Abstract
Despite Food and Drug Administration (FDA) approval of hydroxyurea to reduce the frequency of vaso-occlusive episodes, sickle cell disease (SCD) has continued to be treated primarily with analgesics for pain relief. However, elucidation of the multiple pathophysiologic mechanisms leading to vaso-occlusion and tissue injury in SCD has now resulted in a burgeoning effort to identify new treatment modalities to prevent or ameliorate the consequences of the disease. Development of new drugs as well as investigation of drugs previously used in other settings have targeted cell adhesion, inflammatory pathways, upregulation of hemoglobin F, hemoglobin polymerization and sickling, coagulation, and platelet activation. Although these efforts have not yet yielded drugs ready for FDA approval, several early studies have been extremely encouraging. Moreover, the marked increase in clinical pharmaceutical research addressing SCD and the new and old drugs in the pipeline make it reasonable to expect that we will soon have new treatments for SCD.
Introduction
The simplicity of the genetic mutation that causes sickle cell disease (SCD) belies the complexity of the disease’s pathophysiology. A single base-pair change (A→T), and the ensuing alteration of one amino acid (glutamic acid replaced by valine) in the β chain of hemoglobin (Hb), a protein only expressed in erythrocytes, nevertheless causes a multiorgan disease with many complex pathophysiologic mechanisms (Figure 1). Thus, therapeutic approaches may target the “root cause” (ie, by replacement of the abnormal hemoglobin), as do stem cell transplantation and gene therapy, or one or more of the many damaging and interwoven pathways responsible for the disease’s cardinal manifestations—episodic severely painful vaso-occlusive episodes (VOC), hemolytic anemia, and progressive multiorgan damage.
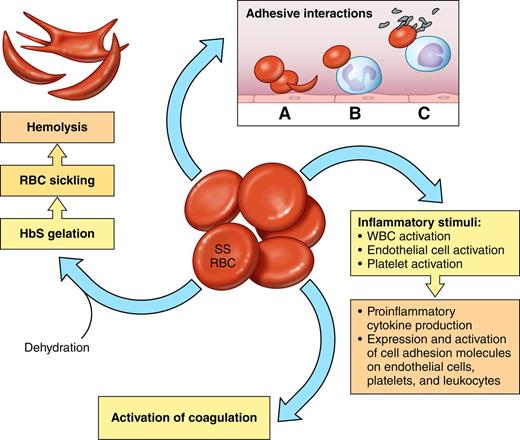
The sickle red blood cell (SS RBC) as source of multiple pathophysiologic pathways. Red cells with predominantly HbS (SS RBCs) become rapidly dehydrated, which increases the propensity of HbS to polymerize when deoxygenated. Pharmacologic reagents that prevent dehydration may therefore also reduce HbS polymerization and hemolysis. Altered lipid sidedness (phosphatidylserine exposure) may play a role in SS RBC adhesion and also promote activation of coagulation. Oxidative damage of red cell membrane proteins likely contributes to altered cell elasticity. Abnormal adhesive properties lead to SS RBC adhesion to endothelial cells (A), SS RBC adhesion to neutrophils (B), and adhesive interactions that result in heterocellular aggregate formation involving SS RBCs, monocytes, and platelets (C). Abnormal intracellular signaling increases the activation state of red cell adhesion molecules, and increased adhesive interactions then lead to abnormally active cell-cell signaling, which leads to activation of both other blood cells and endothelial cells. Both SS RBCs and hypoxia/reperfusion also lead to activation of inflammatory pathways involving both mononuclear and polymorphonuclear leukocytes. Platelet activation also contributes to inflammatory pathways as well as activation of coagulation.
The sickle red blood cell (SS RBC) as source of multiple pathophysiologic pathways. Red cells with predominantly HbS (SS RBCs) become rapidly dehydrated, which increases the propensity of HbS to polymerize when deoxygenated. Pharmacologic reagents that prevent dehydration may therefore also reduce HbS polymerization and hemolysis. Altered lipid sidedness (phosphatidylserine exposure) may play a role in SS RBC adhesion and also promote activation of coagulation. Oxidative damage of red cell membrane proteins likely contributes to altered cell elasticity. Abnormal adhesive properties lead to SS RBC adhesion to endothelial cells (A), SS RBC adhesion to neutrophils (B), and adhesive interactions that result in heterocellular aggregate formation involving SS RBCs, monocytes, and platelets (C). Abnormal intracellular signaling increases the activation state of red cell adhesion molecules, and increased adhesive interactions then lead to abnormally active cell-cell signaling, which leads to activation of both other blood cells and endothelial cells. Both SS RBCs and hypoxia/reperfusion also lead to activation of inflammatory pathways involving both mononuclear and polymorphonuclear leukocytes. Platelet activation also contributes to inflammatory pathways as well as activation of coagulation.
Red cells that contain primarily HbS or HbS with one of the variants that interacts with it, such as HbC, are abnormal in many respects, including that as a result of hemolysis they are overall much younger than normal erythrocytes.1 The fundamental defect in sickle red blood cells (SS RBCs) is the insolubility of HbS when it becomes deoxygenated, leading to formation of polymers that aggregate into tubular fibers and, as they enlarge, deform red cells, causing the characteristic sickle shape. In addition, SS RBCs become dehydrated, have abnormally activated intracellular signaling pathways, have decreased nitric oxide2 and adenosine triphosphate3 content and antioxidant capacity, demonstrate oxidative damage to many cellular components,4 and reflect dysregulation of miRNAs and gene expression during erythropoiesis.5,6 Cellular dehydration contributes to deoxygenated hemoglobin polymer formation and ultimately cell sickling and hemolysis. Signaling pathways downstream of the β2 adrenergic receptor and protein kinase A result in activation of MEK and ERK7 as well as several cell surface adhesion receptors.8-10 Oxidative damage of membrane proteins and aggregation of proteins along the inner surface of the plasma membrane led to further intracellular abnormalities.4,6
At their surfaces, SS RBCs demonstrate altered lipid sidedness, with markedly increased phosphatidylserine exposure.4 Along with the formation of microparticles, phosphatidylserine exposure contributes to the procoagulant activity of SS RBCs. SS RBCs also evince abnormal adhesive properties, including activation of known adhesion receptors (including BCAM/Lu, ICAM-4, and CD44) and increased interactions with leukocytes, platelets, endothelial cells, and extracellular matrix proteins. Abnormal SS RBC cell-cell signaling can activate both leukocytes and endothelial cells,11,12 making both more easily involved in adhesive interactions and also driving endothelial cell expression of procoagulant proteins.
SS RBCs are also stiffer than normal red cells in the circulation. Wide-field digital interferometry (WFDI) examination of normal red cells, normal-appearing SS RBCs, and sickled RBCs has shown that normal-appearing HbSS red cells are 2 to 3 times stiffer than HbAA red cells, and sickled RBCs are about 2 times stiffer than normal-appearing SS RBCs.13
Thus, new drug development as well as trials of existing compounds have targeted one or more of these pathophysiologic factors (Figure 1) in an effort to improve the overall prognosis of SCD as well as to reduce or treat its cardinal manifestation, vaso-occlusion. Given the diversity of therapeutic targets and pharmacokinetics of potential drugs, trials of new therapies have focused on a variety of different outcomes, including prevention of SCD events (such as the frequency of both VOC and acute chest syndrome (ACS), both reduced by hydroxyurea) and the ability to shorten the course of acute VOC. However, because resolution of VOC-related pain is a patient-reported outcome generally not accepted as a clinical outcome for SCD therapies, other end points—such as length of stay, time to discharge, or time to readiness for discharge—have been used. Unfortunately, these end points occur at highly variable time points among patients, so that achievement of statistically significant differences has been quite challenging. Partly for that reason, phase 2 studies have often used more easily quantified and sometimes less variable surrogate end points, although not always with great success.
Development of drugs targeting cell adhesion
Drugs targeting either red cell or leukocyte adhesion (or both) are attractive therapeutic modalities in treating SCD (Table 1), because multiple adhesive interactions have been demonstrated as contributing to vaso-occlusion (Figures 1 and 2) and both ex vivo and in vivo animal studies have suggested the potential efficacy of such an approach.
Adhesive interactions involving SS RBCs. (A) Multiple interactions between SS RBCs and endothelial cells, extracellular matrix, and plasma proteins. Red cells express multiple adhesion molecules that recognize ligands on either plasma protein “bridging molecules” or endothelial cell surfaces. Specific adhesion receptors on red cells that contribute to vaso-occlusion include ICAM-4 (the LW blood group protein, a receptor for several integrins), BCAM/Lu (the Lutheran blood group protein, a receptor for laminins (LAM) containing the α5 chain [laminins-10 and -11]), and CD44 (which bears the Indian blood group antigens), which is involved in binding to fibronectin and E-selectin. SA, sialic acid; FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor. (B) Interactions between SS RBCs and leukocytes adherent to vessel walls. Many stimuli may activate leukocyte adhesion, including cytokines and contact with SS RBCs. Adhesion to endothelial cells initially occurs via selectins expressed by endothelial cells but ultimately likely also involves endothelial integrins. ESL-1, E-selectin ligand-1; PSGL-1, P-selectin glycoprotein ligand-1.
Adhesive interactions involving SS RBCs. (A) Multiple interactions between SS RBCs and endothelial cells, extracellular matrix, and plasma proteins. Red cells express multiple adhesion molecules that recognize ligands on either plasma protein “bridging molecules” or endothelial cell surfaces. Specific adhesion receptors on red cells that contribute to vaso-occlusion include ICAM-4 (the LW blood group protein, a receptor for several integrins), BCAM/Lu (the Lutheran blood group protein, a receptor for laminins (LAM) containing the α5 chain [laminins-10 and -11]), and CD44 (which bears the Indian blood group antigens), which is involved in binding to fibronectin and E-selectin. SA, sialic acid; FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor. (B) Interactions between SS RBCs and leukocytes adherent to vessel walls. Many stimuli may activate leukocyte adhesion, including cytokines and contact with SS RBCs. Adhesion to endothelial cells initially occurs via selectins expressed by endothelial cells but ultimately likely also involves endothelial integrins. ESL-1, E-selectin ligand-1; PSGL-1, P-selectin glycoprotein ligand-1.
Selectin inhibitors
Drugs targeting selectin-mediated adhesion are being especially actively investigated. Such drugs inhibit adhesion and/or activation of leukocytes that are otherwise recruited to inflamed vessels. Human studies were preceded by several animal studies showing that inhibition of both P-selectin–mediated and E-selectin–mediated adhesion led to reduction of vaso-occlusion in in vitro and in murine models.11,14-18 Several selectin inhibitors are now in various stages of investigation.
GMI-1070 (now called rivipansel) is currently in a phase 3 study for acute vaso-occlusive episodes. Study of the drug in sickle mice showed that GMI-1070 could both prevent VOC and reduce the severity of ongoing VOC.15 In phase 1 studies in individuals with SCD in steady state, GMI-1070 was well tolerated, and data suggested that biomarkers of endothelial activation, leukocyte activation, and activation of coagulation were reduced as a result of drug administration.19 A phase 2 study of the drug during VOC then showed a marked reduction in opioid requirements as well as trends toward shorter duration of VOC and shorter length of hospital stays.20 The drug was also well tolerated during VOC. The phase 3 study of rivipansel treatment during VOC (NCT02187003) started in June 2015 and is expected to be completed in July 2018. Although selectin inhibitors have the potential to prevent leukocyte migration to sites of infection, clinical infections related to rivipansel have not been observed to date.
SelG1 is a humanized monoclonal antibody against P-selectin. A phase 1 dose-finding study was reported in abstract form in 2013.21 This single-center, double-blind, placebo-controlled, ascending single and multiple-dose study of IV SelG1 in healthy adult subjects showed the drug to be well tolerated and effective in blocking P-selectin function for approximately one month at well-tolerated dose levels. The phase 2 study (NCT01895361) is designed as a prophylactic study to prevent VOC events during 50 weeks of treatment every 28 days with IV SelG1 and is still actively treating patients.
Heparins as inhibitors of adhesion
Heparins have a well-known ability to inhibit adhesive interactions via P-selectin.16,23 Sevuparin, a derivative of low-molecular-weight heparin (LMWH), is being developed as a P-selectin blocker. Sevuparin retains the P-selectin–binding domain of heparin but largely lacks anticoagulant properties. It has been reported in abstract to inhibit SS RBC adhesion to endothelial cells in vitro and to reduce tumor necrosis factor–induced vaso-occlusion in vivo.24 After in vitro studies showing effect-inhibiting Plasmodium falciparum rosettes,25 sevuparin was first used in a phase 1/2 study as adjunctive therapy for malaria (NCT01442168). Sevuparin is now in an international phase 2 trial (NCT02515838) for the management of acute VOC.
In a single-center, randomized double-blind study of tinzaparin for acute VOC episodes, therapeutic-dose tinzaparin anticoagulation was associated with significantly shorter periods of severe pain, overall duration of pain episode, and overall duration of hospitalization.26 However, issues regarding potential study bias and standard of care make the study difficult to interpret.27 In addition, it is difficult to attribute the effects of tinzaparin to either its anticoagulant or antiadhesive properties.
Targeting signaling pathways that activate adhesion molecules
The role of the β2-adrenergic signaling pathway in activation of both the BCAM/Lu and ICAM-4 (LW) red cell adhesion receptors has suggested the possibility that beta-blockers might reduce RBC adhesion and thus have a salutary effect on SCD. A proof-of-principle phase 1 study in humans showed that oral propranolol was associated with a reduction in the ability of epinephrine to stimulate SS RBC adhesion in vitro.28 A phase 2 double-blind crossover study (NCT01077921) in 25 subjects showed that propranolol treatment was associated with decreased levels of all measured biomarkers (soluble E-selectin, P-selectin, ICAM-1, vascular cell adhesion molecule-1 [VCAM-1]) compared both with baseline values and with placebo, but these differences were not statistically significant.29 A second phase 1 study of propranolol in children without asthma (NCT02012777) is currently enrolling. Given the high prevalence of asthma in children with SCD, concerns remain that beta-blockers may not be tolerated by many patients with SCD.
A second signaling pathway of interest involves the kinases MEK and ERK, which in red cells appear to become activated downstream of the β2-adrenergic receptor and protein kinase A.7 Several MEK inhibitors have been developed as treatment of oncological diseases, as in cancer cells MEK and ERK act downstream of EGFR. In a murine model of vaso-occlusion involving infusion of human SS RBCs, exposure of SS RBCs to a MEK inhibitor reduced red cell adhesion to tumor necrosis factor–stimulated endothelial vessel walls and diminished trapping of SS RBCs in organs, concomitantly improving the circulatory half-life of SS RBCs.30 Thus, although clinical trials of MEK inhibitors in SCD have not yet been undertaken, this remains an interesting pathway for future drug development. Although reportedly well tolerated by most patients, MEK inhibitors have been associated with anemia and thus may exacerbate anemia in SCD.
Nonspecific inhibitors of adhesion
Poloxamer 188 is a surfactant that alters the way cells and molecules interact with water. Intravenous administration of poloxamer 188 acts by multiple mechanisms to improve microvascular blood flow, most notably in ischemic vascular beds, by inhibiting cell adhesion, lowering blood viscosity, and decreasing friction along the vessel wall.31 After successful early-phase clinical studies,32,33 poloxamer 188 was tested for efficacy during acute VOC in a phase 3 clinical trial (NCT00004408), resulting in a modest reduction in duration of painful episodes.34,35 It is now being studied in a second phase 3 trial (NCT01737814) for its ability to reduce the time to resolution of VOC.
Development of drugs targeting inflammatory pathways
Vaso-occlusion not only directly injures tissue via local hypoxemia but also engenders an inflammatory response typical of hypoxia/reperfusion injury,18,36 involving both mononuclear as well as polymorphonuclear leukocytes, along with production of a large array of proinflammatory cytokines. As described elsewhere in this issue, leukocytes, platelets, and multiple proinflammatory pathways contribute to the pathophysiology of SCD. Therefore, several approaches are being studied to determine whether downregulation of inflammatory pathways will ameliorate aspects of SCD (Table 2).
Adenosine and invariant NK T cells
Invariant NKT (iNKT) cells are implicated in the pathogenesis of ischemia/reperfusion injury.37,38 iNKT cell activation can be downregulated by activation of the adenosine A2A receptor (A2AR), and adenosine can reduce activation of and cytokine production by iNKT cells through this receptor.39-41 Expression of the A2AR on pulmonary iNKT cells is markedly increased in sickle mice, and recent studies demonstrate that blockade of iNKT cell activation in mice with SCD reduces pulmonary inflammation and injury.39 Adenosine also normally enhances 2,3-bisphosphoglycerate production via activation of the adenosine A2B receptor on RBCs, thus promoting O2 release. However, this same action, by promoting hemoglobin deoxygenation, might also promote polymerization of HbS and subsequent sickling.42
Regadenoson is a partially selective adenosine A2A receptor agonist. This drug is being studied for usefulness during VOC, which is theorized to involve iNKT cells as important contributors to the inflammatory components of VOC pathophysiology. Regadenoson is currently Food and Drug Administration (FDA)-approved for use in radionuclide myocardial perfusion imaging, similar to injectable formulations of adenosine and dipyridamole. However, regadenoson is more selective than these other agents for the adenosine A2A receptor. Furthermore, animal studies have shown that ATL146e, another adenosine A2A receptor agonist, caused a dose-dependent reversal of pulmonary dysfunction in sickle mice.39 Moreover, a phase 1 human study (NCT01085201) of regadenoson in individuals with SCD found that regadenoson could be safely administered to both children and adults in both steady state and during VOC, despite its demonstrated association with both cardiac and respiratory side effects outside the SCD context. The investigators found that iNKT cells demonstrated increased activation in individuals with SCD in steady state compared with controls; this activation was more marked during VOC. After a 24-hour infusion of regadenoson during VOC, phospho-NF-κB p65 activation in iNKT cells decreased significantly, and administration during VOC was not associated with toxicity.43 A phase 2 study of regadenoson in children and adults experiencing VOC (NCT01788631) is now underway to determine whether regadenoson infusion reduces iNKT cell activation among individuals with SCD VOC or ACS, compared with placebo. This study is also designed to look at length of hospital stay for VOC or ACS with regadenoson or placebo to determine whether regadenoson improves respiratory symptoms and reduces opioid use, and to study the effect of regadenoson on inflammatory biomarkers. The investigators hope to complete accrual by mid to late 2016.
A second drug targeting iNKT cells has also been studied in phase 1 (NCT01783691) and has been granted fast-track status by the FDA. This humanized monoclonal antibody against iNKT cells depletes iNKT cells in vivo in animals. A completed nonrandomized, nonplacebo-controlled phase 1b, dose-escalation single-dose study of the safety, pharmacokinetics, pharmacodynamics, and biological activity of IV NKTT120 in adults with SCD in steady state has been reported in abstract form.44 However, a phase 2 study of this drug is not yet listed as active at ClinicalTrials.gov.
Downregulation of leukotrienes
Given the association of SCD and asthma, along with the evidence that inflammatory pathways are important to both initial vaso-occlusion and tissue injury, targeting inflammatory mediators such as leukotrienes also appears to be a promising approach for the development of novel therapies for SCD.45 Cysteinyl leukotriene (CysLT), leukotriene E4 (LTE4), is elevated in steady-state SCD, and its level is correlated with pain event rate.46 Montelukast, already FDA-approved for treatment of asthma, is a CysLT inhibitor that acts by binding to CysLT receptors. Therefore, to test that LTE4 has a pathogenic role in sickle vasculopathy and vaso-occlusion, an 8-week phase 2 study (NCT01960413) of montelukast is being conducted in patients already on stable doses of hydroxyurea, with the surrogate end point of a reduction in sVCAM-1 levels. In this trial, investigators are looking at whether montelukast has additive effects in patients already taking hydroxyurea, compared with study participants treated with hydroxyurea only. Outcomes to be measured also include markers of tissue injury, lung function, and forearm microvascular blood flow.
Another approach is to target a key leukotriene synthetic enzyme, 5-lipoxygenase (5-LO). Zileuton, also FDA-approved for asthma, acts as a leukotriene inhibitor by inhibiting 5-LO, thus interfering with leukotriene formation. In SCD, placenta growth factor is elevated due to hyperplastic erythropoiesis and leads to activation of both monocytes and endothelial cells by inducing 5-LO,47 which leads to increased production of leukotrienes, including LTB4, a very potent chemoattractant and neutrophil and endothelial cell activator, and LTE4, which is associated with lung inflammation. In addition, interleukin-13 (IL-13) is known to increase expression of VCAM-1, which is elevated in SCD and known to be associated with mortality.48,49 In the context of SCD, zileuton reduces adhesion of PMN and sickle RBC to rat pulmonary vasculature50 ; it has also been shown to reduce IL-13 production by murine splenic lymphocytes, and thus it has been postulated to be potentially beneficial in SCD.51 Zileuton has been examined in a phase 1 study (NCT01136941) in children and adults with SCD, to determine whether it can safely inhibit 5-LO activity in individuals with SCD and if it will significantly reduce leukotrienes and biomarkers of inflammation. Zileuton has also been noted to be associated with increased HbF,52 and its effect on HbF levels in patients with SCD is also being examined.
Neutrophil adhesion and nonspecific antiinflammatory reagents
Intravenous γ globulin (IVIg) reduces inflammation via inhibition of neutrophil adhesion. In sickle mice exposed to an inflammatory challenge as a stimulus for VOC,53,54 IVIg infusion caused a rapid reduction in adherent leukocytes, markedly decreased leukocyte-erythrocyte interactions, and increased microcirculatory blood flow as well as survival. This effect occurs via IVIg’s ability to block the FcγRIII, leading to inhibition of neutrophil adhesion, reduction of RBC capture by leukocytes, and reduced Mac-1 activity as a result of recruitment of Src homology 2–containing tyrosine phosphatase-1.55 Recently reported results of a phase 1/2 study of IVIg for vaso-occlusion (NCT01757418) confirmed that IVIg decreased human neutrophil Mac-1 function. IVIg in this small study was not associated with reduced duration of pain crisis, total opioid use, or time to hospital discharge, although the study was likely not powered to detect such differences.56
Statins decrease endothelial inflammation in cardiovascular disease, so they have also been studied in SCD. A pilot study of 21 days of simvastatin in children with SCD looked at both safety issues as well as the effect of simvastatin on biomarkers of vascular dysfunction in SCD. Simvastatin resulted in dose-dependent statistically increased NOx levels and decreased C-reactive protein and IL-6 levels, while having no effect on vascular endothelial growth factor, sVCAM-1, or tissue factor expression.57 A phase 2 study of the ability of simvastatin to reduce the frequency of acute pain episodes is currently ongoing (NCT01702246).
Induction of HbF and antisickling agents
Irreversible sickling does not appear to be the driving factor for red cell adhesion and subsequent vaso-occlusion that occurs typically in postcapillary venules, because morphologically normal sickle red cells adhere as avidly to the endothelium as do sickled cells. Nevertheless, sickling leads to entrapment, or at least slow passage of cells through capillaries, as well as to hemolysis.
Induction of HbF
Drugs that increase HbF levels are the archetypal antisickling agents, because HbF interferes with polymerization of HbS, thereby lessening hemolytic rate and resulting in the increase in total Hb levels seen with hydroxyurea therapy.58 A number of drugs in addition to hydroxyurea are known to result in elevated HbF levels, at least temporarily (Table 3). For example, histone deacetylase inhibitors, including butyrates, generally increase HbF levels.59-63 Decitabine demethylates and reactivates expression of the methylated γglobin gene. Other drugs, such as thalidomide and related compounds, act by different mechanisms.
Butyrates were the first histone deacetylase inhibitors to be extensively studied in SCD.64-66 Sodium butyrate infusion was effective in a small study if given as pulse rather than continuous therapy.65 However, the orally available sodium dimethyl butyrate (HQK-1001) has produced generally unimpressive results in several studies,60-62 and in one study pain episodes were more frequent in patients receiving sodium dimethyl butyrate.
Pomalidomide has demonstrated the ability to upregulate HbF production in vitro and in sickle mice.67,68 A dose-finding study in individuals with SCD (NCT01522547) has been completed, but results reported only in abstract69 found increases in HbF and total Hb only at the highest dose or with >6 months of exposure.
Overall, optimally efficient induction of HbF may require combined use of drugs with different molecular and epigenetic mechanisms.70
Hemoglobin modification and antisickling agents
Carbon monoxide (CO) is a potent antisickling agent, because it attaches to Hb and, while attached, can prevent or reverse HbS polymerization. At very low levels, CO may also have antiinflammatory effects. Sickling may also be ameliorated by shifting the oxyhemoglobin dissociation curve to the left (Aes-103) or preventing cell dehydration through Gardos channel inhibition (ICA-17043, senicapoc).71-75 Despite its ability to reduce sickling and increase Hb levels, however, senicapoc did not reduce the frequency of VOC in a phase 3 clinical trial,72 perhaps because it did not reduce the sickling-independent adhesivity of the red cells themselves. This problem may also turn out to plague other agents designed to reduce sickling.
Sanguinate is a bovine pegylated Hb product designed to reduce sickling by delivering CO to HbS and then carrying O2. It was given safely to normal healthy subjects in a phase 1 trial,76 as well as to SCD subjects (NCT01848925). It has been granted orphan drug status and is now in a randomized, double-blind placebo-controlled phase 2 study of individuals with SCD experiencing VOC (NCT02411708), examining both safety and time to discharge.
MP4CO, or pegylated carboxyhemoglobin, markedly induced hepatic heme oxygenase-1 activity and inhibited NF-κB activation and microvascular stasis in sickle mice.77 However, although a phase 1 safety study has been completed (NCT01356485), a planned phase 2 study was withdrawn from ClinicalTrials.gov before enrollment, and the sponsor has ceased operations.
SCD-101 is a botanical-derived drug currently in a phase 1 study (NCT02380079) to determine dose and ability to inhibit sickling in vivo. Finally, a phase 1 study of Aes-103 (NCT01597401) (active ingredient 5-hydroxymethyl-2-furfural) has been completed in subjects with stable SCD.78 A phase 2 study in stable SCD (NCT01987908) has also just been completed, but results are not yet available.
Anticoagulants and antiplatelet agents
Although coagulation is chronically activated in SCD,79-83 no specific clinical complication of SCD has yet been clearly associated with a polymorphism of the coagulation pathway, and anticoagulation has an uncertain role in SCD in the absence of clinically apparent thromboembolic disease. Nevertheless, activation of coagulation and platelets is felt to contribute to vascular blockade, endothelial damage, and stimulation of inflammatory pathways. Thus, both anticoagulants and antiplatelet agents have attracted champions who have led or are leading clinical investigations of these agents (Tables 4 and 5). However, concerns remain regarding bleeding risk, especially in association with Moya Moya vascular disease of the central nervous system.
Vitamin K antagonists
In the 1960s, a small study of warfarin anticoagulation in 12 patients, with duration of treatment ranging from 12 to 34 months, reported a slight decrease in VOC rate but a significant number of bleeding episodes.84 Warfarin was subsequently reported to be associated with lower d-dimer levels in individuals with SCD with VOC.85 More recently, a study of anticoagulation with warfarin for pulmonary hypertension (NCT01036802) was terminated because of difficulty accruing patients.
Heparins
Heparins may potentially exert both antiadhesive effects (discussed above) and anticoagulation effects. However, well-designed placebo-controlled studies with various LMWHs are needed to confirm or dismiss the results of the single study of full anticoagulation doses of tinzaparin reported thus far.26 A somewhat more recent study of the LMWH dalteparin (NCT01419977) looked at the effect of prophylactic dosing on change in d-dimer, change in pain score, and change in the thrombin generation assay during VOC. Results, provided in abstract form,86 report that prophylactic dose dalteparin did not significantly affect markers of activation of coagulation, although pain scores at days 1 and 3 decreased more markedly in patients treated with dalteparin than with placebo.
There is also an ongoing feasibility study of unfractionated heparin in ACS (NCT02098993) in which the primary outcome is time to discharge. At this time, however, it is unclear whether this will lead to a larger multicenter phase 3 trial of unfractionated heparin for ACS.
Direct thrombin and factor X inhibitors
With the advent of orally available direct thrombin and factor X inhibitors, investigators interested in the role of coagulation in SCD have begun investigating these newer agents. Current studies are looking at the potential benefit of rivaroxiban and apixiban in outpatients with SCD. The rivaroxaban study (NCT02072668) is looking at the effect on levels of sVCAM-1 and IL-6, whereas the apixaban study is looking at daily pain scores.
Antiplatelet agents
Platelets and platelet activation are thought to contribute to SCD pathophysiology by multiple mechanisms, including participation in formation of heterocellular blood cell aggregates,87,88 release of proinflammatory cytokines,89 and activation of coagulation pathways. Therefore, a number of platelet antagonists have been examined in SCD. In a small study, eptifibatide failed to reduce time to VOC resolution (NCT00834899),90 and no further studies of this drug are currently ongoing. Prasugrel has shown promising results in sickle mice,91 in which it partially attenuated both basal and agonist-stimulated platelet activation. A multicenter phase 2 study of prasugrel in adults with SCD showed that biomarkers of in vivo platelet activation were significantly reduced in individuals with SCD receiving prasugrel compared with placebo; however, only nonsignificant decreases in pain were observed. A phase 3 study of prasugrel (NCT01794000) to determine whether it affects the number of VOC events per year is ongoing. A phase 2 study of ticagrelor (NCT02482298) is also ongoing to determine whether the drug reduces the number of days of pain, pain intensity, and analgesic use.
Outlook for the future
New pharmacotherapeutic approaches to SCD are being explored more actively than ever, and both clinical investigators and pharmaceutical companies are engaged in developing better treatment to prevent VOC as well as to abrogate the process once it occurs. With our expanded knowledge of the pathophysiologic mechanisms of vaso-occlusion, and indeed of how SS RBCs engender the widespread effects we observe in patients, drug therapy targeted to specific pathophysiologic mechanisms is becoming a realistic approach.
However, given the panoply of effects SS RBCs have, it is likely that, ultimately, optimal therapy will only be achieved with a multitargeted approach, such as that now used for acute coronary syndromes. Unfortunately, the limited number of individuals with SCD in developed countries and the dearth of resources available where most of the world’s patients live will likely make definitive studies of many pharmacologic agents difficult to accomplish. Although early phase studies may find biomarkers of inflammation, endothelial activation, or activation of coagulation to be helpful, there is insufficient evidence that any biomarkers are predictive of meaningful clinical response, although some clearly correlate with survival.49,92
Thus, real progress in treating SCD will be critically dependent on participation of clinical sites throughout the world, as well as on the availability of funds to support the arduous research approaches needed to confirm safety and efficacy in this highly variable disease.
Acknowledgments
Because of limitations to length of the paper, many significant studies and contributions to this field have not been included in this review, and the author apologizes to investigators whose work was not included in this report.
This work was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL107608) and by the Doris Duke Charitable Foundation.
Authorship
Contribution: M.J.T. wrote the article.
Conflict-of-interest disclosure: M.J.T. has received research funding from Dilaforette, the maker of sevuparin, and GlycoMimetics, the maker of GMI-1070 (rivipansel), and has consulted for Pfizer, Inc regarding development of the phase 3 clinical trial of rivipansel.
Off-label drug use: Drugs described in this report of research studies are not being recommended for clinical off-label use.
Correspondence: Marilyn J. Telen, Box 2615, Duke University Medical Center, Durham, NC 27710; e-mail: marilyn.telen@duke.edu.


![Figure 2. Adhesive interactions involving SS RBCs. (A) Multiple interactions between SS RBCs and endothelial cells, extracellular matrix, and plasma proteins. Red cells express multiple adhesion molecules that recognize ligands on either plasma protein “bridging molecules” or endothelial cell surfaces. Specific adhesion receptors on red cells that contribute to vaso-occlusion include ICAM-4 (the LW blood group protein, a receptor for several integrins), BCAM/Lu (the Lutheran blood group protein, a receptor for laminins (LAM) containing the α5 chain [laminins-10 and -11]), and CD44 (which bears the Indian blood group antigens), which is involved in binding to fibronectin and E-selectin. SA, sialic acid; FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor. (B) Interactions between SS RBCs and leukocytes adherent to vessel walls. Many stimuli may activate leukocyte adhesion, including cytokines and contact with SS RBCs. Adhesion to endothelial cells initially occurs via selectins expressed by endothelial cells but ultimately likely also involves endothelial integrins. ESL-1, E-selectin ligand-1; PSGL-1, P-selectin glycoprotein ligand-1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/7/10.1182_blood-2015-09-618553/4/m_810f2.jpeg?Expires=1765313955&Signature=dkVND3SHMpCdp4mIlc3QEptCItxSx9-BjxVOsyxzZG1KQD0odekvT3AVT2-sfeJmDBq6p4hVvjs5EJ3cNd-Mmczru0Wb6CHCQAwS6IiFOBX68NdTLCruQ74OlFFajkAArHCJUQF13Q0SmHwqkWdaTB5bWdBQva4gBA0fy0mNUp6biWGV25m8G7fJklBTmANI99zWtP6nAdMZNNwBAPvtqm5RlKB5tDdrhZy~6T-f38yN4-cP-OQ4a9lnPpMfZHOyANZuebPNilCsL6300~GjRWmBR81iDZd5rfr9JVYsi4beWNSD-lSI1kvUfqr4lGNcIXF4v8IiZxksddq2yrDx5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Adhesive interactions involving SS RBCs. (A) Multiple interactions between SS RBCs and endothelial cells, extracellular matrix, and plasma proteins. Red cells express multiple adhesion molecules that recognize ligands on either plasma protein “bridging molecules” or endothelial cell surfaces. Specific adhesion receptors on red cells that contribute to vaso-occlusion include ICAM-4 (the LW blood group protein, a receptor for several integrins), BCAM/Lu (the Lutheran blood group protein, a receptor for laminins (LAM) containing the α5 chain [laminins-10 and -11]), and CD44 (which bears the Indian blood group antigens), which is involved in binding to fibronectin and E-selectin. SA, sialic acid; FBN, fibronectin; TSP, thrombospondin; ULvWF, ultra-large von Willebrand factor. (B) Interactions between SS RBCs and leukocytes adherent to vessel walls. Many stimuli may activate leukocyte adhesion, including cytokines and contact with SS RBCs. Adhesion to endothelial cells initially occurs via selectins expressed by endothelial cells but ultimately likely also involves endothelial integrins. ESL-1, E-selectin ligand-1; PSGL-1, P-selectin glycoprotein ligand-1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/7/10.1182_blood-2015-09-618553/4/m_810f2.jpeg?Expires=1765666279&Signature=0JZju4qo7xcka6raALiKcR2Kzy57cu9fXmjjCaYb-b7cs0cxyU9u5jJRuXupIKhiEIPErw52xOV-NcJ7bE8Z8gsvQp86h8MpEgFWIiJ2OW3ojDy~Z~-WSM4TW5u~qprXEDNUBM4yeUHbnreQ-N85DO~OnXuPO2JQfSWl5NcPfXobhKzm37m11kpoDyy0mbu97VvSKV0z6IThRWltoiVdtONrv2od6FIgWc5~snv3nUPcb3G8raQ6Qmj2I1-Jr3TnNhqBuP2ZemWofS2mPhDQ9UuzILGMmtIdnaLn6dC7Pv5H9zJhmfmuV3WwwZKKfeL1P8MBhXOqd3lUKoFFPB8bIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)