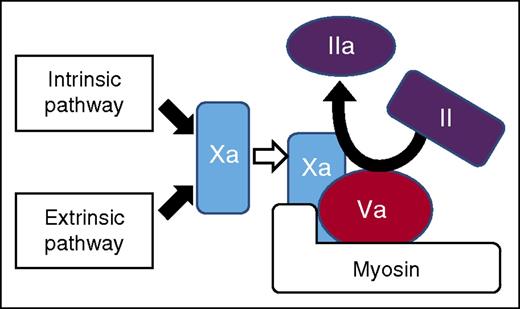
Model of prothrombinase assembly on myosin. Factor Xa and factor Va can colocalize on purified myosin and activate prothrombin. This activity requires factor Va and myosin cannot promote factor Xa independent activity. The Gla domain of factor Xa is not absolutely required for interaction, which provides evidence that factor Xa binding to myosin is different than binding to phospholipids. See Figure 7 in the article by Deguchi et al that begins on page 1870.
Model of prothrombinase assembly on myosin. Factor Xa and factor Va can colocalize on purified myosin and activate prothrombin. This activity requires factor Va and myosin cannot promote factor Xa independent activity. The Gla domain of factor Xa is not absolutely required for interaction, which provides evidence that factor Xa binding to myosin is different than binding to phospholipids. See Figure 7 in the article by Deguchi et al that begins on page 1870.
A prohemostatic effect of muscle has been reported in the surgical literature going back to the start of the 20th century. Surgeons reported using muscle grafts to control troublesome bleeding. For example, in 1935, Howard Clute wrote that “It has been repeatedly demonstrated to me that troublesome bleeding may be readily stopped by the use of free muscle grafts.”2(p746) Muscle was found to be more effective at stopping oozing than fat or fascia.
Had I been asked about the mechanism by which muscle grafts promote hemostasis, I would have speculated, incorrectly, that the damaged muscle might present an appropriate lipid surface for coagulation. Lipids surfaces have been thought to be the dominant surface on which coagulation occurs since Chargaff et al showed that the procoagulantly active component of platelets could be found in the lipid extract and not in the defatted protein.3 This idea was further strengthened when the group from Maastricht showed in the 1980s that one specific lipid, phosphatidylserine, is essential for lipid surface activity of the IXa/VIIIa and Xa/Va complexes.4
Deguchi et al came at the problem from a different direction. They previously reported in an abstract at the American Society of Hematology meetings that polymorphisms in the myosin gene could be found in patients with recurrent venous thromboembolism.5 Deguchi et al follow up on that observation with the studies reported in the current issue of Blood. Through direct binding studies, Deguchi et al establish that factor Xa and Va can bind to myosin. Through thrombin generation assays, they establish that this binding can result in prothrombin activation. Thus, as shown in the figure (taken from Deguchi et al), a functional prothrombinase complex can be formed on isolated myosin without a lipid surface. This finding establishes that it is biochemically possible for myosin to promote thrombin generation.
However, there are many reactions that are biochemically possible without being physiologically relevant. Deguchi et al go on to use whole blood flow assays that monitor platelet and fibrin deposition to show that myosin actively promotes thrombus formation. They also show that antibodies against myosin can block thrombin generation. They used these antibodies to study thrombin generation in plasma from trauma patients. Anti-myosin antibodies decreased thrombin generation in patients from these trauma patients more than thrombin generation in plasma from nontrauma patients. This result suggests that circulating myosin from damaged muscle might contribute to the thrombotic risk seen in some trauma patients. This result also suggests the possibility that antibodies or small molecules that target the myosin binding site have potential as antithrombotic therapies.
In addition to the insight that this paper gives us regarding a possible antithrombotic target to help trauma patients, the work of Deguchi et al adds to our understanding of how surfaces other than phospholipids can contribute to hemostasis at sites of injury. Factor IX binds tightly and specifically to the structural protein collagen IV6 ; this binding has been implicated in promoting hemostasis. The inorganic polymer polyphosphate has been shown to bind factor XI and thrombin, leading to factor XI activation.7 Deguchi et al showed that, in a purified system with added factor Xa and factor Va, myosin, a structural protein, has as much activity as an equimolar amount of lipid. Future studies in complex systems like whole blood and in animal models will establish the relative contribution of myosin to in vivo hemostasis and thrombosis. At the very least, these studies may increase the surgical awareness of an old technique that still holds relevance in our current era.8
Conflict-of-interest disclosure: The author declares no competing financial interests.