Key Points
Dogs with an FVII G96E mutation (FVII-G96E) represent the most common human FVII mutation type and are ideal for testing new therapies.
cFVII gene delivery in FVII-G96E dogs via AAV at a dose effective in humans showed stable and clinically therapeutic FVII expression.
Abstract
Factor VII (FVII) deficiency is a rare autosomal recessive bleeding disorder treated by infusion of fresh-frozen plasma, plasma-derived FVII concentrates and low-dose recombinant activated FVII. Clinical data suggest that a mild elevation of plasma FVII levels (>10% normal) results in improved hemostasis. Research dogs with a G96E missense FVII mutation (FVII-G96E) have <1% FVII activity. By western blot, we show that they have undetectable plasmatic antigen, thus representing the most prevalent type of human FVII deficiency (low antigen/activity). In these dogs, we determine the feasibility of a gene therapy approach using liver-directed, adeno-associated viral (AAV) serotype 8 vector delivery of a canine FVII (cFVII) zymogen transgene. FVII-G96E dogs received escalating AAV doses (2E11 to 4.95E13 vector genomes [vg] per kg). Clinically therapeutic expression (15% normal) was attained with as low as 6E11 vg/kg of AAV and has been stable for >1 year (ongoing) without antibody formation to the cFVII transgene. Sustained and supraphysiological expression of 770% normal was observed using 4.95E13 vg/kg of AAV (2.6 years, ongoing). No evidence of pathological activation of coagulation or detrimental animal physiology was observed as platelet counts, d-dimer, fibrinogen levels, and serum chemistries remained normal in all dogs (cumulative 6.4 years). We observed a transient and noninhibitory immunoglobulin G class 2 response against cFVII only in the dog receiving the highest AAV dose. In conclusion, in the only large-animal model representing the majority of FVII mutation types, our data are first to demonstrate the feasibility, safety, and long-term duration of AAV-mediated correction of FVII deficiency.
Introduction
Factor VII (FVII) deficiency is an orphan autosomal recessive coagulation disorder (1 in 500 000 people1 ) caused by mutations that affect the plasma levels and/or activity of blood coagulation FVII. FVII deficiency is genetically categorized as type I (low activity and antigen) and type II (low activity but normal or near normal antigen levels). Type I is the most common form, affecting ∼70% of patients.2 Although there is some variability in the clinical symptomatology as it relates to the genetic lesion, ∼70% of FVII-deficient patients are symptomatic, and among those, ∼40% have severe deficiency (≤1% plasma levels).3 Extensive hemarthrosis and gastrointenstinal and central nervous system (CNS) bleeds are among the manifestations in patients with severe FVII deficiency. Additional symptoms include epistaxis, muscle hematomas, menorrhagia, and postoperative bleeding.3,4 Currently, acute bleeding episodes are treated by infusion of fresh-frozen plasma, plasma-derived FVII concentrates, prothrombin complex concentrates, and low-dose recombinant activated human FVII (rhFVIIa).5
Ten percent of FVII-deficient children have a severe bleeding tendency (eg, CNS bleeds) in the first year of life.3 In this clinically relevant population subset, early prophylactic treatment can have a substantial benefit. Consequently, there is increased focus on patients afflicted with a severe phenotype, where prophylaxis is the most appropriate therapeutic option. Unfortunately, and in contrast to hemophilia, studies on prophylaxis for FVII deficiency are scarce and fragmented into case reports or meta-analyses of patient treatment data. Despite this, it is generally accepted that doses of FVII (10-30 IU/kg) or rhFVIIa (20-30 µg/kg) administered 2 to 3 times per week are associated with effective outcomes in severe patients.4,6 It is therefore recommended that such high-risk patients be placed on long-term prophylaxis, initiating when the first severe bleed occurs (CNS or gastrointenstinal), often happening at birth.
In contrast to on-demand or prophylactic protein administration, gene therapy has the potential for long-term, stable expression of a therapeutic protein. Hemophilia B has been the archetypal coagulation disorder to potentially be treated by this mode of gene-based prophylaxis. Liver-directed administration of a recombinant serotype 8 adeno-associated viral (AAV8) vector (2E12 vector genomes [vg] per kg) expressing human factor IX in severe hemophilia B patients (≤1 activity) resulted in stable and multiyear expression of human factor IX at ∼6% normal (∼300 ng/mL). This resulted in a significant reduction of bleeding episodes (>90%) and use of prophylactic factor IX protein post–gene transfer.7,8 However, the transient increase in liver enzymes observed in most of the patients treated with 2E12 vg/kg, albeit resolved with a short course of prednisolone, has set an upper limit of dosing in humans using AAV8.
The short half-life of rhFVIIa (∼3 hours9 ) makes the need for gene-based prophylaxis for FVII deficiency especially attractive. Toward that goal, previous work has demonstrated the potential for AAV-mediated gene expression of FVII in vivo. Specifically, administration of AAV8 (mouse FVII transgene) in mice with very low FVII levels (∼1% normal) resulted in stable transgene expression of up to 260% normal.10 In contrast, AAV administration (human FVII transgene) in adult nonhuman primates leads to transgene expression peaking at 2 weeks and declining to a plateau of ∼7% normal.10 In utero gene transfer (AAV serotype 5) in nonhuman primates resulted in rapidly declining expression of human FVII after birth (<5% normal); an effort to increase plasmatic levels by readministration (AAV8) in adulthood of these animals also resulted in the rise/fall of transgene expression to a plateau of ∼7% normal.10 Notably, the observed loss of expression in nonhuman primates was not because of inhibitory antibody formation.10 A rise of FVII activity >10% to 15% normal can achieve hemostasis in FVII-deficient patients.11-14 Therefore, based on the current nonhuman primate data,10 the translational potential of AAV-mediated gene therapy for FVII deficiency remains unclear. A further complication is the lack of data in large-animal models of FVII deficiency, in contrast to hemophilia, and the very limited use of species-specific FVII transgene(s).
Using hemophilic dogs, we have previously established the therapeutic efficacy of continuous expression of activated FVII via AAV8 gene transfer, as a potential treatment of hemophilia patients with inhibitors to factor VIII or IX.15 The importance of canine models of disease for novel therapeutic approaches is further exemplified from liver-directed gene transfer studies in hemophilia B dogs that have accurately predicted the AAV vector dose required to produce a given circulating factor level in humans.16 We have previously described a naturally occurring canine model of FVII deficiency where a single mutation (G96E) in the EGF-2 domain of canine FVII (cFVII) results in very low FVII activity.17 Here, using research animals, we further confirm that this particular genetic defect represents the most prevalent mutation type in humans (type I). This allowed us to use these animals and determine the efficacy of AAV-mediated gene delivery of cFVII as a model of gene-based prophylaxis in FVII deficiency. Although the animals in this study were asymptomatic, like some human patients, the existing correlation of FVII levels and hemostatic outcome in human patients was used as an index for the efficacy of AAV-mediated FVII gene transfer. As described previously, the previous experimental data expressing human FVII by AAV in nonhuman primates10 have unclear predictive value in terms of therapeutic efficacy for FVII deficiency. Addressing this limitation, the overall novelty of our data here is encompassed in the choice of the most appropriate large-animal model of FVII deficiency as well as the longevity, stability, and safety (hemostatic, immunologic, and physiological) of the observed FVII expression at therapeutic levels by AAV.
Methods
Protein purification and AAV production
Cell transfection and western blotting
Human embryonic kidney (HEK293) cells were grown as previously described18 and transfected with a pcDNA3-based construct expressing cFVII or cFVII-G96E17 using Lipofectamine LTX (Life Technologies, Carlsbad, CA). Cell lysates were obtained with RIPA buffer (Life Technologies) as per the manufacturer’s recommendations. Cell lysates (or pooled plasma from wild-type (WT) FVII and FVII-G96E dogs diluted with phosphate-buffered saline [PBS]) were subjected to polyacrylamide gel electrophoresis [4% to 12% Bis-acrylamide/tris(hydroxymethyl)aminomethane (Tris) polyacrylamide gel in 2-(N-morpholino)ethanesulfonic acid buffer] under reducing conditions and western blotting using a rabbit custom polyclonal anti-cFVII antibody (Green Mountain Antibodies, Burlington, VT) and a secondary donkey anti-rabbit immunoglobulin (Ig) G (H+L) antibody (IR-Dye 680, 1:10 000 dilution, Cat. 926-32223; Li-Cor Biosciences, Lincoln, NE). β-actin was used as a loading control detected by a mouse monoclonal antibody (1 µg/mL, Cat. A1978; Sigma-Aldrich, St. Louis, MO) and visualized with a secondary goat anti-mouse IgG (H+L) antibody (IR-Dye 800CW, 1:10 000 dilution, Cat. 926-32210; Li-Cor Biosciences). In all cases, nitrocellulose membranes were blocked with western blot blocking reagent (1:10 in Tris-buffered saline; Roche, Indianapolis, IN) overnight (4°C), and primary and secondary antibodies were incubated at room temperature for 2 hours and 1 hour, respectively, diluted in Tris-buffered saline. Blots were imaged at 169 μm with the Odyssey imager (Li-Cor Biosciences).
Clotting assays and cFVII antigen determination
Clotting assays are described in the supplemental Methods (available on the Blood Web site). cFVII antigen in canine plasma was determined by an enzyme-linked immunosorbent assay (ELISA), using custom antibodies generated against recombinant cFVII (Green Mountain Antibodies). Specifically, a sandwich ELISA was performed with a rabbit polyclonal coating antibody (2.5 µg/mL [capture]) and a rat monoclonal antibody (1 µg/mL [detection]), followed by an anti-rat IgG/horseradish peroxidase–labeled antibody (1:1000 dilution, Cat. 61-9520; Life Technologies). All samples were assayed at a 1:80 dilution using blocking buffer (blocking reagent [Roche Diagnostics] diluted 1:10 in PBS) as a diluent. A standard curve of recombinant cFVII in FVII-G96E pooled plasma was used to interpolate cFVII antigen in the FVII-G96E dogs, with each standard point diluted 1:80 in blocking buffer.
The d-dimer, fibrinogen, and IgG quantification and FVII inhibition assays
These assays are described in the supplemental Methods.
AAV vector administration in FVII-G96E dogs
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Dogs received a single infusion of AAV. Portal vein infusion was performed in N24, the first dog of the study, according to methodology we previously used in hemophilia A and B dogs infused with AAV expressing canine activated FVII.15 The successful liver transduction and expression of cFVII evident in N24 allowed us to use a less invasive route of infusion in the remaining 3 dogs (peripheral vein), as previously described.19 All dogs received infusions of normal canine plasma during AAV administration (and for 3-4 days after). Canine serum chemistries and platelet counts were analyzed in an automated clinical pathology laboratory.
Statistical analysis
Two-tailed Mann-Whitney U tests were performed using GraphPad Prism v5.0b (Macintosh). Statistical differences were considered significant when P < .05.
Results
FVII-G96E dogs are a model of type I FVII deficiency
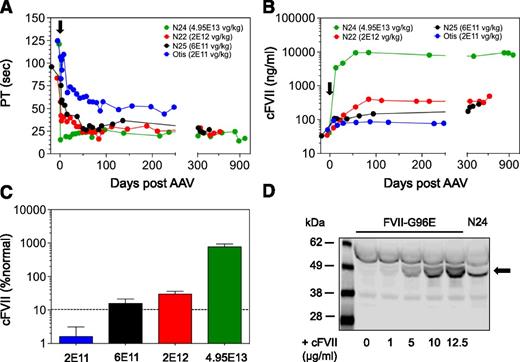
We have previously identified dogs with very low FVII activity because of a missense mutation in the canine F7 gene (G96E) that results in a molecule with a secretory defect.17 Here, 4 research animals with the same defect (FVII-G96E) exhibited an average prothrombin clotting time of 106.4 ± 20 seconds, significantly prolonged compared with normal dogs (26 ± 7.2 seconds, P < .05; Figure 1A), corresponding to <1% cFVII activity (data not shown). However, in order to determine circulating antigen levels in these dogs, we developed an ELISA using recombinant cFVII15 as standard. Using this assay, we determined that the circulating antigen levels in normal dogs were 1040 ± 380 ng/mL (n = 5, WT), whereas FVII-G96E dogs had ∼40 ng/mL (n = 4; Figure 1A). The discrepancy between the baseline activity and antigen levels in the FVII-G96E dogs is likely because of the lower sensitivity of the ELISA vs a PT clotting assay used. Our data are in good agreement with a previous report,20 but the secretory defect of the FVII-G96E protein has only been demonstrated in vitro.17 In order to directly confirm this specific defect in vivo, we used a polyclonal antibody that could detect both WT cFVII and cFVII-G96E (Figure 1B). A western blot of normal canine and FVII-G96E plasma demonstrated that FVII-G96E dogs have no detectable circulating antigen (Figure 1C). Therefore, based on all the data, the FVII-G96E dogs are a model of the most prevalent type I FVII deficiency. As such, these dogs are an ideal model for the evaluation of gene-based therapeutic approaches for congenital FVII deficiency.
Dogs with FVII-G96E mutation show reduced prothrombin time (PT) and minimal cFVII antigen by ELISA and western blot. (A) Using a PT clotting assay, hemostatically normal dogs (n = 5, WT) exhibited a clot time of 26 ± 7.2 seconds. In contrast, FVII-G96E dogs (n = 4) had a clot time of 106.4 ± 20 seconds (P < .05), corresponding to <1% cFVII activity. An ELISA was developed to detect cFVII antigen in canine plasma. Hemostatically normal dogs (n = 5, WT) were found to express 1040 ± 380 ng/mL, in contrast to FVII-G96E dogs (n = 4) that expressed ∼40 ng/mL. (B) Detection of cFVII-G96E or cFVII-WT in cell lysates by fluorescence-based western blot (shown in gray scale). HEK 293 cells were transiently transfected with a plasmid expressing cFVII-G96E (p-cFVII-G96E17 ). Untransfected control cell lysates without or with added recombinant cFVII-WT protein (1 µg) are shown. Black arrow indicates cFVII-WT or FVII-G96E. White arrow indicates the β-actin loading control. (C) Detection of endogenous canine WT FVII or FVII-G96E in canine plasma by fluorescence-based western blot (shown in gray scale). Lane 1, FVII-G96E plasma with 1 µg of recombinant cFVII-WT added (final sample diluted 1:16 in PBS); lanes 2 to 4, normal canine plasma diluted 1:16, 1:32, and 1:64 in PBS (respectively); lanes 5 to 7, FVII-G96E plasma diluted 1:16, 1:32, and 1:64 in PBS (respectively). Black arrow indicates canine WT FVII or FVII-G96E.
Dogs with FVII-G96E mutation show reduced prothrombin time (PT) and minimal cFVII antigen by ELISA and western blot. (A) Using a PT clotting assay, hemostatically normal dogs (n = 5, WT) exhibited a clot time of 26 ± 7.2 seconds. In contrast, FVII-G96E dogs (n = 4) had a clot time of 106.4 ± 20 seconds (P < .05), corresponding to <1% cFVII activity. An ELISA was developed to detect cFVII antigen in canine plasma. Hemostatically normal dogs (n = 5, WT) were found to express 1040 ± 380 ng/mL, in contrast to FVII-G96E dogs (n = 4) that expressed ∼40 ng/mL. (B) Detection of cFVII-G96E or cFVII-WT in cell lysates by fluorescence-based western blot (shown in gray scale). HEK 293 cells were transiently transfected with a plasmid expressing cFVII-G96E (p-cFVII-G96E17 ). Untransfected control cell lysates without or with added recombinant cFVII-WT protein (1 µg) are shown. Black arrow indicates cFVII-WT or FVII-G96E. White arrow indicates the β-actin loading control. (C) Detection of endogenous canine WT FVII or FVII-G96E in canine plasma by fluorescence-based western blot (shown in gray scale). Lane 1, FVII-G96E plasma with 1 µg of recombinant cFVII-WT added (final sample diluted 1:16 in PBS); lanes 2 to 4, normal canine plasma diluted 1:16, 1:32, and 1:64 in PBS (respectively); lanes 5 to 7, FVII-G96E plasma diluted 1:16, 1:32, and 1:64 in PBS (respectively). Black arrow indicates canine WT FVII or FVII-G96E.
AAV delivery of cFVII in FVII-G96E dogs results in long-term cFVII expression
We generated an AAV8 vector that harbors the complementary DNA for zymogen cFVII under the control of a liver-specific promoter/enhancer15,18,21 (AAV8-cFVII). Four FVII-G96E dogs (Otis, N25, N22, and N24) received escalating doses of AAV8-cFVII ranging from 2E11 vg/kg to 4.95E13 vg/kg (Table 1). This range covers the AAV doses employed in a recent liver-directed hemophilia B gene therapy clinical trial, where long-term human coagulation factor IX expression was attained.8 At baseline, all treated dogs had <1% cFVII activity levels. Following AAV8-cFVII administration, we observed a reduction in the PT that reached a plateau (Figure 2A): from 121.1 ± 1.0 seconds to 22.1 ± 3.2 seconds (4.95E13 vg/kg, N24), 83.5 ± 18.4 seconds to 25.0 ± 3.6 seconds (2E12 vg/kg, N22), and 96.0 ± 5.8 seconds to 28.7 ± 3.7 seconds (6E11 vg/kg, N25). Correlating with the PT, we observed an increase in cFVII expression that reached a plateau of 8320 ± 1800, 307 ± 56, and 170 ± 62 ng/mL for the highest to the 6E11 vg/kg administered AAV vector dose, respectively (Figure 2B). These antigen levels corresponded to 770 ± 167%, 29.7 ± 6.2%, and 15.7 ± 5.7% of normal, respectively, and showed AAV vector dose dependency (Figure 2C). Although the 2E11 vg/kg dose administered in Otis resulted in a shortening of the PT from 124.8 ± 4.6 seconds to a plateau of 50.4 ± 4.0 seconds (Figure 2A), total cFVII antigen levels in this dog were close to or at assay sensitivity. Dog N24 exhibited levels of expression higher than those we previously achieved in hemophilia dogs expressing activated cFVII (∼2 µg/mL15 ). To confirm this finding, we used a western blot method to visualize transgene-derived cFVII expression in this dog. At day 84 (well-beyond reaching the expression plateau; see Figure 2B), N24 expressed 9.3 µg/mL based on ELISA that was successfully confirmed by western blot (Figure 2D).
AAV-mediated delivery of cFVII zymogen in FVII-G96E dogs results in stable transgene expression. (A) A PT clotting assay was used to indicate biological activity of the expressed cFVII transgene, following AAV administration (day 0, black arrow) in FVII-G96E dogs. The symbol for the AAV vector dose for each of the FVII-G96E dogs is shown. The first antigen level quantification after AAV administration was within 8 to 13 days. The time to reach the plateau of expression following AAV administration was as follows: N24, day 28; N25, day 50; N22, day 42; and Otis, day 87. (B) AAV administration (day 0, black arrow) in FVII-G96E dogs resulted in cFVII protein expression, determined by ELISA. The symbol for the AAV vector dose for each of the FVII-G96E dogs is shown. (C) Graphical representation of the relationship of AAV vector dose administered and the cFVII antigen levels (% normal) at the expression plateau in the FVII-G96E dogs. Values are shown as mean ± 1 standard deviation, obtained from the individual cFVII antigen levels at each time point after expression reached a plateau for each dog. The dotted line indicates the percent normal FVII that, when achieved, improves hemostasis in FVII-deficient patients. (D) Detection of cFVII transgene expression in N24 plasma (day 84) by fluorescence-based western blot (shown in gray scale). For comparison, recombinant WT cFVII (black arrow) was added in FVII-G96E canine plasma at the indicated concentration. All plasma samples were diluted 1:32 in PBS.
AAV-mediated delivery of cFVII zymogen in FVII-G96E dogs results in stable transgene expression. (A) A PT clotting assay was used to indicate biological activity of the expressed cFVII transgene, following AAV administration (day 0, black arrow) in FVII-G96E dogs. The symbol for the AAV vector dose for each of the FVII-G96E dogs is shown. The first antigen level quantification after AAV administration was within 8 to 13 days. The time to reach the plateau of expression following AAV administration was as follows: N24, day 28; N25, day 50; N22, day 42; and Otis, day 87. (B) AAV administration (day 0, black arrow) in FVII-G96E dogs resulted in cFVII protein expression, determined by ELISA. The symbol for the AAV vector dose for each of the FVII-G96E dogs is shown. (C) Graphical representation of the relationship of AAV vector dose administered and the cFVII antigen levels (% normal) at the expression plateau in the FVII-G96E dogs. Values are shown as mean ± 1 standard deviation, obtained from the individual cFVII antigen levels at each time point after expression reached a plateau for each dog. The dotted line indicates the percent normal FVII that, when achieved, improves hemostasis in FVII-deficient patients. (D) Detection of cFVII transgene expression in N24 plasma (day 84) by fluorescence-based western blot (shown in gray scale). For comparison, recombinant WT cFVII (black arrow) was added in FVII-G96E canine plasma at the indicated concentration. All plasma samples were diluted 1:32 in PBS.
Taken together, these data suggest that AAV-mediated, long-term (up to 2.6 years, ongoing) expression of cFVII is feasible in dogs with type I FVII deficiency. More importantly, an AAV dose as low as 6E11 vg/kg resulted in stable (1.3 years, ongoing) and clinically therapeutic cFVII antigen levels of ≥15% normal.
AAV-mediated expression of cFVII in FVII-G96E dogs is safe
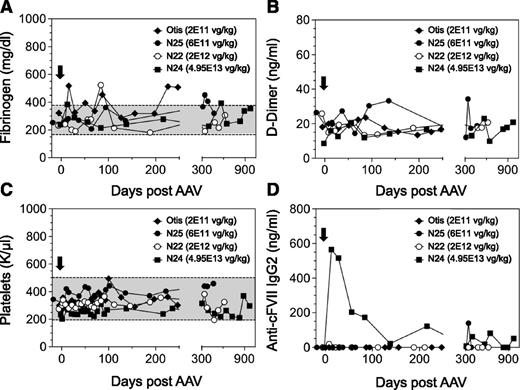
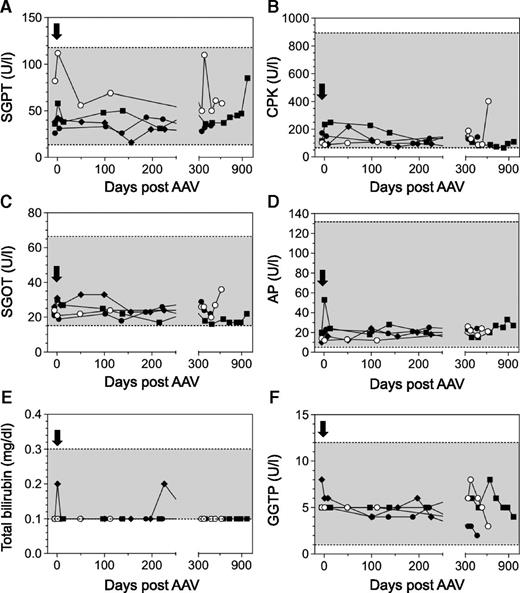
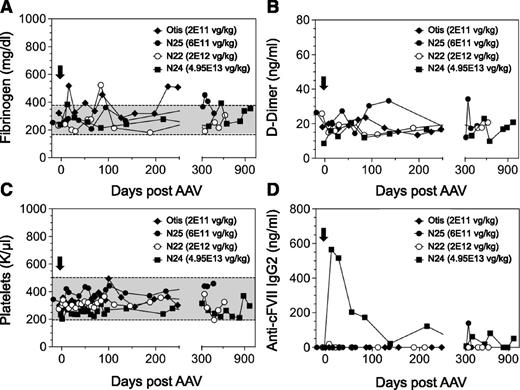
In order to establish the safety of a FVII gene therapy approach in a large-animal model of type I FVII deficiency, we monitored an extensive array of parameters throughout this study. Using markers of liver and kidney function, serum chemistries were found to be within the normal range in all dogs (Figure 3A-F). We also wanted to evaluate any prothrombotic effects in the AAV-treated dogs as a function of time because (1) sustained cFVII expression levels in the AAV-treated FVII-G96E dogs were as high as ∼8.3 µg/mL, and (2) FVII-G96E dogs have a functional intrinsic coagulation pathway (through which the bulk of thrombin is normally generated). We monitored d-dimer and fibrinogen levels that have been used as markers of inadvertent coagulation activation and cardiovascular disease in dogs,22-24 which were found to be within the normal range (Figure 4A-B). Together with the stable platelet counts (Figure 4C) and serum chemistries, this indicates that continuous cFVII expression, even in N24 averaging 8.3 µg/mL for almost 3 years, did not result in evident activation of the endogenous coagulation system or induce a chronic pathological state.
Serum chemistries in FVII-G96E dogs expressing cFVII. Serum glutamyl pyruvic transaminase (SGPT) (A), serum creatine phosphokinase (CPK) (B), serum glutamyl oxaloacetic transaminase (SGOT) (C), alkaline phosphatase (AP) (D), total bilirubin (E), and γ glutamyl transpeptidase (GGTP) (F) levels were monitored throughout the study, before and after AAV administration (black arrow, day 0). A shaded box indicates the normal range of values for each marker. The following symbols are used for the FVII-G96E dogs: closed squares (N24, 4.95E13 vg/kg), open circles (N22, 2E12 vg/kg), closed circles (N25, 6E11 vg/kg), and closed rhombi (Otis, 2E11 vg/kg).
Serum chemistries in FVII-G96E dogs expressing cFVII. Serum glutamyl pyruvic transaminase (SGPT) (A), serum creatine phosphokinase (CPK) (B), serum glutamyl oxaloacetic transaminase (SGOT) (C), alkaline phosphatase (AP) (D), total bilirubin (E), and γ glutamyl transpeptidase (GGTP) (F) levels were monitored throughout the study, before and after AAV administration (black arrow, day 0). A shaded box indicates the normal range of values for each marker. The following symbols are used for the FVII-G96E dogs: closed squares (N24, 4.95E13 vg/kg), open circles (N22, 2E12 vg/kg), closed circles (N25, 6E11 vg/kg), and closed rhombi (Otis, 2E11 vg/kg).
Hemostatic and immunologic markers in FVII-G96E dogs expressing cFVII following AAV administration. Fibrinogen (A), d-dimer (B), and platelet counts (C) were monitored throughout the study, before and after AAV administration (black arrow, day 0). A shaded box indicates the normal range of values for each marker. All d-dimer levels were within normal range. (D) Anti-cFVII IgG2 levels were determined in all FVII-G96E dogs that received AAV8-cFVII. IgG2 levels are depicted following background correction (for each dog). The following symbols are used for the FVII-G96E dogs (in all panels): closed squares (N24, 4.95E13 vg/kg), open circles (N22, 2E12 vg/kg), closed circles (N25, 6E11 vg/kg), and closed rhombi (Otis, 2E11 vg/kg).
Hemostatic and immunologic markers in FVII-G96E dogs expressing cFVII following AAV administration. Fibrinogen (A), d-dimer (B), and platelet counts (C) were monitored throughout the study, before and after AAV administration (black arrow, day 0). A shaded box indicates the normal range of values for each marker. All d-dimer levels were within normal range. (D) Anti-cFVII IgG2 levels were determined in all FVII-G96E dogs that received AAV8-cFVII. IgG2 levels are depicted following background correction (for each dog). The following symbols are used for the FVII-G96E dogs (in all panels): closed squares (N24, 4.95E13 vg/kg), open circles (N22, 2E12 vg/kg), closed circles (N25, 6E11 vg/kg), and closed rhombi (Otis, 2E11 vg/kg).
The FVII-G96E dogs treated here have ∼40 ng/mL of endogenous FVII antigen. Moreover, this material harbors a missense mutation that, as a molecule, could potentially be immunologically different to the cFVII expressed following AAV gene delivery. Therefore, it was imperative to determine the presence and extent of an immunologic response to cFVII transgene expression facilitated by AAV administration. Using purified recombinant cFVII protein as the “immunogen” in ELISA-based IgG detection assays, we found that dog N24 exhibited a transient elevation of anti-cFVII specific IgG2 that was absent in the other AAV-treated dogs that achieved clinically therapeutic cFVII levels (N22 and N25) or in Otis (Figure 4D). We did not detect any anti-cFVII IgG1 for any of the treated FVII-G96E dogs (data not shown). Based on a “Bethesda” type assay, we found that the transient IgG2 response to cFVII in N24 was not inhibitory (supplemental Figure 1) and did not affect the transgene expression plateau (see Figure 2B).
Discussion
Monogenic disorders with an established clinical phenotype, a well-characterized molecular and genetic defect and a defined target tissue are ideal candidates for gene-based therapies. These treatment modalities are further supported by data from protein replacement therapies with long-term efficacy data when they exist. Hemophilia B is an exemplary disorder that fulfills these requirements with the additional advantage of available large-animal models for preclinical efficacy studies. As a result, hemophilia B has been extensively used in gene therapy clinical trials25 that have recently defined the efficacy and upper limit of AAV8 vector dosing in humans.7,8 In contrast to hemophilia, clinical data on long-term protein prophylaxis and adequate animal models of FVII deficiency are limited. Nevertheless, a rise >10% to 15% normal should provide adequate hemostasis in patients with a severe phenotype.11-14 Here, we used this threshold as a therapeutic metric in a unique canine model of type I FVII deficiency. We show for the first time that expression of cFVII via AAV gene transfer is safe, is long-term, and can reach therapeutic levels using vector doses that are considered acceptable in humans.
Mice are routinely used in preclinical studies for novel therapeutics. The only viable mouse model of FVII deficiency consists of a targeted insertion of a tetracycline-regulated mouse FVII complementary DNA expression cassette that results in <1% FVII levels in the uninduced state.26 In these animals, AAV-mediated delivery of FVII was shown to result in long-term expression that was hemostatic and improved animal survival.10 In the same study, however, FVII transgene expression in nonhuman primates after AAV administration exhibited a transient peak, resulting in a plateau of ∼7% normal within a few weeks. Similar patterns were observed following in utero AAV administration or even after alternate AAV serotype readministration in the same animals during adulthood.10 The reason for this temporal expression pattern was not apparently because of formation of anti-human FVII antibodies and remains unclear. Although AAV-mediated FVII expression was indeed demonstrated in nonhuman primates,10 it was short term, below what is considered the therapeutic threshold for FVII deficiency, and in a genetic context different to the majority of FVII-deficient patients that have FVII missense mutations. Therefore, we believe previous work10 has unclear predictive value in terms of AAV-mediated gene therapy for FVII deficiency.
There are 3 additional key parameters that differentiate the data presented here. First, stable and therapeutic transgene expression was attained with a single AAV infusion, using vector doses that are considered acceptable in humans. Second, the defect in the FVII-G96E dogs arises from a naturally occurring mutation resulting in impaired FVII secretion and activity, a representative of the most prevalent mutation type in human FVII deficiency.2 Therefore, in this only available large-animal model of FVII deficiency, AAV-mediated expression of WT FVII simulates a clinical scenario in the proper and most likely genetic context. Lastly, the use of a species-specific transgene here ensures that the known (or yet unknown) interactions of the AAV-expressed FVII with the host system would avoid potential species-specific incompatibilities. For example, human FVII interacts poorly with mouse tissue factor.27 Notably, the interaction of the endothelial protein C receptor (EPCR) with human FVII in mice may be involved in the FVII biodistribution after bolus infusion.28 It is unclear if human FVII can interact with endogenous EPCR in animals other than mice, such as dogs or nonhuman primates, or if endogenous EPCR can modulate FVII levels when the latter is expressed continuously via AAV. Nevertheless, and in contrast to previous studies using a human FVII transgene in nonhuman primates,10 the use of cFVII in the FVII-G96E dogs ensures that the endogenous EPCR, tissue factor, or other interaction with AAV-expressed FVII retains its species specificity. In aggregate, we believe that the FVII-G96E dogs in the present study provided the proper context (genetic and molecular) to evaluate and validate our gene therapy approach for human FVII deficiency.
Manifestation and management of symptoms begins early in the life of patients with congenital bleeding defects. Therefore, there is considerable value in prophylaxis studies in young patients. In the case of hemophilia, early prophylaxis has demonstrated added clinical benefits compared with on-demand therapy.29,30 Specifically, for FVII deficiency, and based on limited data, early prophylaxis with rhFVIIa is recommended.6 Information on the threshold of FVII circulating levels that are hemostatic is limited, but levels >10% to 15% normal are generally considered sufficient for improved hemostasis. In accordance with this finding, and based on use of rhFVIIa in surgery for FVII-deficient patients, those with <10% normal should receive replacement therapy, whereas those with >30% normal likely should not.11 Our data suggest that AAV-mediated gene transfer can achieve and sustain therapeutic FVII levels. Unfortunately, the FVII-G96E dogs in our study did not exhibit spontaneous bleeds. Therefore, we could not directly establish the relationship between FVII circulating levels attained here and hemostatic efficacy. However, the lack of bleeds here should be evaluated considering the following: First, in the initial description of cFVII deficiency 2 research animals were also asymptomatic, in contrast to the 3 companion animals that were symptomatic.17 It is possible that the living environment and daily activities of companion animals are more likely to result in bleeds compared with animals in a research colony. Second, FVII-deficient patients can also be asymptomatic with regard to bleeds. Lastly, the therapeutic threshold for human FVII deficiency was based on factor levels after infusion; here, the AAV-treated dogs expressed FVII at therapeutic levels continuously. Taken together, we believe that in spite of the asymptomatic nature of the AAV-treated dogs in this study, attaining such steady-state levels in humans would likely be hemostatic.
Treatment-associated complications in FVII deficiency include thrombotic events. However, these are rare and usually associated with type II mutations.31 Here, dogs N22 and N25 expressed therapeutic levels of FVII for >1 year; dog N24 represents a case where FVII levels have been sustained at 770% normal for almost 3 years. Notably, the mode of treatment presented here is drastically different and more provocative than that to which human patients have typically been exposed. However, our data provide an opportunity to study for the first time the long-term effects of FVII overexpression in vivo in a large-animal model of the disorder. No thrombotic complications or other events suggesting a compromised animal physiology were observed. These data demonstrate the remarkably wide therapeutic window and the elevated safety of a FVII gene-based prophylaxis. In contrast to hemophilia, inhibitory antibodies in FVII-deficient patients are extremely sporadic6 but can occur against FVII or rhFVIIa.32 Unfortunately, limited available data do not allow for establishing cause-effect relationship(s) in the affected patients. Here, an IgG2 response to the FVII transgene product was seen in dog N24, which expressed supraphysiological levels of FVII. The canine IgG2 corresponds to human IgG4, a subclass with a strong association with high-titer FVIII inhibitors in hemophilia33 that is also reported predominant in the anti-rhFVIIa antibody response in FVII deficiency.34 The reasons for the observed immune response here remain unclear. All dogs in this study received plasma infusions during and for 3 to 4 days after AAV administration; our empirical evidence with 1 hemophilia A dog suggested that normalizing hemostasis during AAV administration may lead to increased transgene expression.35 Dog N24 also received a single prophylactic plasma infusion 3 weeks prior to AAV administration and at 2 and 5 months after AAV administration, all for an unrelated procedure. It is possible that the elevation of IgG2 observed may have resulted from the prior plasma exposure. However, the infusions post–AAV administration did not cause another elevation of anti-cFVII IgG2. Nevertheless, the immune response in N24 was transient, was noninhibitory, and resolved within a few weeks post–AAV administration, without affecting the FVII transgene expression plateau. Most importantly, none of the dogs with clinically relevant expression exhibited an immune response to the cFVII transgene.
Data from the recent hemophilia B gene therapy trial are now the comparator for AAV8 gene-based therapeutic approaches, and in particular for bleeding disorders.7,8 Based on the relationship of AAV vector dose and the transgene expression plateau, our data suggest that AAV8 administration at a vector dose considered acceptable in humans (6E11 vg/kg) would result in stable and clinically therapeutic levels of FVII in human patients. The hemophilia B dogs have accurately described the relationship between AAV vector dose and a given factor level expression of a human factor IX transgene in human clinical trials.16 In lieu of this, and considering that endogenous FVII levels in normal dogs are twice that of humans, the 15% normal levels attained here using 6E11 vg/kg would correspond to 30% normal in humans. Modifications such as in the transcriptional regulation of the FVII transgene may provide an even more favorable relationship between AAV vector dose and vector-derived FVII expression. For example, recent data using conserved transcription factor motifs in a combinatorial approach36 provide evidence for a further strategy to enhance liver-specific expression of a FVII transgene. However, additional preclinical studies will be needed to document the benefit of such modifications.
In conclusion, we have provided the first evidence of the efficacy and safety of a long-term gene therapy approach in a large-animal model of the most prevalent mutation type in FVII deficiency. Moreover, the therapeutic AAV vector doses employed are within the limits considered acceptable in humans, further supporting the feasibility of this approach in humans. Like FVII deficiency, Leber congenital amaurosis 2 and familial lipoprotein lipase deficiency are also examples of rare or orphan disorders with a limited patient base. However, this has not prohibited development of successful AAV gene therapy clinical applications for these disorders.37,38 As such, we believe that our data are clinically relevant and hold considerable promise for gene-based prophylaxis in human FVII deficiency.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following colleagues: Drs Sriram Krishnaswamy, Rodney M. Camire, and Valder R. Arruda at the University of Pennsylvania for their help throughout the study and manuscript preparation.
Funding was provided by the National Institutes of Health (National Heart, Lung and Blood Institute grants P01HL064190 [P.M.] and HL63098 [T.C.N.]), the Howard Hughes Medical Institute (K.A.H.), and the Pennsylvania Department of Health.
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Authorship
Contribution: O.A.M.-C. performed research, analyzed data, and wrote the manuscript; S.M.S., D.A.B., R.A.R., E.M., and A.F. performed research; G.P. assisted in the development of the cFVII antigen ELISA; S.Z. manufactured the research-grade AAV vector; T.C.N. performed research and analyzed data; K.A.H. designed research; and P.M. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: K.A.H. is currently an employee of Spark Therapeutics. P.M. receives research funding through competitive grants from “Bayer Hemophilia Awards Program,” additional research funding from NovoNordisk A/S, and salary (spouse) from Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Paris Margaritis, 5024 Colket Translational Research Building, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: margaritis@email.chop.edu.