Abstract
Pulmonary hypertension affects ∼10% of adult patients with sickle cell disease (SCD), particularly those with the homozygous genotype. An increase in pulmonary artery systolic pressure, estimated noninvasively by echocardiography, helps identify SCD patients at risk for pulmonary hypertension, but definitive diagnosis requires right-heart catheterization. About half of SCD-related pulmonary hypertension patients have precapillary pulmonary hypertension with potential etiologies of (1) a nitric oxide deficiency state and vasculopathy consequent to intravascular hemolysis, (2) chronic pulmonary thromboembolism, or (3) upregulated hypoxic responses secondary to anemia, low O2 saturation, and microvascular obstruction. The remainder have postcapillary pulmonary hypertension secondary to left ventricular dysfunction. Although the pulmonary artery pressure in SCD patients with pulmonary hypertension is only moderately elevated, they have a markedly higher risk of death than patients without pulmonary hypertension. Guidelines for diagnosis and management of SCD-related pulmonary hypertension were published recently by the American Thoracic Society. Management of adults with sickle-related pulmonary hypertension is based on anticoagulation for those with thromboembolism; oxygen therapy for those with low oxygen saturation; treatment of left ventricular failure in those with postcapillary pulmonary hypertension; and hydroxyurea or transfusions to raise the hemoglobin concentration, reduce hemolysis, and prevent vaso-occlusive events that cause additional increases in pulmonary pressure. Randomized trials have not identified drugs to lower pulmonary pressure in SCD patients with precapillary pulmonary hypertension. Patients with hemodynamics of pulmonary arterial hypertension should be referred to specialized centers and considered for treatments known to be effective in other forms of pulmonary arterial hypertension. There have been reports that some of these treatments improve SCD-related pulmonary hypertension.
Introduction
Sickle cell disease (SCD) is caused by homozygosity for a Glu6Val mutation in HBB (sickle cell anemia; hemoglobin SS) or to compound heterozygous forms like hemoglobin SCD and hemoglobin S-β thalassemia. This mutation results in the synthesis of a structurally abnormal hemoglobin (hemoglobin S). Hemoglobin S polymerization is ultimately responsible for adverse effects of hemolysis, vaso-occlusion, chronic inflammation, anemia, and upregulation of hypoxic responses.1,2 Pulmonary hypertension is relatively common in SCD and should be considered both in the broader picture of pulmonary hypertension in patients without SCD and also with the view that SCD pathophysiology affects the pathogenesis, classification, and prognosis of this disorder.
Pulmonary hypertension—its classification and pathology
The World Symposium on Pulmonary Hypertension defines pulmonary hypertension as mean pulmonary artery pressure ≥25 mm Hg at rest as determined by right heart catherization.3 The upper level of normal for mean pulmonary artery pressure is 20 mm Hg, and the clinical significance of patients with mean pulmonary artery pressure between 21 and 24 mm Hg is under discussion. Some authorities consider this range to specify borderline pulmonary hypertension, and this range may identify subjects with reduced exercise capacity and poor outcomes.4
Clinically, pulmonary hypertension is divided into 5 broad categories as shown in Table 1.5 In its guidelines published in 2009, the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology and the European Respiratory Society included pulmonary hypertension associated with SCD and other hemolytic anemias in Group 1, pulmonary arterial hypertension.5 At the Fifth World Symposium on Pulmonary Hypertension in 2013, the decision was made to move pulmonary hypertension associated with chronic hemolytic anemia including SCD from Group 1 to Group 5, pulmonary hypertension with unclear or multiple etiologies.6
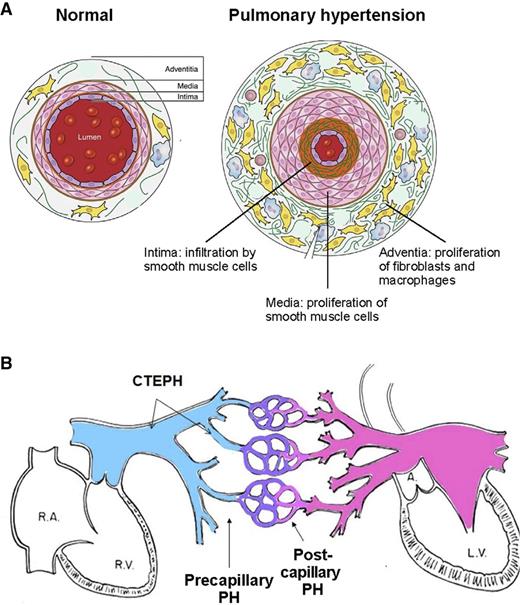
Pathologically, all 3 layers of the pulmonary arteriolar wall—intima, media, and adventitia—are affected in Group 1, pulmonary arterial hypertension (Figure 1).5,7 An increase in the number and size of smooth muscle cells in the media of the vessel wall leads to medial hypertrophy. Migration of smooth muscle cells from the media to the layer of endothelial cells that normally lines the lumen of the vessel leads to intimal proliferation. Plexiform lesions in the vessel lumen consist of a proliferation of endothelial cells and an interstitial layer of myogenic cells.8 Expansion of cells surrounding the media—fibroblasts, progenitor cells, macrophages, and other immune cells—leads to thickening of the adventitia, a layer that serves as a resource for the repair of vessel injury.9 Changes typical of pulmonary arterial hypertension may be found to varying degrees in the other forms of pulmonary hypertension as well. In addition, Group 2 pulmonary hypertension caused by left heart disease features enlarged pulmonary veins and capillaries. In Group 4 chronic thromboembolic pulmonary hypertension (CTEPH),5 organized thrombi replace the intima of proximal or distal elastic pulmonary arteries and attach to the medial layer, causing variable degrees of stenosis or complete occlusion of the lumen. Changes similar to pulmonary arterial hypertension can develop in the nonoccluded areas, and collateral vessels from the systemic circulation may grow to reperfuse areas of complete obstruction.5
Schematic representations of pulmonary hypertension. (A) Schematic cross-sectional representation of a normal pulmonary arteriole and a pulmonary arteriole affected by pulmonary hypertension. (Adapted from Pugliese et al.7 ) (B) Schematic representation of site of initiation of elevated pulmonary arterial pressure of precapillary pulmonary hypertension, postcapillary pulmonary hypertension, and CTEPH. L.V., left ventricle; PH, pulmonary hypertension; R.A., right atrium; R.V., right ventricle.
Schematic representations of pulmonary hypertension. (A) Schematic cross-sectional representation of a normal pulmonary arteriole and a pulmonary arteriole affected by pulmonary hypertension. (Adapted from Pugliese et al.7 ) (B) Schematic representation of site of initiation of elevated pulmonary arterial pressure of precapillary pulmonary hypertension, postcapillary pulmonary hypertension, and CTEPH. L.V., left ventricle; PH, pulmonary hypertension; R.A., right atrium; R.V., right ventricle.
Hemodynamically, pulmonary hypertension is subdivided as precapillary pulmonary hypertension if the pulmonary capillary wedge pressure (PCWP) or left ventricular end-diastolic pressure (LVEDP) at right heart catheterization is ≤15 mm Hg and as postcapillary pulmonary hypertension if the PCWP or LVEDP is >15 mm Hg (Figure 1B).5 For practical purposes, the hemodynamic grouping of postcapillary pulmonary hypertension and the clinical grouping of Group 2 pulmonary hypertension refer to the same entity.
Echocardiography to screen for pulmonary hypertension
Echocardiography to screen for pulmonary hypertension in patients without SCD
In general, the tricuspid regurgitation velocity (TRV) measured during echocardiography in combination with estimated right atrial pressure is considered to be a valid estimate of systolic pulmonary artery pressure.10,11 The normal TRV is considered to be <2.5 m/sec, but elevation of TRV and estimated systolic pulmonary artery pressure does not reliably identify subjects with pulmonary hypertension defined as mean pulmonary arterial pressure ≥25 mm Hg. Rather, an elevated TRV can help identify subjects who should be examined definitively with right heart catheterization. Pulmonary hypertension is considered to be unlikely if the TRV is ≤2.8 m/sec and there are no other echocardiographic changes suggestive of pulmonary hypertension such as enlargement of right-sided chambers and right ventricular systolic dysfunction. The diagnosis is considered to be possible if the TRV is 2.9-3.4 m/sec and to be likely if it is >3.4 m/sec.5
Echocardiography to screen for pulmonary hypertension in SCD
Several studies have documented a positive correlation of TRV and echocardiography-estimated systolic pulmonary artery pressure with systolic pulmonary pressure measured at right heart catheterization in patients with SCD. In these studies, the correlation coefficient r has ranged from 0.645 to 0.77, P < .001.12-14 Several studies, some overlapping, have also addressed the positive predictive value of specific TRV values for right heart catheterization–documented pulmonary hypertension, and these are summarized in Table 2. Based on these studies, more than half of SCD patients with TRV ≥2.9 m/sec have pulmonary hypertension on right heart catheterization, whereas <15% of those with TRV 2.5 to 2.8 m/sec have pulmonary hypertension. However, performing right heart catheterizations only in SCD patients with TRV ≥2.9 m/sec leads to a failure to diagnose pulmonary hypertension in about 4 of 10 patients with the condition.12 The accuracy of noninvasive diagnosis of pulmonary hypertension in SCD could be improved by a multimodality approach: the combination of TRV 2.5 to 2.8 m/s, NT-proBNP >164.5 pg/mL, and 6-minute walk distance <333 m had a positive predictive value of 62% with a false-negative rate of 7%.12 Therefore, SCD patients with TRV of 2.5 to 2.8 m/sec should be considered for right heart catheterization if there are other findings that suggest pulmonary hypertension, such as dyspnea on exertion, increased brain natriuretic peptide, right ventricular hypertrophy seen by echocardiography, and/or limited exercise capability.
Prevalence and mortality of pulmonary hypertension in patients with SCD
Three studies conducted based on screening for pulmonary hypertension with echocardiography, and confirming the diagnosis with right heart catheterization, have indicated a minimum prevalence of pulmonary hypertension in adults with SCD of 6.0% to 10.4% (Table 3). An emphasis should be placed on the fact that these are minimum estimates. In the study from France, which reports the lowest pulmonary hypertension prevalence, >10% of the patients from the background population were excluded from the study because of kidney disease (creatinine clearance <30 mL/min) and other severe manifestations12 ; a substantial proportion of these subjects would be expected to have pulmonary hypertension on right heart catheterization. In all 3 studies, not all of the subjects who were eligible for a right heart catheterization underwent the procedure (Table 3) and undoubtedly some cases of pulmonary hypertension were missed because of this.
These and other studies have documented a marked increase in mortality rate in SCD patients with pulmonary hypertension.12,14,16 In the report from France, 3 of 24 (12.5%) patients with pulmonary hypertension died over 3 years compared with 3 of 379 (0.8%) without the diagnosis.12 In the report from Brazil, mortality for up to 3 years of follow-up was 37.5% among 8 patients with pulmonary hypertension compared with 5.6% among 72 without (P = .0005).14 In the report from the United States, the 6-year mortality rate was 37% among 55 patients with pulmonary hypertension compared with 17% among 447 without (P = .006).16 In an earlier report from the United States of patients undergoing right heart catheterization, 11 of 20 (55.0%) patients with pulmonary hypertension died over up to 9 years of follow-up compared with 3 of 14 (21.4%) without pulmonary hypertension.17
Two of the 3 studies reporting prevalence of right heart catheterization–documented pulmonary hypertension in SCD included only patients with severe sickling genotypes—hemoglobin SS and hemoglobin Sβ0-thalassemia.12,14 The third study16 did include 92 patients (17.3%) with the milder hemoglobin SC genotype; 14 of these subjects underwent cardiac catheterization and 10 had pulmonary hypertension. The low number of hemoglobin SC patients with catheterization-proven pulmonary hypertension does not allow reliable prognosis estimates. However, a study using echocardiography to estimate elevated systolic pulmonary artery pressure in the various sickle genotypes showed that even though the frequency of elevated systolic pulmonary artery pressure was relatively low in hemoglobin SCD, the risk of death in hemoglobin SC patients who had this abnormality was of the same magnitude as in hemoglobin SS patients.18
Pathways of precapillary pulmonary hypertension in SCD
Slightly more than half of the reported cases of right heart catheterization–documented pulmonary hypertension in SCD have precapillary pulmonary hypertension (Table 4). The diagnosis of pulmonary arterial hypertension in patients without SCD traditionally includes the additional criterion of elevated pulmonary vascular resistance as defined by >3 Wood units.20 However, it is recognized that the elevation in cardiac output and reduced blood viscosity associated with SCD results in a lower baseline pulmonary vascular resistance than among nonanemic subjects and that SCD subjects with precapillary pulmonary hypertension often do not have elevation in pulmonary vascular resistance by the conventional definition. The American Thoracic Society’s Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease recommended a revised definition of elevated pulmonary vascular resistance in SCD of >2 Wood units.21 It is hemodynamically possible to have both pre- and postcapillary pulmonary hypertension in the same patient, and patients with postcapillary pulmonary hypertension should be reevaluated after effective therapy for left ventricular dysfunction to determine whether elevation of the mean pulmonary artery pressure persists. Mortality with precapillary pulmonary hypertension is at least as high as mortality with postcapillary pulmonary hypertension.12,14,17 An analysis of the National Institutes of Health cohort indicated that mortality in SCD adults with pulmonary hypertension is driven primarily by the severity of their precapillary pulmonary vascular pathology: those who died had significantly higher mean pulmonary artery pressure, transpulmonary pressure gradient, and pulmonary vascular resistance.19
Plausible pathways for the development of precapillary pulmonary hypertension in SCD include hemolysis, hypoxia, thromboembolism, or a combination of these factors. The explanted lung of a SCD patient with precapillary pulmonary hypertension was reported to show histopathologic features of pulmonary arterial hypertension, CTEPH, and pulmonary venous obstructive disease.22
Hemolysis and precapillary pulmonary hypertension
A large body of literature supports the potential role of intravascular hemolysis and abnormal NO signaling in the pathogenesis of precapillary pulmonary hypertension in SCD.23-25 Intravascular hemolysis leads to the release to the plasma of cell-free hemoglobin, submicron red blood cell microparticles that contain hemoglobin and heme, and arginase-1. The emptying of these red blood cell contents into plasma has the potential to inhibit NO signaling and impair vascular endothelial function.26-29 Cell-free plasma hemoglobin rapidly reacts with NO to form nitrate27 and erythrocyte-derived arginase-1 limits the availability arginine, the obligate substrate of the NO synthases.30 Release of heme from the hemoglobin molecule in plasma leads to activation of TLR4 and inflammasome pathways.31,32 Hemolysis also drives platelet and hemostatic activation and is proposed to generate reactive oxygen species and activate vascular oxidases.33-36 Clinical and translational studies have found associations of markers of hemolysis, including cell-free hemoglobin and red blood cell microparticles, with endothelial dysfunction, increased estimated systolic pulmonary artery pressure, and right heart catheterization–documented pulmonary hypertension.13,16,37
A trial of the 5′-phosophdiesterase inhibitor, sildenafil, to increase downstream mediators of the NO signaling pathway such as soluble guanylate cyclase and cyclic GMP in SCD patients with pulmonary hypertension or elevated TRV was conducted. The trial was stopped early because of high painful crisis rate in the sildenafil arm, possibly related to effects of inhibition of 5′-phosophdiesterase on limb pain, myalgia, and back pain.15 Although this study did not show a reduction in mean pulmonary artery pressure or TRV in subjects receiving sildenafil at the time it was stopped, other recent publications continue to offer support for the “hemolytic vasculopathy” view of SCD.
In a study of sickle cell and control mice, induction of intravascular hemolysis in control mice stimulated systemic and vascular inflammatory responses in conjunction with NO consumption, similar to the basal responses in sickle mice.38 Vascular inflammatory effects included decreased leukocyte rolling and increased adhesion and extravasation. The role of cell-free hemoglobin was affirmed by the finding that administration of the free hemoglobin scavenger, haptoglobin, completely inhibited the inflammatory response. Acute administration of hydroxyurea at the time of induction of hemolysis inhibited the effects of cell-free hemoglobin on these inflammatory responses in conjunction with activation of NO/cGMP signaling.
In a study of patients with SCD, elevated TRV correlated with impaired flow-mediated vasodilation, a test of endothelial NO synthase activity, and both measures were associated with measures of hemolysis including cell-free plasma hemoglobin.39 Acute red blood cell transfusion led to improved flow-mediated vasodilation in this study and chronic transfusion, which lowers plasma-free hemoglobin in SCD,40 was associated with both lower TRV and improved flow-mediated vasodilation, suggesting improved vascular function over time.39
Thromboembolism and precapillary pulmonary hypertension
SCD is a condition with a predilection for thrombotic complications including pulmonary embolism,41 and CTEPH is a major category of pulmonary hypertension (Table 1). Therefore, consideration needs to be given to the possibility that chronic thromboembolism may contribute to pulmonary hypertension in SCD. In one series, scintigraphic evidence suggestive of CTEPH occurred in approximately 12% of SCD patients with pulmonary hypertension.42 The clinical presentation is similar to Group 1, pulmonary arterial hypertension, but mismatched segmental defects on ventilation/perfusion scanning is suggestive of the diagnosis of CTEPH, and pulmonary angiography is confirmatory of the diagnosis. The interpretation of ventilation/perfusion scans can be complex in the setting of preexisting lung abnormalities,42 and pulmonary angiography is an invasive procedure. Nevertheless, making the diagnosis of CTEPH is important because pulmonary endarterectomy can be curative for patients with proximal obstructive lesions, and the precise diagnosis can guide medical therapy required for patients with distal lesions who do not qualify for a surgical approach.43 Pulmonary endarterectomy poses a challenge to SCD patients because of the tendency for increased sickling during cardiopulmonary bypass, hypothermia, and circulatory arrest associated with the procedure.44 It is wise to reduce hemoglobin S to ∼10% by exchange blood transfusion before the procedure, and with this approach a number of pulmonary endarterectomy procedures have been successfully performed in SCD patients with CTEPH.45-49
Hemolysis promotes platelet and hemostatic activation in the setting of SCD,33-36 and chronic inflammation is another potentially contributing factor to a hypercoagulable state.50 Furthermore, most hemoglobin SS patients have autosplenectomy, and splenectomy is a recognized risk factor for thrombosis and CTEPH.51 In keeping with these observations, autopsy studies suggest the possibility of a contribution of thromboembolism in some cases of SCD pulmonary hypertension. Microthrombotic and/or thromboembolic lesions are common findings at postmortem examination of patients with SCD.52,53 Acute or organizing thrombi in the pulmonary arteries, predominantly distal, was a common fining at autopsy in a series of 11 SCD patients with the diagnosis of pulmonary hypertension.54 Plexiform lesions were reported to be another common finding,54 but at least some of these lesions may have actually represented recanalyzed thrombi.6 A significant association was found between the postmortem diagnoses of pulmonary hypertension and thromboembolism in another autopsy series.55
Hypoxia and precapillary pulmonary hypertension
Chronic hypoxia influences diverse cellular and metabolic processes56 and is recognized to be an important cause of pulmonary hypertension (Table 1).57,58 Nevertheless, relatively little attention has been given to upregulation of the hypoxic response as a potential factor in SCD pulmonary hypertension. Erythropoietin expression sensitively reflects tissue oxygenation status,59 and hypoxia inducible factor (HIF)-α, the master regulator of the body’s response to hypoxia, was discovered by studying the regulation of the erythropoietin gene.60 SCD is characterized by high circulating erythropoietin concentrations under basal circumstances,61 indicating that this chronic anemia is accompanied by chronic upregulation of the hypoxic response. In addition, normoxic activation of HIF-1α is involved in the etiology of various forms of pulmonary hypertension through changes in mitochondrial redox signaling, fission, and numbers, and contributes to the development of a proliferative, apoptosis-resistant phenotype in pulmonary vascular cells.62-64 Furthermore, placental growth factor activates HIF-1α in normoxia and has been associated with elevated systolic pulmonary artery pressures in SCD.65 We postulated that the upregulated hypoxic response in SCD might contribute to altered gene expression and to the development of pulmonary hypertension. To address this hypothesis, we prospectively compared clinical data and genomic profiles of subjects with SCD to subjects with Chuvash polycythemia, a monogenic hematologic disorder characterized by an upregulated hypoxic response and elevated systolic pulmonary artery pressures.66,67 Specifically, homozygosity for VHLR200W leads to posttranslational stabilization of the α subunits of HIF at normoxia via decreased binding of these subunits to the mutant VHL protein, which normally marks them for destruction through the proteasome.66,68 As the result, increased levels of HIF-1 and HIF-2 lead to altered transcription of a number of genes.66
We observed a strong correlation of altered gene expression profiles in hemoglobin SS subjects and VHLR200W homozygote polycythemic subjects with global congenital upregulation of hypoxic responses, suggesting that >50% of PBMC gene expression variation in hemoglobin SS patients may be related to the hypoxic response.2 Interestingly, MAPK8, a hypoxia-downregulated gene that has a prominent role in promoting apoptosis,69 appeared to play an important role in hypoxic gene regulation, and an eQTL of this gene (rs10857560) that further downregulated expression was associated with right heart catheterization–documented precapillary pulmonary hypertension in SCD. Abnormal proliferation of pulmonary vascular smooth muscle cells and resistance to apoptosis is a prominent feature of pulmonary arterial hypertension.70 Expression of MAPK8 was downregulated in SCD and Chuvash polycythemia where the hypoxic response is upregulated, and the A allele of rs10857560 was associated with a further decrease in expression in SCD. Homozygosity for the A allele was present in all 14 of the patients with precapillary pulmonary hypertension that we examined, suggesting that the dosage effect of the A allele on MAPK8 gene expression might contribute to the pathogenic mechanism of precapillary pulmonary hypertension in SCD.2
Postcapillary pulmonary hypertension and left ventricular dysfunction in SCD
From a hemodynamic standpoint, almost half of the cases of SCD pulmonary hypertension reported in the literature have postcapillary or venous pulmonary hypertension (Table 4), which overlaps with Group 2, pulmonary hypertension caused by left heart disease (Table 1). Left ventricular diastolic dysfunction is common in patients with SCD, possibly related to ventricular dilatation and concentric hypertrophy of the myocardium as a response to chronic anemia and relative systemic hypertension.71,72 Also known as left ventricular failure with preserved ejection fraction, this finding is an independent predictor of decreased exercise tolerance61 and of mortality73 in SCD. Mortality in patients with postcapillary pulmonary hypertension appears to be substantial.19 Making the diagnosis of postcapillary pulmonary hypertension is important, because treatment needs to be directed at left ventricular failure and, if effective, can lead to a marked improvement in symptomatology and a resolution of pulmonary hypertension. Management guidelines for left ventricular failure with preserved ejection fraction are available,74 and the approach focuses on treatment of systemic hypertension and correction of fluid overload.
Clinical importance of elevated systolic pulmonary artery pressure
Elevated systolic pulmonary artery pressure, as estimated by TRV during Doppler echocardiography, occurs in ∼30% of hemoglobin SS and 10% to 25% of hemoglobin SC adults. Furthermore, more than half of hemoglobin SS adults without elevated TRV at rest develop abnormally high PA systolic pressure with exercise.75 Hemoglobin SS adults with even a modest increase in systolic pulmonary pressure have reduced exercise tolerance61 and decreased survival.13,76,77 In addition, 10% to 15% of the pediatric SCD population have elevated systolic pulmonary artery pressure78 ; although the effects on survival are unclear, there appears to be reduced exercise tolerance with this finding.79 Adults with elevated systolic pulmonary artery pressure who are found not to have pulmonary hypertension at right heart catheterization have similar survival to SCD patients without elevated TRV,12,14,16 suggesting that the elevated mortality with high TRV is largely driven by the subset of patients that do have pulmonary hypertension.
A remarkable clinical feature of sickle-related pulmonary hypertension is its high mortality despite relatively low mean pulmonary artery pressures. In contrast to other patients with pulmonary arterial hypertension (eg, idiopathic, scleroderma-associated) where morbidity and mortality are typically associated with mean pulmonary arterial pressures in the range of 50 to 60 mm Hg, in patients with SCD these phenomena are observed with mean pulmonary pressures in the range of 30 to 40 mm Hg, with mild elevations in pulmonary vascular resistance.17,19 In non-SCD patients with pulmonary arterial hypertension, the cardiac output is inversely related to mortality.80 In SCD patients with or without pulmonary hypertension, the cardiac output is considerably above the normal range for nonanemic individuals. From this perspective, SCD pulmonary hypertension patients might be expected to have a longer survival than pulmonary arterial hypertension patients without SCD, but this is not the case. It appears that in patients with anemia at the limits of cardiac output compensation, any degree of pulmonary hypertension is poorly tolerated and results in significant morbidity and mortality. In addition, complications that occur frequently in SCD, such as pain crises81 and especially acute chest syndrome,82 also acutely raise pulmonary pressures so that SCD patients with pulmonary hypertension develop “acute on chronic” pulmonary hypertension, making them less likely to survive these sickle-related complications.
Diagnosis and management of pulmonary hypertension in SCD
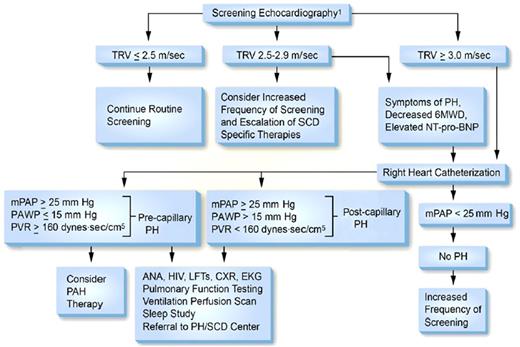
In 2014 an Expert Panel convened by the National Heart, Lung and Blood Institute concluded that “there is insufficient evidence to make a recommendation supporting regular screening with Doppler echocardiography”83 for pulmonary hypertension because studies demonstrating benefit of treating pulmonary hypertension are not available. In contrast, in the same year the American Thoracic Society’s Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease reported that the “committee members routinely perform risk stratification on their patients with SCD by measuring the TRV via Doppler echocardiography” and suggested a frequency of screening of every 1 to 3 years.21 The focus of the American Thoracic Society’s Ad Hoc Committee was to institute accepted methods for treating high-risk SCD in those patients who are found to have pulmonary hypertension. The guidelines for evaluation of SCD patients for pulmonary hypertension as proposed by the Ad Hoc Committee are summarized in Figure 2.84 The recommendations for evaluation of SCD patients for pulmonary hypertension and for the management of pulmonary hypertension by the authors of this review are summarized in Table 5.85-87
Proposed algorithm for evaluation of pulmonary hypertension related to sickle cell disease. 6MWD, 6-minute walk distance; ANA, anti-nuclear antibody; CXR, chest X-ray; EKG, electrocardiogram; LFTs, liver function tests; mPAP, mean pulmonary artery pressure; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SCD, sickle cell disease; TRV, tricuspid regurgitation velocity. Note: Echocardiography should be performed while patients are clinically stable. PAH therapy is to be considered on the basis of a weak recommendation and very low-quality evidence. Reprinted with permission of the American Thoracic Society.84 The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Proposed algorithm for evaluation of pulmonary hypertension related to sickle cell disease. 6MWD, 6-minute walk distance; ANA, anti-nuclear antibody; CXR, chest X-ray; EKG, electrocardiogram; LFTs, liver function tests; mPAP, mean pulmonary artery pressure; NT-pro-BNP, N-terminal pro–brain natriuretic peptide; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; SCD, sickle cell disease; TRV, tricuspid regurgitation velocity. Note: Echocardiography should be performed while patients are clinically stable. PAH therapy is to be considered on the basis of a weak recommendation and very low-quality evidence. Reprinted with permission of the American Thoracic Society.84 The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Future directions
Even minor degrees of anemia adversely affect survival in non-SCD pulmonary arterial hypertension patients.88 Anemia was associated with higher mortality even though it increased cardiac output, a hemodynamic feature that predicts longer survival. It seems possible that the anemia of SCD patients could contribute to high mortality of SCD patients with pulmonary hypertension, although as discussed previously, the degree of elevation in mean pulmonary artery pressure is modest.19 Case reports suggest that exchange blood transfusion, an intervention that improves anemia, is beneficial to SCD patients with pulmonary hypertension.89 Randomized, controlled trials of exchange transfusions in SCD patients with severe pulmonary hypertension are needed to address their high mortality. Though such trials are still lacking, it seems probable that in the United States the median survival of adult SCD patients with pulmonary hypertension has improved in the past decade in association with echocardiographic screening and better care of patients with this complication. Currently the median survival of SCD pulmonary hypertension patients is >6 years,16 or more than 3 times longer than a median of 2.1 years reported for a corresponding group of SCD pulmonary hypertension patients studied more than a decade ago.17 That the longer survival is not caused by more severe patients being studied in the 2003 report is supported by the similarity in their pulmonary hemodynamics (mean pulmonary artery pressure of ∼36 mm Hg in both series). Nor is it likely that the longer SCD pulmonary hypertension patient survival is merely a result of advances in general SCD medical care: for patients whose right heart catheterization results did not show pulmonary hypertension, the survival was similar in both studies. It seems possible that routine ECHO screening of SCD adults over the last decade has identified a group of patients who have a higher risk of death and whose referral to SCD pulmonary hypertension–specialized centers has allowed interventions such as intensification of SCD-specific treatment and/or in selected cases pulmonary arterial hypertension–specific treatment that may have lowered their mortality. This encouraging trend should serve as a stimulus to conduct more controlled trials for reducing the mortality of pulmonary hypertension in SCD, such as transfusion therapy and new pharmacologic agents.
Acknowledgments
This study was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (1R01HL111656-01) (R.F.M.).
Authorship
Contribution: V.R.G. wrote the first draft of the paper; and O.L.C. and R.F.M. made substantial additions and revisions.
Conflict-of-interest disclosure: V.R.G. has served as a consultant for Incyte Corporation, La Jolla Pharmaceuticals, and Alexion. The remaining authors declare no competing financial interests.
Correspondence: Victor R. Gordeuk, Sickle Cell Center, University of Illinois at Chicago (MC 712), 820 S Wood St, Chicago, IL 60612; e-mail: vgordeuk@uic.edu.